长期缺硒对大鼠血浆氨基酸的影响
2022-05-28余海立张小丽张彦阮之阳唐铜张双庆
余海立 张小丽 张彦 阮之阳 唐铜 张双庆
摘 要:目的:探讨长期缺硒对大鼠血浆氨基酸的影响。方法:选取20只SPF级3 w龄雄性断乳Sprague-Dawley大鼠随机分为2组:对照组喂养正常饲粮(0.18 mg Se/kg)、缺硒组喂养低硒饲粮(0.02 mg Se/kg)。应用L-8900氨基酸分析仪检测血浆氨基酸水平。结果:第300天缺硒组大鼠血浆磷酸丝氨酸、牛磺酸、天门冬氨酸含量显著降低;第532天缺硒组大鼠血浆丝氨酸、缬氨酸、异亮氨酸、亮氨酸、苯丙氨酸、组氨酸含量显著升高,甘氨酸含量显著降低。结论:长期缺硒引起大鼠氨基酸代谢发生显著变化。
关键词:硒;氨基酸;代谢
补硒对于防治疾病和维持健康的重要意义[1-2]。硒水平与脂质代谢、葡萄糖代谢的研究逐渐增多,Fang等[3]研究了血硒水平和糖脂代谢的关系,发现血硒水平和血清胆固醇水平呈正相关。Zhao等[4]研究发现,硒可以调节2型糖尿病小鼠空腹血糖、糖化血红蛋白、胰岛素和瘦素水平,改善糖耐量,并调节脂质代谢。硒可以调节血浆和肝脏中胰岛素水平,并以组织特异性调节肝脏和和肌肉中蛋白质、脂质代谢水平[5]。硒水平与糖脂代谢的研究逐渐增多,但缺硒或者补硒对于氨基酸代谢的研究较少。Sun等[6]利用富硒小球藻喂养褶皱臂尾轮虫建立的长寿轮虫模型,发现富硒轮虫体内谷胱甘肽过氧化物酶和过氧化氢酶活性升高,氧化应激水平降低,说明硒可以增加轮虫氨基酸代谢水平,可能通过将能量代谢途径从三羧酸循环转向糖酵解途径,从而减少活性氧的生成,对轮虫起到抗衰老作用。富硒酵母利用无机硒经过生物转化合成有机硒,与普通酵母相比,富硒酵母中赖氨酸、亮氨酸、缬氨酸含量显著升高,富硒酵母适应环境压力的能力显著提升[7],目前缺少长期缺硒对氨基酸代谢影响的研究。本课题组前期研究发现,缺硒300 d对大鼠血浆、小脑中D-丝氨酸、L-丝氨酸水平无显著影响[8],本研究应用氨基酸分析仪检测长期缺硒大鼠氨基酸代谢变化,筛选代谢途径变化,分析硒水平对代谢通路变化的影响和生物学意义。
1 材料与方法
1.1 主要材料與试剂
SPF级雄性3 w龄断乳Sprague-Dawley大鼠,体质量(48.3±5.9)g,湖北省三峡大学实验动物中心,生产许可证号:SCXK(鄂)2017-0012,饲养于三峡大学实验动物中心(使用许可证号:SYXK(鄂)2017-0061)。
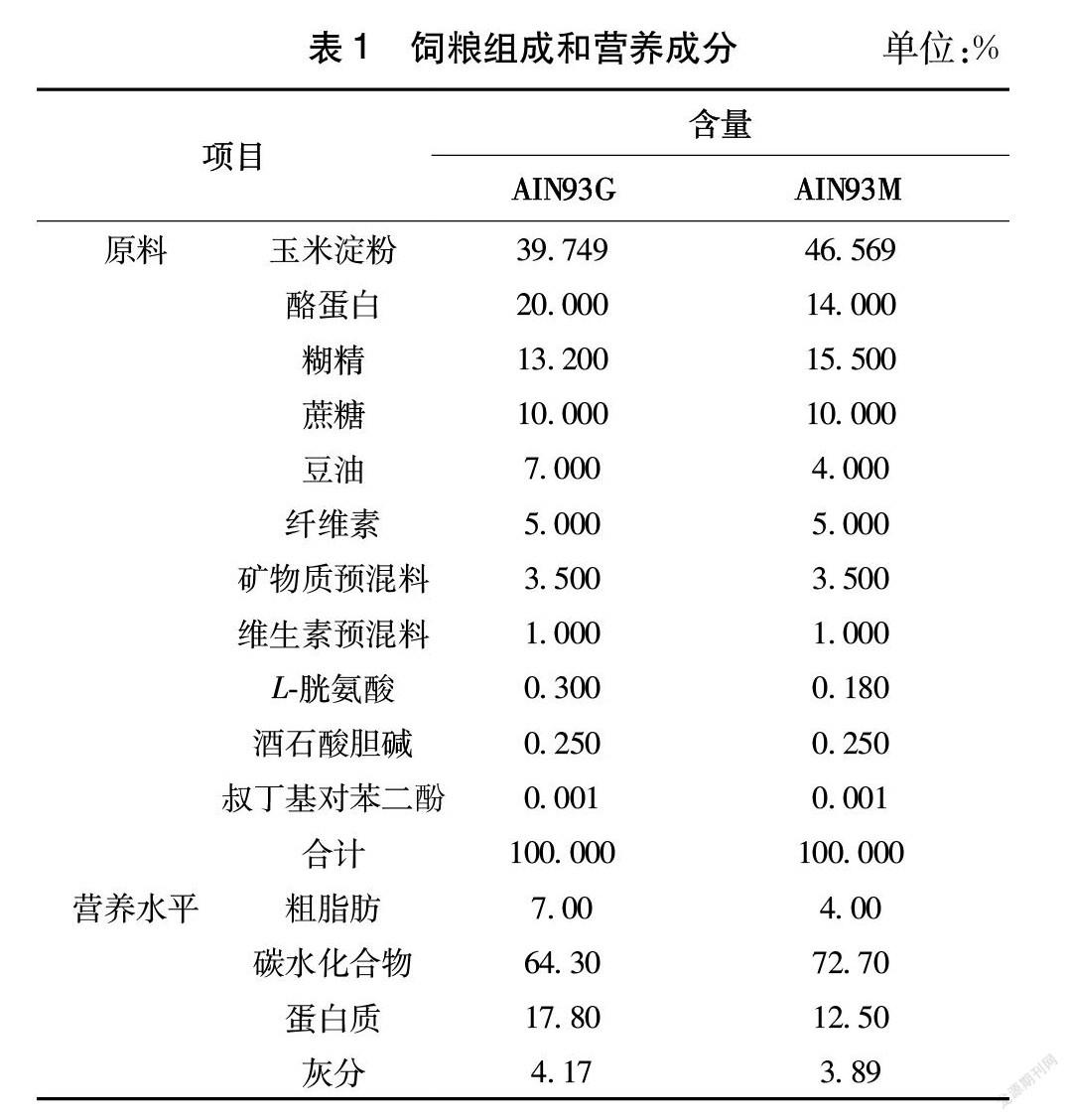
饲料:大鼠低硒饲粮和正常饲粮,由南通特洛菲有限公司按照美国AIN93标准生产纯化型标准饲粮,经60Co辐照灭菌,-20 ℃保存,其饲粮组成和营养成分见表1。经检测,低硒饲粮和正常饲粮硒水平分别为0.02、0.18 mg/kg。氨基酸标准混合溶液、PF系列标准缓冲溶液、氨基酸分析仪配套茚三酮显色液,日本和光纯药工业株式会社;磺基水杨酸,国药控股有限公司。
1.2 主要仪器与设备
L-8900全自动氨基酸分析仪,株式会社日立制作所;LEOPARD C1450-230V高速离心机,莱普特科学仪器(北京)有限公司。
1.3 方法
1.3.1 动物饲养及分组 雄性3 w龄断乳大鼠按照体质量随机等分为缺硒组和对照组,每组10只,分别饲喂低硒饲粮和正常饲粮,试验开始至第60天使用G型饲粮,试验第61~532天使用M型饲粮,自由饮水,试验期532 d。
1.3.2 样品收集 大鼠禁食不禁水12 h后,第300天和532天眼静脉丛采血,置于肝素钠抗凝离心管中,1 520×g离心10 min,分离血浆,-80 ℃保存用于游离氨基酸含量检测。
1.3.3 饲料硒含量测定 参照GB/T 13883-2008 第一法氢化物原子荧光光谱法进行测定。
1.3.4 血浆游离氨基酸含量测定 取血浆1 000 μL,加入1 000 μL的50 g/L磺基水杨酸,涡旋10 min,4 ℃条件下14 100×g离心10 min沉淀蛋白,取500 μL上清加入500 μL 的50 g/L磺基水杨酸混匀,过0.22 μm滤膜后用于氨基酸含量检测,进样量20 μL。
1.3.5 统计分析 采用软件SPSS 23.0,试验数据以平均值±标准差表示,组间比较采用t检验分析,P < 0.05表示差异具有统计学意义。
2 结果与分析
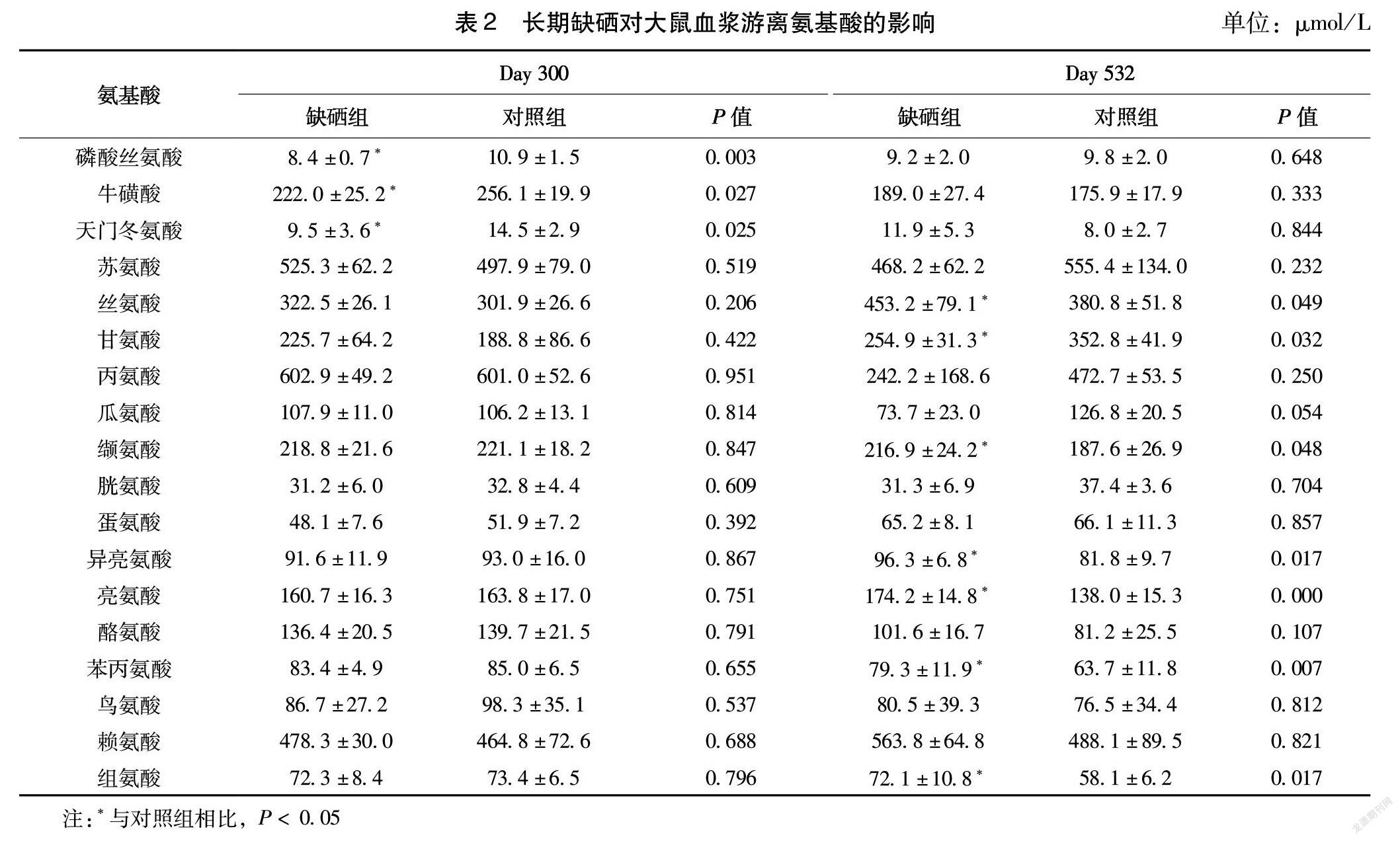
由表2可知,饲养至第300天,与对照组相比,缺硒组大鼠血浆磷酸丝氨酸、牛磺酸、天门冬氨酸含量显著下降(P < 0.05)。饲养至第532天,与对照组相比,缺硒组大鼠血浆丝氨酸、缬氨酸、异亮氨酸、亮氨酸、苯丙氨酸、组氨酸含量显著升高(P < 0.05),甘氨酸含量显著降低(P < 0.05)。
3 结论
硒是维持生理代谢的重要元素之一,Beata等[9]研究发现,缺硒小鼠血清中15种代谢物发生显著性变化,多数变化的生物途径与氨基酸代谢有关。缺硒32 w C57BL/6小鼠丝氨酸、苏氨酸、苯丙氨酸、蛋氨酸、脯氨酸、甘氨酸和异亮氨酸的水平升高,表明缺硒改变了小鼠甘氨酸,丝氨酸和苏氨酸代谢,苯丙氨酸,酪氨酸和色氨酸的生物合成。Yim等[10]研究发现,缺硒5 w C57BL / 6J雄性小鼠肝脏和大脑代谢物变化,硒缺乏导致硒蛋白表达急剧下降,多种代谢物显著变化,影响了小鼠体内氨基酸生物合成、氨基酸代谢、氮代谢、谷胱甘肽代谢等生物途径。前期研究发现,缺硒300 d对大鼠血浆、小脑中D-丝氨酸、L-丝氨酸水平无显著影响[8]。本研究检测缺硒300 d和532 d大鼠血浆氨基酸水平,发现长期缺硒引起了部分氨基酸代谢的显著改变。缺硒饮食会显著降低甲硫氨酸亚砜还原酶B1、谷胱甘肽过氧化物酶1等硒蛋白的表达[10]。硒蛋白缺乏引起体内活性氧水平增加,导致氧化应激[11],氨基酸可以降低氧化应激水平,调节代谢稳态[12]。磷酸丝氨酸参与硒蛋白的合成过程[13],天门冬氨酸促进硒蛋白发挥抗氧化作用[14],牛磺酸可以减少微量元素通过尿液的流失,调节体内硒元素稳态。丝氨酸是良好的抗氧化剂,可以降低体内氧化应激水平[15],本研究发现,缺硒大鼠体内丝氨酸显著增加,可能是机体应对体内硒水平下降的代偿机制。另外,丝氨酸为硒蛋白的合成提供碳骨架,硒蛋白合成减缓也可能是丝氨酸升高的原因。硒和甘氨酸均能降低过氧化氢诱导的细胞损伤[16],体内硒水平下降可能会引起甘氨酸水平的变化。异亮氨酸[17]、亮氨酸[18]、缬氨酸[19]具有良好的抗氧化活性,可以调节机体氧化应激水平。苯丙氨酸[20]、组氨酸[21]是机体必需氨基酸,参与机体多种代谢途径。缺硒使硒蛋白表达降低,氨基酸代谢的变化可能是机体调节氧化还原稳态的代偿机制,有待进一步研究。
参考文献
[1]Roman M,Jitaru P,Barbante C.Selenium biochemistry and its role for human health[J].Metallomics:Integrated Biometal Science,2014,6(1):25-54.
[2]余海立,陈智仙,张彦,等.硒与认知功能[J].中国药事,2019,33(4):385-390.
[3]Fang H,He X,Wu Y,et al.Association between selenium level in blood and glycolipid metabolism in residents of enshi prefecture,China[J].Biological Trace Element Research,2020,199(7):2456-2466.
[4]Zhao D,Zhu H,Gao F,et al.Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice[J].Food & Function,2020,11(7):6528-6541.
[5]Zhao Z,Barcus M,Kim J,et al.High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs[J].The Journal of Nutrition,2016,146(9):1625-1633.
[6]Sun X,Cui Y,Wang Q,et al.Proteogenomic analyses revealed favorable metabolism pattern alterations in rotifer brachionus plicatilis fed with selenium-rich chlorella[J].Journal of Agricultural and Food Chemistry,2018,66(26):6699-6707.
[7]Kieliszek M,Btazejak S,Bzducha-WróBel A,et al.Effect of selenium on lipid and amino acid metabolism in yeast cells[J].Biological Trace Element Research,2019,187(1):316-327.
[8]余海立,張凤伟,张彦,等.长期缺硒对大鼠体内D-丝氨酸、L-丝氨酸水平的影响[J].动物营养学报,2020,32(8):3869-3876.
[9]Mickiewicz B,Villemaire M,Sandercock L,et al.Metabolic changes associated with selenium deficiency in mice[J].Biometals,2014,27(6):1137-1147.
[10]Yim S H,Clish C B,Gladyshev V N.Selenium deficiency is associated with pro-longevity mechanisms[J].Cell Reports,2019,27(9):2785-2797.
[11]Li S,Zhao Q,Zhang K,et al.Selenium deficiency-induced pancreatic pathology is associated with oxidative stress and energy metabolism disequilibrium[J].Biological Trace Element Research,2021,199(1):154-165.
[12]Ruocco C,Segala A,Valerio A,et al.Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress[J].Current Opinion in Clinical Nutrition and Metabolic Care,2020,24(1).
[13]Manhas R,Gowri V S,Madhubala R.Leishmania donovani encodes a functional selenocysteinyl-tRNA synthase[J].Journal of Chemical Biology,2016,291(3):1203-1220.
[14]Marciel M P,Hoffmann P R.Molecular mechanisms by which selenoprotein K regulates immunity and cancer[J].Biological Trace Element Research,2019,192(1):60-68.
[15]He L,Liu Y,Long J,et al.Maternal serine supply from late pregnancy to lactation improves offspring performance through modulation of metabolic pathways[J].Food & Function,2020,11(9):8089-8098.
[16]Ndoni S A,Okoko T.Comparative effect of selenium and glycine on hydrogen peroxide-induced cell death and activation of macrophage U937 cells[J].Journal of Genetic Engineering and Biotechnology,2017,15(2):521-526.
[17]Zhao J,Liu Y,Jiang J,et al.Effects of dietary isoleucine on the immune response,antioxidant status and gene expression in the head kidney of juvenile Jian carp (Cyprinus carpio var.Jian)[J].Fish Shellfish Immunol,2013,35(2):572-80.
[18]Zhou C,Lin H,Huang Z,et al.Effects of dietary leucine levels on intestinal antioxidant status and immune response for juvenile golden pompano (Trachinotus ovatus)involved in Nrf2 and NF-κB signaling pathway[J].Fish Shellfish Immunol,2020,107(6):336-345.
[19]Luo J B,Feng L,Jiang W D,et al.Physical and flavor characteristics,fatty acid profile,antioxidant status and Nrf2-dependent antioxidant enzyme gene expression changes in young grass carp (Ctenopharyngodon idella)Fillets fed dietary valine[J].PLoS One,2017,12(1):eo169270.
[20]Akimitsu O,Wada K,Noji T,et al.The relationship between consumption of tyrosine and phenylalanine as precur sors of catecholamine at breakfast and the circadian typology and mental health in Japanese infants aged 2 to 5 years[J].Journal of Physiological Anthropology,2013,32(6):13.
[21]Holeek M.Histidine in health and disease:metabolism,physiological importance,and use as a supplement[J].Nutrients,2020,12(3):848-866.
Effects of Long-Term Selenium Deficiency on Amino Acid Levels in Rats
YU Hai-li 1,ZHANG Xiao-li1,ZHANG Yan1,RUAN Zhi-yang1,TANG Tong1,ZHANG Shuang-qing2
(1 The Hubei Provincial Key Laboratory of Yeast Function,Yichang 443003,China;
2 National Institute for Nutrition and Health,Chinese Center for Disease Control and Prevention,Beijing 100050,China)
Abstract:Objective To investigate effects of long-term selenium (Se)deficiency on amino acids in rats.Method Totally 20 male weaned rats aged 3 weeks were randomly divided into two groups.Se-deficient group was fed low Se diet (0.02 mg Se/kg)and normal group was fed normal diet (0.18 mg Se/kg).L-8900 amino acid analyzer was used to analyze the levels of plasma amino acids.Result The contents of phosphoric acid,taurine and aspartic acid in selenium-deficient rats were significantly decreased on day 300.The levels of serine,valine,isoleucine,leucine,phenylalanine,histidine and glycine in the selenium-deficient group were significantly increased and the levels of glycine were significantly decreased on day 532.Conclusion Long-term selenium deficiency can cause significant changes in amino acid metabolism in rats.
Keywords:selenium;amino acid;metabolism
