海洋环境中油气管道的微生物腐蚀研究进展
2022-05-28娄云天何盛宇陈旭东钱鸿昌张达威
娄云天,何盛宇,陈旭东,钱鸿昌,张达威
海洋钻采设施的腐蚀及防护
海洋环境中油气管道的微生物腐蚀研究进展
娄云天1,2,何盛宇1,2,陈旭东1,2,钱鸿昌1,张达威1,2
(1.北京科技大学 新材料技术研究院,北京 100083;2.北京科技大学顺德研究生院,广东 佛山 528399)
海上油气集输管道的腐蚀能够导致严重的环境风险和经济损失,其中微生物腐蚀一直以来被认为是造成该问题的主要因素之一。针对海洋环境油气管网中腐蚀性微生物的来源进行了分类,包括油藏内源性微生物、外注海水以及微生物采油(MEOR)引入的外源性微生物。分析了海底油藏储层中流体化学物质特性,确认其富含甲烷、硫化物、挥发性脂肪酸等,并依据内源微生物代谢及产物特征进行了分类,包括硫酸盐还原菌(SRB)、产甲烷菌、发酵菌以及铁还原菌(IRB)。同时,通过举例分析某油田采出水中微生物群落丰度特征,阐明了外源微生物长期受到油田开采环境胁迫后微生物群落的变化规律。在此基础上,进一步针对海上油气集输管网内涉及的微生物代谢产物理论、电活性微生物腐蚀理论以及腐蚀性微生物之间的协同与拮抗作用进行了全面的归纳总结。最后,对目前以纯培养或模式菌株混合培养为主要方式的微生物腐蚀研究中存在的问题进行了讨论,并对基于生物技术的新型防腐手段进行了展望。
油气集输管道;微生物腐蚀;油藏微生物;腐蚀机理
随着我国海洋能源产业不断发展,加速海洋油气资源的合理开发利用以及油气开采装置的升级势在必行[1]。海上油气占全世界油气资源总量的30%以上,并且开采量逐年增加[2]。海底管道作为海上油气集输系统中重要的组成部分,被誉为海洋油气生产系统的“动脉”[3]。据统计,由腐蚀造成的海底管道事故占比高达37%[4]。在长时间服役过程中海底管线不可避免地遭受腐蚀甚至失效的危害,一旦出现损伤或破裂将造成严重的安全事故和经济损失[5-7]。轰动一时的美国阿拉斯加Prudhoe Bay油管泄漏事件造成原油日减产40万桶,导致国际油价一度上升,当地8 000 m2的土壤和水源受到严重污染。同时,由于管道泄露,油气运输被迫中断,27 km的管线被更换。事故调查报告显示,管道内部检测到大量微生物、硫化物/氯化物沉积和腐蚀性气体(CO2、H2S)[8-9]。
海上油气开采过程中通常使用采出水回注的方式进行生产,该过程极易向原油中混入微生物和沙石等腐蚀因素,加之大量来自海水中的有机物质、无机盐以及油藏内可溶性碳氢化合物,为微生物的生长繁殖提供了必要的能量来源,管道腐蚀失效的风险被进一步增加。油气储集层中的微生物群落结构以及代谢方式复杂且多样,其中不乏典型的腐蚀性微生物,油气集输系统中多相流集输管线、回注水管线以及污水处理装置等组成部分极易受到微生物腐蚀(Microbiologically Influenced Corrosion,MIC)的危害。相关事故分析显示,超过20%油气管线故障和原油泄漏直接或间接地与MIC有关,其中超过70%由硫酸盐还原菌引起[10-12]。
目前,有关MIC的研究大部分还依靠实验室条件下选取模式菌株或者实地环境采样的方式进行纯培养或混菌测试[13-16]。然而,依靠纯培养的方式几乎无法还原油气管线在服役过程中复杂环境微生物代谢所引起的腐蚀行为,以及与之相匹配的高腐蚀速率。长期以来,由于受限于检测手段,MIC领域一直缺乏有关基于生物膜复杂性、材料与腐蚀产物之间关系的研究。近些年,环境微生物组学的快速兴起似乎能够用于解决以上问题,宏基因组学、转录组学以及代谢组学能够实现对实验室条件下无法复制的严苛环境中微生物种类、分布和代谢特征,以及腐蚀性/非腐蚀性代谢产物等因素进行多维度的监检测,结合新型MIC缓解和治理方法,必然会为海上油气管线MIC相关研究的进展提供有价值的信息。
1 腐蚀性微生物的来源
1.1 内源性微生物
早在1926年,Bastin等[17]从Illinois盆地的油田中成功分离出硫酸盐还原菌,提出了油气储集层中可能存在微生物的假设。在海洋环境中,沉积物1 m以下的生态系统被称为“深部生物圈”,极限深度可达4 000 m以上,其中就包含了大量的海底油气资源[18]。海底沉积层覆盖了地球表面近70%的面积,还栖息着超过90%的海洋微生物,为海底油气储集层中微生物的多样性提供了可能[19-20]。Spark等[21]在欧洲北海油田中位于井下深度约4.5 km的岩心中发现了微生物群落。通过16s RNA序列比对,岩心和钻井泥浆中的微生物种类完全不同,证明了油藏极端环境下内源微生物的存在。
海底油藏环境严苛,根据储层中流体化学物质特性(如甲烷、硫化物、挥发性脂肪酸等)以及微生物群落分析,表明油气储集层主要为无氧或低氧环境,油藏微生物群落为了适应恶劣的生存环境衍生出了复杂的代谢方式。值得注意的是,许多储集层中的微生物能够进行铁还原以及硫酸盐还原呼吸。在高温储集层中,产甲烷菌通常占据生态位成为优势群落,协同乙酸氧化过程在油藏内物质循环中发挥着重要的作用。当环境中硫酸盐含量较低时,微生物群落以发酵反应为主要驱动力,H2、CO2和乙酸等发酵菌代谢产物作为底物,为产甲烷菌提供能量来源,通过共代谢和互养作用维持生长,微生物群落之间保持一定程度的代谢多样性是一种重要的生存机制[22-23]。依据细菌代谢方式及产物的不同可以分为以下几类。
1.1.1 硫酸盐还原菌
硫酸盐还原菌(Sulfate-Reducing Bacteria,SRB)在自然界的分布十分广泛,并且在石油生态系统中扮演着重要的角色[24]。SRB是一类能够通过将硫酸盐、亚硫酸盐和硫代硫酸盐作为最终电子受体还原成H2S从而获得能量的原核微生物[25]。SRB能够利用糖类、氨基酸、脂肪酸等百余种化合物作为电子供体,通过还原多种价态的含硫化合物最终完成新陈代谢过程。油藏中分离得到的SRB属于嗜温菌或嗜热菌,甚至在一些油井中分离得到了超嗜热SRB,其最适生长温度超过80 ℃[26]。同时,部分SRB还能够耐受较高浓度的NaCl,其浓度最高可达23%[27]。目前,在油藏中分离得到的SRB超过40个属,主要分为四大类[28-29]:(1)变形菌门(),代表菌包括脱硫弧菌属()和脱硫杆菌属();(2)嗜热脱硫杆菌属();(3)嗜热脱硫弧菌属();(4)硫酸盐还原古菌,代表菌有古生球菌属()和暖枝菌属()。
1.1.2 产甲烷菌
产甲烷菌是一类专性厌氧菌,能够通过代谢氢、CO2、乙酸盐、甲胺等低分子量物质获取能量,其最终产物为甲烷[30]。产甲烷菌的生长活动受到温度、盐含量、pH和氧含量等因素的影响,甚至10‒6量级的氧浓度都会对其生长产生显著的抑制作用[31]。目前,在油气藏生态系统中分离得到的产甲烷菌主要分布于古菌域广古菌门的5个目,包括甲烷微菌目()、甲烷杆菌目()、甲烷球菌目()、甲烷炙热古菌目()和甲烷八叠球菌目()。产甲烷菌代谢底物包括3种类型:氢营养型、乙酸营养型和甲基营养型[30]。已发现的产甲烷菌并不完全严格依照以上叙述的底物进行代谢,据统计超过70%的产甲烷菌能够利用H2作为电子供体还原CO2产生CH4,如、、等,同时还能够利用CO、丙酮酸盐或乙二醇等代替H2,但是其效率显著下降,仅为H2作为电子供体时的1%~4%[32-33]。甲基营养型产甲烷菌如不仅代谢甲醇、甲胺等简单甲基化合物,而且一些较为复杂的甲基胺类化合物(如胆碱、甜菜碱等)同样可以维持其代谢需要。
1.1.3 发酵菌
发酵菌是一类能够通过代谢糖类、多肽等底物,产生有机酸、CO2和H2等发酵产物的微生物。发酵菌在产能反应过程中无需外源电子受体,通过将发酵底物的氧化过程与菌体内次级代谢产物的还原过程相互耦合获取能量。作为油藏微生物群落中重要的组成部分,发酵菌可以分为嗜热菌和嗜盐菌2个大类。已分离出的嗜热发酵菌大多数属于热袍菌属()、石衣菌属()、栖热腔菌属(),其最适生长温度为50~70 ℃。通过比对分析从全世界范围内不同油藏分离得到的嗜热发酵菌的生存环境和代谢特点,其底物种类和生长温度都与油气储集层的原生环境高度相关,这一现象表明该类微生物为油藏原生细菌[34]。嗜热发酵菌不但能够在高温条件下正常生存,在营养物质缺乏的情况下依然能够保持较好的活力。Takahata等通过检测发现日本Kubiki油田的石油产出水中的细菌浓度高达4.6×104cells/mL,即使在饥饿状态下也能够保持细胞活性长达200 d以上,这一特性对其能够在油藏环境中长期生存至关重要。在有些含盐量较高的油藏环境中,嗜盐发酵菌能够通过积累可溶性有机质维持细胞与环境之间的渗透压平衡,而并非仅仅使用Na+、K+等离子[35-36]。
1.1.4 铁还原菌
铁还原菌(Iron-reducing bacteria,IRB)是一类能够将H2、有机物等作为电子供体,Fe3+作为终端电子受体的一类严格厌氧或兼性厌氧的细菌或古菌[37-38]。研究人员在不同的油藏中分离得到了如脱铁杆菌属()、地芽孢杆菌属()等典型嗜热铁还原菌,其能够使用乳酸、氨基酸、醋酸盐等作为电子供体[39]。在近中性的厌氧环境中,化学和生物过程中的三价铁氧化物作为电子收集单元极易被还原,研究证明三价铁氧化物在非硫化物沉积过程中主要受铁还原菌的代谢过程控制[40]。在一些含硫化物的环境中,如油藏、海底沉积物等,较早的研究结果显示Fe3+的还原过程是由于微生物成因的H2S导致的。最新的研究已经证实了铁还原菌能够利用三价铁还原酶直接进行反应,并且占总还原量的90%[41]。
1.2 外源性微生物
在海上石油开采过程中,为了保持油气储集层的压力,需要以不断注入海水或回注水的方式驱动原油的开采。同时,为了确保长距离油气管道的完整性以及安全运行,需要进行水压测试或周期性的管道停输检修。在以上操作过程中,海水中种类丰富且组成复杂的微生物不可避免地被引入到集输管道中,必然会在复杂的管网系统中形成生物膜且造成严重的微生物腐蚀[42-43]。相较于陆地,海洋环境中微生物对于高盐、高压、高温等较为严苛的环境因素的耐受能力普遍更强,这意味着海上油气管道内的微生物及其生物膜的适应性更强且难以杀灭。Zhou等[44]利用环境基因组测序分析手段对中国渤海某油田采出水中的微生物群落多样性进行了分析。该油田由于长期注入海水或采出水回注,导致储集层中被引入大量外源微生物。以该研究中样本1基于RNA的结果分析为例,丰度及活性排在前十的菌属包括博斯氏菌属(,68.8%)、不动杆菌属(,7.0%)、鞘氨醇单胞菌属(,3.2%)、嗜氢菌属(,4.7%)、无色杆菌属(,3.0%)、短波单胞菌属(,2.0%)、甲基杆菌属(,1.9%)、埃希氏杆菌属(,1.7%)、假单胞菌属(,1.4%)、伯克氏菌科(,0.4%)。不难发现,以上菌属中包含多种如博斯氏菌属()、甲基杆菌属()和热硫还原杆菌属()等硫氧化细菌(Sulfur- oxidizing bacteria,SOB),具有将不同价态的含硫化合物氧化为硫酸盐的能力[45-46]。同时,该油井由于长期受到SRB及其产生的H2S的污染,用于缓解该问题的硝酸盐类抑制剂的注入促进了硝酸盐还原菌(Nitrate-Reducing Bacteria,NRB)的生长,通过竞争摄取电子供体的方式与SRB形成竞争性抑制,其中代表性的菌属有假单胞菌属()、嗜氢菌属()、不动杆菌属()和无色杆菌属()等[47-48]。
另外一种涉及油藏内引入外源微生物的方式是微生物采油(Microbial Enhanced Oil Recovery,MEOR)。作为一种主要基于微生物学、分子生物学技术的三次采油方法,通过向油藏内注入特定的菌种或营养物质,利用其自身生长代谢特性或产生功能性产物(产酸、产气或产生物溶剂)来改变油气储集层内环境和微生物种群结构,进而降低原油黏度或溶解岩层以增加储层渗透率,从而达到提高采油率的目的[49-51]。MEOR相关微生物,包括醋酸杆菌属()、芽孢杆菌属()以及部分产甲烷菌()等,能够在代谢过程中产生有机酸或生物表面活性剂。Kato等[52]分离得到了一株产乙酸菌GT1,其不仅可以利用有机物发酵产生乙酸,还能直接从铁单质中摄取电子,以上代谢特征极易引起微生物腐蚀。
2 生物膜对腐蚀的影响
生物膜的形成是微生物抵御外界环境变化维持群落内稳态的基本生存机制,如图1所示,处于悬浮状态的微生物通过附着、聚集等步骤逐渐成为复杂且稳定的混合微生物群落。相比于悬浮状态,微生物嵌入由胞外聚合物(EPS)构成的基质后不仅能够提高代谢过程的稳定性,还能加强互养微生物种群之间的协同作用,这一特性使得腐蚀性微生物的危害进一步增加[53-54]。海上油气集输系统由复杂的管道网络构成,多相流的传输形式以及部分管网中较低的流速加快了腐蚀性生物膜以及沉积物在弯头、焊缝和阀门等腐蚀敏感区域的形成和堆积,进一步减缓了管道内物料的流速,最终导致油气运输停滞。

图1 生物膜形成的一般过程示意图[53]
在实际工况下,腐蚀性生物膜由微生物胞外聚合物(蛋白、多糖、核酸等)和腐蚀产物(FeS、FePO4和FeCO3等)共同构成。由于EPS所构成的三维网状结构(宏观上多呈现出黏液状),强化了细菌之间以及细菌与腐蚀产物之间的黏附性。同时,细菌生物膜中往往含有如氨基酸、糖醛酸等含有大量负电荷基团的有机物,能够通过螯合、吸附等方式沉淀金属阳离子,进一步刺激腐蚀性微生物与金属离子之间的相互作用以及电子传递过程[55]。由于环境微生物种类复杂,所构成的微生物群落以及代谢产物多样,在生物膜中往往包含不同化学浓度梯度以及氧化还原电位的微环境。从微生物代谢多样性的层面分析,这种结构使生物膜内的不同代谢类型的微生物之间建立了更有利的共生条件,促进了共代谢和互养作用。但是从微生物腐蚀的角度分析,浓差电池的产生极易造成金属表面局部阴阳极的形成,是引起油气管线局部腐蚀主要的原因之一。腐蚀性微生物对于油气管网的影响可以总结为以下3点:(1)微生物及其分泌的EPS作为有机沉积物率先附着并沉积,使管道内环境的理化性质发生改变;(2)腐蚀性微生物的代谢活动及其产物会加速管道内腐蚀产物的堆积;(3)腐蚀性生物膜的沉积会改变原有沉积物的性质,从而进一步加速腐蚀。
3 微生物腐蚀机理
3.1 微生物代谢产物腐蚀理论
SRB、发酵菌等微生物能够产生具有腐蚀性的代谢产物,如硫化物或有机酸等,该过程被称为化学微生物腐蚀(Chemical Microbiologically Influenced Corrosion,CMIC)。这些腐蚀性代谢产物与金属材料发生反应后极易在管网内形成沉积物,在促进内腐蚀进一步发展的同时还会造成管网堵塞。以SRB为例,其产生的H2S微溶于水后产生HS‒使局部环境呈酸性,造成管网内部的局部腐蚀穿孔,解离出的氢也会富集在材料的缺陷处,造成氢渗透或开裂。同时,H2S扩散到金属表面发生反应生成具有导电性的无定形FeS产物层,随后经过反复溶解Fe(HS)+/HS‒再沉积,腐蚀产物层中积累了更多的HS‒,进一步加速阳极溶解速率[56-58]。有机酸的产生同样对油气管网具有很强的腐蚀性,发酵菌及其代谢产物在腐蚀性生物膜中的作用近些年得到了广泛关注。研究发现,醋酸菌能够在厌氧条件下借助Wood–Ljungdahl通路中的金属蛋白/金属酶以H2和CO2为底物生成乙酸,即使生物膜中有机酸的浓度很低,也能增加金属腐蚀的风险[59]。
3.2 电活性微生物腐蚀理论
具有电活性代谢能力的微生物通过胞外电子传递(Extracellular Electron Transfer,EET)的方式从金属氧化过程中提取电子,或者将细胞内有机物彻底氧化后释放的电子传递到细胞外的电子受体(如硫酸盐、硝酸盐或金属难溶物等),以上过程能够诱发或加速腐蚀[60-62],被称为电化学微生物腐蚀(Electrochemical Microbially Influenced Corrosion,EMIC)。目前,胞外电子传递主要有3种机制:直接电子传递(Direct Electron Transfer,DET)、间接电子传递(Mediated Electron Transfer,MET)和电运动机制(Electrokinesis)[63-65]。直接电子传递是指细菌通过外膜的细胞色素C直接与电子受体接触,其电子传递效率较高,但生物膜与电子受体之间的接触面积直接决定了电子传递效率的上限,且无法进行较远距离的电子传输[66]。间接电子传递是指细菌通过内源或外源的电子穿梭体,在细菌与电子受体之间通过往复的氧化还原反应实现较长距离的电子转移。电运动机制是指细菌通过将电子传递到细胞膜表面,然后依靠布朗运动或鞭毛驱使细胞撞击电子受体表面,撞击瞬间完成电子传递过程[67]。
CMIC与EMIC在油气管道内腐蚀性生物膜中存在着平衡与转化。CMIC很大程度上依靠碳氢化合物等有机碳源的降解与硫酸盐或硝酸盐的还原反应耦合驱动腐蚀的发生,而EMIC则是微生物主动驱使腐蚀过程,两者之间的转化取决于可用的有机碳源是否充足。以典型腐蚀性微生物为例,当作为有机碳源的乳酸充足时,的CMIC过程导致FeS为主的腐蚀产物堆积,而当环境中可用的乳酸含量逐渐减少时,的代谢模式发生了转变,利用从Fe0直接获取电子的方式对CMIC过程中的能量缺口进行代偿。从局部腐蚀形貌变化情况来看,可用有机碳源减少至10%时,其腐蚀程度最严重。而将有机碳源全部去除后,腐蚀程度显著减弱,表明EMIC过程并不能维持全部的代谢需求,仅可以作为腐蚀性微生物解决环境突变的一种应对策略[68]。
3.3 腐蚀微生物之间的协同与拮抗
由于实际腐蚀环境中多变的物理化学因素,腐蚀性微生物膜中涉及到十分复杂的代谢过程。如图2所示,环境微生物的多样性结构使得不同种类微生物之间在应对外界环境变化时存在协同和拮抗的相互作用,最终通过群落演变、生物膜成熟直至形成具有腐蚀性的复杂微生物群落。

图2 涉及海底油气管道微生物腐蚀机理汇总[73]
在油气集输系统中,腐蚀性生物膜中微生物之间相互协同互补的代谢模式扮演着重要的角色。实际服役环境中,在同一区域的腐蚀产物沉积层中往往能够同时分离得到发酵产酸菌以及具有氢代谢特征的菌种,此类微生物多出现在含有采出水的油气生产设施中,通过发酵反应代谢挥发性脂肪酸、醇或碳氢化合物来生长,发酵过程中产生的氢被生物膜的氢营养型微生物消耗,如产甲烷菌[69-70]。有机酸类代谢产物不仅能够直接对金属造成腐蚀,发酵产生的H2还能够还原单质硫,从而产生大量的硫化氢[71]。以上过程表明,发酵产酸菌能够通过产生甲酸、乙酸等途径来刺激电活性微生物的生长,从而加速金属腐蚀进程[72]。此外,当铁还原菌和铁氧化菌(Iron-oxidizing bacteria,IOB)同时存在于生物膜中时,IOB能够通过促进金属氧化析出腐蚀产物(铁氧化物和铁硫化物),形成保护性腐蚀产物层,限制金属表面与腐蚀环境直接接触。然而,IRB利用金属氧化沉积物作为电子受体,导致金属表面再次暴露于腐蚀产物和腐蚀性微生物。
4 微生物腐蚀的减缓与防治
油气管道中清理内部MIC广泛使用物理刮擦与非氧化性杀菌剂相结合的方法。传统的杀菌剂有戊二醛、三氯异氰脲酸(TCCA)和四羟甲基硫酸磷(THPS)等。然而,由于使用机械破坏的方式导致腐蚀性微生物从破损的生物膜中扩散出来,重新弥散到管网中导致腐蚀性微生物充分地扩散,反而进一步加剧了腐蚀[74]。同时,部分管道内长期形成的保护性铁氧化物沉积层也会被清除,导致基体重新暴露在外面。长期使用杀菌剂对油气集输管网进行清理,不仅会导致管内微生物群落的改变,且新的微生物群落的种群结构不可预测,不一定具有更低的腐蚀性。此外,杀菌剂的使用对于部分顽固微生物会产生耐药性,还有污染环境的风险[75]。
为了解决海上石油设施以及油气集输管网中由微生物导致的H2S酸化问题,向系统内注入硝酸盐被认为是一种低成本、高效率的解决方案。外注硝酸盐可以刺激NRB的生长,NRB和SRB都可以利用乙酸、乳酸或长链脂肪酸作为能源,能够与SRB竞争电子供体。对于相同的电子供体,硝酸盐还原过程能够获得更多的能量。以乙酸为例,被NRB和SRB氧化的自由能变化见式(1)—(2)[76]。
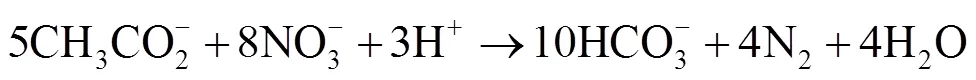
Δo= ‒495 kJ/mol (1)
Δo= ‒47 kJ/mol (2)
掠食性微生物(and Like Organisms,BALOs)和噬菌体作为一种新型的基于微生物手段的防腐方法得到了广泛关注[80]。当MIC系统引入BALOs或噬菌体时,腐蚀性微生物及其生物膜会被作为猎物而被捕获并遭到破坏。它们有着相似的生存方式,首先通过入侵或感染进入宿主体内,然后分泌各种裂解酶分解并吸收宿主体内的营养物质完成自身的繁殖和复制,直到宿主死亡破裂后进行下一次“捕猎”[81]。Qiu等发现SRB的活性在与BALOs共存的条件下受到显著抑制,浸泡60 d后,X70钢的腐蚀速率由19.17 mg/(dm2·d)下降到3.75 mg/(dm2·d)。相比于传统杀菌剂的方法,BALOs和噬菌体首先克服了微生物耐药性的问题,BALOs可以侵入由混合细菌组成的微生物群落,破坏顽固的生物膜,从而削弱生物膜对外环境的抵抗。此外,BALOs和噬菌体还可以避免重复接种,并通过增殖在腐蚀体系中长期保持有效浓度直至目标微生物被杀灭,显著降低了由于杀菌剂过量使用造成的环境污染[82-84]。
5 总结与展望
海上石油天然气生产设备及管网的MIC不仅防治成本高,而且可能造成严重的生态环境危害。长期的研究积累使得研究者们对其中涉及的油藏内微生物演化规律以及油气集输系统中的MIC机理有了更全面的理解。但是,目前MIC研究中面临的主要问题是单纯依靠实验室条件下的纯培养手段,无法准确地检测和评估具有腐蚀性微生物及其生物膜中的化学、电化学和微生物代谢过程。
针对海上油气管道MIC的防治,工业杀菌剂依然是最经济且高效的防腐手段,如季铵盐类、有机溴类、杂环类或复配类杀菌剂等。然而,工业杀菌剂的使用面临着如微生物耐药性增加、难降解、降低油品等诸多问题。未来的MIC相关研究应该利用先进的生物学检测技术,如环境基因组学、微流控以及高通量检测技术,对油气集输系统中微生物群落的结构和多样性进行检测和分析,从组学的角度了解环境微生物在腐蚀过程中的潜在功能、代谢特征以及对于原位环境实时变化的响应机制,更好地了解石油生产中的微生物过程。根据实际腐蚀环境制定特异性的杀菌防腐方案,有针对性地选择杀菌剂的种类和用量,将有助于精准地预防和缓解MIC,为新型防腐技术的实施提供有价值的信息和数据支撑。
[1] 谢玉洪. 中国海油“十三五”油气勘探重大成果与“十四五”前景展望[J]. 中国石油勘探, 2021, 26(1): 43-54.
XIE Yu-hong. Major Achievements in Oil and Gas Exploration of CNOOC in the 13thFive-Year Plan Period and Prospects in the 14thFive-Year Plan Period[J]. China Petroleum Exploration, 2021, 26(1): 43-54.
[2] XIE Xiao-rong, ZHONG Jian-liang, SUN Ying-yun, et al. Online Optimal Power Control of an Offshore Oil-Platform Power System[J]. Technology and Economics of Smart Grids and Sustainable Energy, 2018, 3(1): 1-13.
[3] 李秋扬, 赵明华, 任学军, 等. 中国油气管道建设现状及发展趋势[J]. 油气田地面工程, 2019, 38(S1): 14-17.
LI Qiu-yang, ZHAO Ming-hua, REN Xue-jun, et al. Construction Status and Development Trend of Chinese Oil & Gas Pipeline[J]. Oil-Gas Field Surface Engineering, 2019, 38(S1): 14-17.
[4] 王红红, 刘国恒. 中国海油海底管道事故统计及分析[J]. 中国海上油气, 2017, 29(5): 157-160.
WANG Hong-hong, LIU Guo-heng. Statistics and Analysis of Subsea Pipeline Accidents of CNOOC[J]. China Offshore Oil and Gas, 2017, 29(5): 157-160.
[5] LI Xiao-gang, ZHANG Da-wei, LIU Zhi-yong, et al. Materials Science: Share Corrosion Data[J]. Nature, 2015, 527(7579): 441-442.
[6] HOU Bao-rong, LI Xiao-gang, MA Xiu-min, et al. The Cost of Corrosion in China[J]. NPJ Materials Degradation, 2017, 1: 4.
[7] 李鑫, 尚东芝, 于浩波, 等. 油气管道SRB腐蚀研究新进展[J]. 表面技术, 2021, 50(2): 211-220.
LI Xin, SHANG Dong-zhi, YU Hao-bo, et al. Research Progress on Oil & Gas Pipeline Corrosion Induced by SRB[J]. Surface Technology, 2021, 50(2): 211-220.
[8] 50 ABDULLAH A, YAHAYA N, MD NOOR N, et al. Microbial Corrosion of API 5L X-70 Carbon Steel by ATCC 7757 and Consortium of Sulfate-Reducing Bacteria [J]. Journal of Chemistry, 2014, 2014: 130345.
[9] JACOBSON G. Corrosion at Prudhoe Bay-a Lesson on the Line[J]. Materials Performance, 2007, 46(8): 26-34.
[10] TALEB-BERROUANE M, KHAN F, HAWBOLDT K, et al. Model for Microbiologically Influenced Corrosion Potential Assessment for the Oil and Gas Industry[J]. Corrosion Engineering, Science and Technology, 2018, 53(5): 378-392.
[11] USHER K M, KAKSONEN A H, COLE I, et al. Critical Review: Microbially Influenced Corrosion of Buried Carbon Steel Pipes[J]. International Biodeterioration & Biodegradation, 2014, 93: 84-106.
[12] 朱立国, 王秀平, 孟科全, 等. 海上油田微生物堵调体系对管网腐蚀的研究[J]. 化学与生物工程, 2016, 33(3): 53-55.
ZHU Li-guo, WANG Xiu-ping, MENG Ke-quan, et al. Pipeline Corrosion Produced by Microbial Plugging and Profile Control System in an Offshore Oilfield[J]. Chemistry & Bioengineering, 2016, 33(3): 53-55.
[13] LEKBACH Y, DONG Yu-qiao, LI Zhong, et al. Catechin Hydrate as an Eco-Friendly Biocorrosion Inhibitor for 304L Stainless Steel with Dual-Action Antibacterial Properties AgainstBiofilm[J]. Corrosion Science, 2019, 157: 98-108.
[14] DOU Wen-wen, JIA Ru, JIN Peng, et al. Investigation of the Mechanism and Characteristics of Copper Corrosion by Sulfate Reducing Bacteria[J]. Corrosion Science, 2018, 144: 237-248.
[15] CHEN Shi-qiang, WANG Peng, ZHANG Dun. Corrosion Behavior of Copper under Biofilm of Sulfate-Reducing Bacteria[J]. Corrosion Science, 2014, 87: 407-415.
[16] LIU Hong-wei, GU Ting-yue, ZHANG Guo-an, et al. Corrosion of X80 Pipeline Steel under Sulfate-Reducing Bacterium Biofilms in Simulated CO2-Saturated Oilfield Produced Water with Carbon Source Starvation[J]. Corrosion Science, 2018, 136: 47-59.
[17] BASTIN E S, GREER F E, MERRITT C A, et al. The Presence of Sulphate Reducing Bacteria in Oil Field Waters[J]. Science, 1926, 63(1618): 21-24.
[18] EHRENBERG S N, NADEAU P H. Sandstone vs. Carbonate Petroleum Reservoirs: A Global Perspective on Porosity-Depth and Porosity-Permeability Relationships[J]. AAPG Bulletin, 2005, 89(4): 435-445.
[19] BAR-ON Y M, PHILLIPS R, MILO R. The Biomass Distribution on Earth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(25): 6506-6511.
[20] KALLMEYER J, POCKALNY R, ADHIKARI R R, et al. Global Distribution of Microbial Abundance and Biomass in Subseafloor Sediment[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(40): 16213-16216.
[21] SPARK I, PATEY I, DUNCAN B, et al. The Effects of Indigenous and Introduced Microbes on Deeply Buried Hydrocarbon Reservoirs, North Sea[J]. Clay Minerals, 2000, 35(1): 5-12.
[22] GITTEL A, SØRENSEN K B, SKOVHUS T L, et al. Prokaryotic Community Structure and Sulfate Reducer Activity in Water from High-Temperature Oil Reservoirs with and without Nitrate Treatment[J]. Applied and Environmental Microbiology, 2009, 75(22): 7086-7096.
[23] LI Guo-qiang, GAO Pei-ke, WU Yun-qiang, et al. Microbial Abundance and Community Composition Influence Production Performance in a Low-Temperature Petroleum Reservoir[J]. Environmental Science & Technology, 2014, 48(9): 5336-5344.
[24] MA Ting-ting, LIU Lai-yan, RUI Jun-peng, et al. Coexistence and Competition of Sulfate-Reducing and Methanogenic Populations in an Anaerobic Hexadecane- Degrading Culture[J]. Biotechnology for Biofuels, 2017, 10: 207.
[25] HU Ping, TOM L, SINGH A, et al. Genome-Resolved Metagenomic Analysis Reveals Roles for Candidate Phyla and other Microbial Community Members in Biogeochemical Transformations in Oil Reservoirs[J]. mBio, 2016, 7(1): 1669.
[26] BROCK T D. Micro-Organisms Adapted to High Temperatures[J]. Nature, 1967, 214(5091): 882-885.
[27] BELIAKOVA E V, ROZANOVA E P, BORZENKOV I A, et al. The New Facultatively Chemolithoautotrophic, Moderately Halophilic, Sulfate-Reducing BacteriumGen. Nov., Sp. Nov., Isolated from an Oil Field[J]. Mikrobiologiia, 2006, 75(2): 201-211.
[28] WIDDEL F. The Dissimilatory Sulfate-and Sulfur-Reducing Bacteria[J]. The Prokaryotes a Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications, 1992, 54: 583-624.
[29] MORI K, KIM H, KAKEGAWA T, et al. A Novel Lineage of Sulfate-Reducing Microorganisms:Fam. Nov.,, Gen. Nov., Sp. Nov., a New Thermophilic Isolate from a Hot Spring[J]. Extremophiles: Life Under Extreme Conditions, 2003, 7(4): 283-290.
[30] OKORO C C, AMUND O O. Microbial Community Structure of a Low Sulfate Oil Producing Facility Indicate Dominance of Oil Degrading/Nitrate Reducing Bacteria and Methanogens[J]. Petroleum Science and Technology, 2018, 36(4): 293-301.
[31] YANG Guang-chao, ZHOU Lei, MBADINGA S M, et al. Activation of CO2-Reducing Methanogens in Oil Reservoir after Addition of Nutrient[J]. Journal of Bioscience and Bioengineering, 2016, 122(6): 740-747.
[32] YANG Yu-ling, LADAPO J, WHITMAN W B. Pyruvate Oxidation by Methanococcus Spp[J]. Archives of Microbiology, 1992, 158(4): 271-275.
[33] DANIELS L, FUCHS G, THAUER R K, et al. Carbon Monoxide Oxidation by Methanogenic Bacteria[J]. Journal of Bacteriology, 1977, 132(1): 118-126.
[34] BIRKELAND N K. Chapter 14 the Microbial Diversity of Deep Subsurface Oil Reservoirs[J]. Studies in Surface Science and Catalysis, 2004, 151: 385-403.
[35] BHUPATHIRAJU V K, MCINERNEY M J, WOESE C R, et al.Sp. Nov., an Obligately Halophilic, Anaerobic Bacterium from an Oil Brine [J]. International Journal of Systematic Bacteriology, 1999, 49 Pt 3: 953-960.
[36] RENGPIPAT S, LANGWORTHY T A, ZEIKUS J G.Sp. Nov., a New Obligately Anaerobic Halophile Isolated from Deep Subsurface Hypersaline Environments[J]. Systematic and Applied Microbiology, 1988, 11(1): 28-35.
[37] JIANG Zhou, SHI Mei-mei, SHI Liang. Degradation of Organic Contaminants and Steel Corrosion by the Dissimilatory Metal-Reducing MicroorganismsandSpp[J]. International Biodeterioration & Biodegradation, 2020, 147: 104842.
[38] MOHAPATRA B R, DINARDO O, GOULD W D, et al. Biochemical and Genomic Facets on the Dissimilatory Reduction of Radionuclides by Microorganisms - A Review [J]. Minerals Engineering, 2010, 23(8): 591-599.
[39] GREENE A C, PATEL B K, SHEEHY A J.Gen. Nov., Sp. Nov., a Novel Thermophilic Manganese- and Iron-Reducing Bacterium Isolated from a Petroleum Reservoir[J]. International Journal of Systematic Bacteriology, 1997, 47(2): 505-509.
[40] LOVLEY D. Dissimilatory Fe(III)- and Mn(IV)-Reducing Prokaryotes[M]. New York: Springer New York, 2006: 635- 658.
[41] CLÉMENT J C, SHRESTHA J, EHRENFELD J G, et al. Ammonium Oxidation Coupled to Dissimilatory Reduction of Iron under Anaerobic Conditions in Wetland Soils[J]. Soil Biology and Biochemistry, 2005, 37(12): 2323-2328.
[42] ABDULSHAHEED A, MUSTAPHA F, GHAVAMIAN A. A Pressure-Based Method for Monitoring Leaks in a Pipe Distribution System: A Review[J]. Renewable and Sustainable Energy Reviews, 2017, 69: 902-911.
[43] MACHUCA L L, MURRAY L, GUBNER R, et al. Evaluation of the Effects of Seawater Ingress into 316L Lined Pipes on Corrosion Performance[J]. Materials and Corrosion, 2014, 65(1): 8-17.
[44] ZHOU Lei, LU Yu-wei, WANG Da-wei, et al. Microbial Community Composition and Diversity in Production Water of a High-Temperature Offshore Oil Reservoir Assessed by DNA- and RNA-Based Analyses[J]. International Biodeterioration & Biodegradation, 2020, 151: 104970.
[45] FRIEDRICH C G, ROTHER D, BARDISCHEWSKY F, et al. Oxidation of Reduced Inorganic Sulfur Compounds by Bacteria: Emergence of a Common Mechanism?[J]. Applied and Environmental Microbiology, 2001, 67(7): 2873-2882.
[46] JAVAHERDASHTI R. Microbiologically Influenced Corrosion (MIC) Microbiologically Influenced Corrosion[M]. New York: Springer, 2008: 29-79.
[47] FIDA T T, CHEN Chuan, OKPALA G, et al. Implications of Limited Thermophilicity of Nitrite Reduction for Control of Sulfide Production in Oil Reservoirs[J]. Applied and Environmental Microbiology, 2016, 82(14): 4190-4199.
[48] WANG Xiao-tong, LI Xi-zhe, YU Li, et al. Distinctive Microbial Communities Imply the Main Mechanism in a MEOR Trial in High Pour-Point Reservoir[J]. Journal of Petroleum Science and Engineering, 2019, 175: 97-107.
[49] S J G, BANAT I M, JOSHI S J. Biosurfactants: Production and Potential Applications in Microbial Enhanced Oil Recovery (MEOR)[J]. Biocatalysis and Agricultural Biotechnology, 2018, 14: 23-32.
[50] CÂMARA J M D A, SOUSA M A S B, BARROS NETO E L, et al. Application of Rhamnolipid Biosurfactant Produced byin Microbial- Enhanced Oil Recovery (MEOR)[J]. Journal of Petroleum Exploration and Production Technology, 2019, 9(3): 2333- 2341.
[51] ZHANG Jun-hui, GAO Hui, XUE Quan-hong. Potential Applications of Microbial Enhanced Oil Recovery to Heavy Oil[J]. Critical Reviews in Biotechnology, 2020, 40(4): 459-474.
[52] KATO S, YUMOTO I, KAMAGATA Y. Isolation of Acetogenic Bacteria that Induce Biocorrosion by Utilizing Metallic Iron as the Sole Electron Donor[J]. Applied and Environmental Microbiology, 2015, 81(1): 67-73.
[53] FLEMMING H C, WUERTZ S. Bacteria and Archaea on Earth and Their Abundance in Biofilms[J]. Nature Reviews Microbiology, 2019, 17(4): 247-260.
[54] DANG Hong-yue, LOVELL C R. Microbial Surface Colonization and Biofilm Development in Marine Environments[J]. Microbiology and Molecular Biology Reviews: MMBR, 2015, 80(1): 91-138.
[55] BEECH I B, SUNNER J. Biocorrosion: Towards Understanding Interactions between Biofilms and Metals[J]. Current Opinion in Biotechnology, 2004, 15(3): 181-186.
[56] WEN Xiang-li, BAI Peng-peng, LUO Bing-wei, et al. Review of Recent Progress in the Study of Corrosion Products of Steels in a Hydrogen Sulphide Environment [J]. Corrosion Science, 2018, 139: 124-140.
[57] LIU T, LIU H, HU Y, et al. Growth Characteristics of Thermophile Sulfate-Reducing Bacteria and Its Effect on Carbon Steel[J]. Materials and Corrosion, 2009, 60(3): 218-224.
[58] JIA Ru, TAN Jie long, JIN Peng, et al. Effects of Biogenic H2S on the Microbiologically Influenced Corrosion of C1018 Carbon Steel by Sulfate Reducing[J]. Corrosion Science, 2018, 130: 1-11.
[59] PROCÓPIO L. Microbially Induced Corrosion Impacts on the Oil Industry[J]. Archives of Microbiology, 2022, 204(2): 138.
[60] ENNING D, VENZLAFF H, GARRELFS J, et al. Marine Sulfate-Reducing Bacteria Cause Serious Corrosion of Iron under Electroconductive Biogenic Mineral Crust[J]. Environmental Microbiology, 2012, 14(7): 1772-1787.
[61] HUANG Lu-yao, CHANG Wei-wei, ZHANG Da-wei, et al. Acceleration of Corrosion of 304 Stainless Steel by Outward Extracellular Electron Transfer of[J]. Corrosion Science, 2022, 199: 110159.
[62] QIAN Hong-chang, LIU Shang-yu, LIU Wen-long, et al. Microbiologically Influenced Corrosion of Q235 Carbon Steel by Aerobic[J]. Acta Metallurgica Sinica (English Letters), 2022, 35(2): 201-211.
[63] HARRIS H W, EL-NAGGAR M Y, BRETSCHGER O, et al. Electrokinesis is a Microbial Behavior that Requires Extracellular Electron Transport[J]. PNAS, 2010, 107(1): 326-331.
[64] LOU Yun-tian, DAI Chun-duo, CHANG Wei-wei, et al. Microbiologically Influenced Corrosion of FeCoCrNiMo0.1High-Entropy Alloys by Marine[J]. Corrosion Science, 2020, 165: 108390.
[65] LI Zi-yu, CHANG Wei-wei, CUI Tian-yu, et al. Adaptive Bidirectional Extracellular Electron Transfer during Accelerated Microbiologically Influenced Corrosion of Stainless Steel[J]. Communications Materials, 2021, 2: 67.
[66] KIM H J, PARK H S, HYUN M S, et al. A Mediator-less Microbial Fuel Cell Using a Metal Reducing Bacterium,[J]. Enzyme and Microbial Technology, 2002, 30(2): 145-152.
[67] HARRIS H W, EL-NAGGAR M Y, BRETSCHGER O, et al. Electrokinesis is a Microbial Behavior that Requires Extracellular Electron Transport[J]. PNAS, 2010, 107(1): 326-331.
[68] XU Da-ke, GU Ting-yue. Carbon Source Starvation Triggered more Aggressive Corrosion Against Carbon Steel by theBiofilm[J]. International Biodeterioration & Biodegradation, 2014, 91: 74-81.
[69] LYLES C N, LE H M, BEASLEY W H, et al. Anaerobic Hydrocarbon and Fatty Acid Metabolism by Syntrophic Bacteria and Their Impact on Carbon Steel Corrosion[J]. Frontiers in Microbiology, 2014, 5: 114.
[70] VIGNERON A, ALSOP E B, CHAMBERS B, et al. Complementary Microorganisms in Highly Corrosive Biofilms from an Offshore Oil Production Facility[J]. Applied and Environmental Microbiology, 2016, 82(8): 2545-2554.
[71] GU T, GALICIA B. Can Acid Producing Bacteria Be Responsible for very Fast MIC Pitting[C]. International Corrosion Conference. Houston: HACE, 2012.
[72] KATO S. Microbial Extracellular Electron Transfer and Its Relevance to Iron Corrosion[J]. Microbial Biotechnology, 2016, 9(2): 141-148.
[73] VIGNERON A, HEAD I M, TSESMETZIS N. Damage to Offshore Production Facilities by Corrosive Microbial Biofilms[J]. Applied Microbiology and Biotechnology, 2018, 102(6): 2525-2533.
[74] DUNCAN K E, DAVIDOVA I A, NUNN H S, et al. Design Features of Offshore Oil Production Platforms Influence Their Susceptibility to Biocorrosion[J]. Applied Microbiology and Biotechnology, 2017, 101(16): 6517-6529.
[75] ENNING D, SMITH R, STOLLE J. Evaluating the Efficacy of Weekly THPS and Glutaraldehyde Batch Treatment to Control Severe Microbial Corrosion in a Simulated Seawater Injection System[C]. International Corrosion Conference. Houston: HACE, 2016
[76] THAUER R K, JUNGERMANN K, DECKER K. Energy Conservation in Chemotrophic Anaerobic Bacteria[J]. Bacteriological Reviews, 1977, 41(3): 809.
[77] VOORDOUW G, GRIGORYAN A A, LAMBO A, et al. Sulfide Remediation by Pulsed Injection of Nitrate into a Low Temperature Canadian Heavy Oil Reservoir[J]. Environmental Science & Technology, 2009, 43(24): 9512-9518.
[78] KASTER K M, GRIGORIYAN A, JENNEMAN G, et al. Effect of Nitrate and Nitrite on Sulfide Production by Two Thermophilic, Sulfate-Reducing Enrichments from an Oil Field in the North Sea[J]. Applied Microbiology and Biotechnology, 2007, 75(1): 195-203.
[79] XU Da-ke, LI Ying-chao, SONG Feng-mei, et al. Laboratory Investigation of Microbiologically Influenced Corrosion of C1018 Carbon Steel by Nitrate Reducing Bacterium[J]. Corrosion Science, 2013, 77: 385-390.
[80] LOU Yun-tian, CHANG Wei-wei, CUI Tian-yu, et al. Microbiologically Influenced Corrosion Inhibition Mechanisms in Corrosion Protection: A Review[J]. Bioelectrochemistry, 2021, 141: 107883.
[81] RENDULIC S, JAGTAP P, ROSINUS A, et al. A Predator Unmasked: Life Cycle offrom a Genomic Perspective[J]. Science, 2004, 303(5658): 689-692.
[82] SCHOOLEY R T, BISWAS B, GILL J J, et al. Development and Use of Personalized Bacteriophage- Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection[J]. Antimicrobial Agents and Chemotherapy, 2017, 61(10): e00954-e00917.
[83] FORTI F, ROACH D R, CAFORA M, et al. Design of a Broad-Range Bacteriophage Cocktail that ReducesBiofilms and Treats Acute Infections in Two Animal Models[J]. Antimicrobial Agents and Chemotherapy, 2018, 62(6): e02573-e02517.
[84] YANG Yu-hui, SHEN Wei, ZHONG Qiu, et al. Development of a Bacteriophage Cocktail to Constrain the Emergence of Phage-Resistant[J]. Frontiers in Microbiology, 2020, 11: 327.
Research Progress on Microbiologically Influenced Corrosion of Oil and Gas Pipelines in Marine Environment
1,2,1,2,1,2,1,1,2
(1. Institute for Advanced Materials and Technology, University of Science and Technology Beijing, Beijing 100083, China; 2. Shunde Graduate School of University of Science and Technology Beijing, Guangdong Foshan 528399, China)
Corrosion of offshore oil-gas gathering and transportation pipelines can lead to serious environmental risks and economic losses. With the continuous development of China's marine energy industry, it is imperative to accelerate the rational exploitation and utilization of offshore oil-gas resources as well as upgrade the oil-gas production equipment. As an important part of oil-gas gathering and transportation networks, submarine pipeline is known as the "artery" of offshore oil-gas production systems. Under actual working conditions, submarine pipelines are inevitably exposed to corrosion and failure. Microbiologically influenced corrosion (MIC) has been considered one of the main factors causing this problem. According to statistics, more than 20% of the oil-gas pipeline corrosion and oil leakage accidents are directly or indirectly related to MIC. The vast ocean includes a wide range of extreme environments such as high salt, high pressure, and low temperature environments. Marine environments are more diverse and complex than terrestrial environments, indicating that marine microbes are more tolerant to extreme conditions. Herein, the sources of corrosive microorganisms in offshore oil-gas pipelines, including reservoir endogenous microorganisms, exogenous microorganisms introduced by seawater injection, and microbial enhanced oil recovery, were classified. The characteristics of fluid chemical substances in submarine reservoirs were analyzed. It was confirmed that they were rich in methane, sulfides, and volatile fatty acids, and they were classified according to the characteristics of endogenous microbial metabolism and products, including sulfate-reducing bacteria, methanogens, fermentative bacteria, and iron-reducing bacteria. Moreover, the characteristics of microbial community abundance in the produced water of an oilfield were analyzed with an example, and the evolution rule of the microbial community under long-term oilfield environmental stress was clarified. Complex gathering and transport networks are particularly prone to biofilm formation and metabolite accumulation, which may cause or exacerbate corrosion problems. A corrosive biofilm, composed of various environmental microorganisms, is a general life form used by microorganisms to resist changes in the external environment and maintain homeostasis of the internal environment, which includes a complex symbiotic relationship between microorganisms with different metabolic characteristics. Accordingly, theories of metabolite-MIC, extracellular electron transfer-MIC, and synergism/antagonism among corrosive microorganisms in offshore pipeline networks were further reviewed. Pure/mixed culture in laboratory conditions can hardly represent the complexity of in situ biofilms in oil-gas pipelines; therefore, it is almost impossible to reconstruct the corrosion behavior of microorganisms in a real service environment. Industrial bactericides are one of the most widely used strategies for MIC in oil-gas pipeline networks. Advanced composite bactericides often possess broad-spectrum antibacterial properties, low toxicity, and sustained bactericidal activity. However, bactericides have drawbacks such as increased microbial resistance, difficulty in degradation, and deterioration of crude oil quality. Therefore, it is extremely challenging to detect the corrosive microbial community and the metabolic processes leading to corrosion accurately under actual working conditions. Advanced biological detection technologies, including environmental genomics, microfluidics, and high-throughput rapid detection technology, should be fully utilized in future research on the MIC of oil-gas pipelines. In this paper, the types of potential microbial species, types of MIC, and the corrosion mechanisms are summarized in detail, and novel anti-corrosion methods based on biotechnology are proposed.
offshore oil and gas pipeline; microbiologically influenced corrosion; reservoir microorganism; MIC mechanism
TG174
A
1001-3660(2022)05-0129-10
10.16490/j.cnki.issn.1001-3660.2022.05.014
2022–03–12;
2022–04–21
2022-03-12;
2022-04-21
国家自然科学基金面上项目(52071015)
General Program of the National Natural Science Foundation of China (52071015)
娄云天(1990—),男,博士,主要研究方向为微生物腐蚀。
LOU Yun-tian (1990-), Male, Doctor, Research focus: microbiological influenced corrosion.
张达威(1984—),男,博士,教授,主要研究方向为智能耐蚀材料、微生物腐蚀与材料腐蚀大数据预测评价。
ZHANG Da-wei (1984-), Male, Ph. D., Professor, Research focus: intelligent corrosion resistant material, microbiological influenced corrosion, prediction and evaluation of material corrosion with big data.
娄云天, 何盛宇, 陈旭东, 等. 海洋环境中油气管道的微生物腐蚀研究进展[J]. 表面技术, 2022, 51(5): 129-138.
LOU Yun-tian, HE Sheng-yu, CHEN Xu-dong, et al. Research Progress on Microbiologically Influenced Corrosion of Oil and Gas Pipelines in Marine Environment[J]. Surface Technology, 2022, 51(5): 129-138.
责任编辑:万长清
