Molecular characterization and functional analysis of daf‑8 in the pinewood nematode, Bursaphelenchus xylophilus
2022-05-26JinghanWangHuanHongRongXieJingjingJiKaiGuoLiqunBaiJiaTangHongshiYuJianrenYeJiafuHu
Jinghan Wang· Huan Hong· Rong Xie· Jingjing Ji· Kai Guo· Liqun Bai· Jia Tang· Hongshi Yu,4· Jianren Ye· Jiafu Hu
Received: 23 November 2020 / Accepted: 17 February 2021 / Published online: 31 May 2021 © Northeast Forestry University 2021, corrected publication 2022
Abstract Bursaphelenchus xylophilus, causal agent of pine wilt disease, causes extensive damage worldwide. Strategies are needed to inhibit population growth or block the spread of the invasive nematode to control pine wilt disease. The gene daf-8 plays crucial roles in larval development and dauer formation in Caenorhabditis elegans, but little is known about its orthologue in B. xylophilus. In the present molecular characterization and functional analysis of daf-8 in B. xylophilus (Bx-daf-8), RT-qPCR showed that the expression of Bx-daf-8 gradually increased during the embryonic stage, peaked in the second-stage juvenile (J2), then dramatically dropped in the J3, and remained at that low level for the rest of its life. Bx-daf-8-transgenic C. elegans was employed to mimic the spatiotemporal expression of Bx-daf-8, which was expressed close to the pharynx during early embryogenesis and weakly throughout the whole body during late embryogenesis. It was observed in head neurons and tail ganglions throughout all postembryonic stages. B. xylophilus embryos were severely abnormal, and hatching rate decreased sharply after Bx-daf-8 knockdown. daf-16-1 and da-f16-2, downstream genes in the IIS pathway, also dropped sharply after Bx-daf-8 knockdown. We propose that TGFβ may crosstalk with the IIS pathway upstream of Bxdaf-16 and that daf-8 may act as a master regulator of daf-16 in B. xylophilus. However, knockdown of Bx-daf-8 did not lead to constitutive developmental arrest at the dauer larval stage, indicating that dauer entry in B. xylophilus might be controlled by several genes and is more complicated than in C. elegans. Bx-daf-8 alone did not control the dauer entry in B. xylophilus, but it was indispensable for embryogenesis, providing a potential target for suppressing population growth of B. xylophilus.
Keywords Daf-8·Expression pattern·RNAi·Embryogenesis·Bursaphelenchus xylophilus
Introduction
The pine wood nematode,Bursaphelenchus xylophilus, causal agent of pine wilt disease causes severe losses, threatening the stability of pine ecosystems worldwide (Zhao et al. 2016). The nematode has two distinct life stages, dispersive and propagative. In a favorable environment, the nematode develops rapidly through four juvenile stages (J1–J4) to the reproductive adult, which feeds on parenchyma cells lining the resin ducts of the pine trees, causing wilt (Mamiya and Kiyohara 1972). However, in harsh conditions such as low temperatures in the winter, the nematode enters the dispersal phase, molting from J2 into dispersal-specialized third-stage dauer larvae (DL3) (Mamiya 1983). The nematodes cannot travel independently outside of the host trees, and therefore rely on vector beetles (Monochamusspp.) for dispersal (Futai 2013). DL3 are sensitive to the volatiles released by the pupa of vector beetles and aggregate around their pupal chambers (Kikuchi et al. 2011). When the pupae become callow adults, DL3 are induced to develop into fourth-stage dauer larvae (DL4). Dauer nematodes enter the trachea of the adult beetles and are transferred to a new host when the vector beetle feeds or oviposits on host trees (Wu et al. 2019). However, the regulatory mechanisms of dauer formation inB. xylophilusare still not clear.
InCaenorhabditis elegans, the transforming growth factor-β (TGFβ) signalling pathway plays a crucial role in normal larval growth and dauer formation. The TGFβ superfamily members are involved in a wide range of biological activities such as cell proliferation, differentiation, development and apoptosis (Massague and Gomis 2006). Genes involved in the dauer TGFβ signalling pathway aredaf-1(TβR-I),daf-3(Co-Smad),daf-4(TβR-II),daf-5(Sno/Ski),daf-7(which encodes a member of TGFβ superfamily member),daf-8(R-Smad) anddaf-14(Smad) (Georgi et al. 1990; Estevez et al. 1993; Schackwitz et al. 1996; Savage-Dunn et al. 2003). Thedaf-1,daf-4,daf-7,daf-8anddaf-14are dauer formation constitutive (daf-c) genes, and mutations in these genes cause dauer formation under non-dauerinducing conditions. The genesdaf-3 and daf-5are dauer formation defective (daf-d) genes, mutations in these genes prevent dauer formation under dauer-inducing conditions. Mutations indaf-3anddaf-5suppress the phenotypes of thedaf-1,daf-4,daf-7,daf-8anddaf-14mutants, suggesting they are downstream of thedaf-cgenes in the genetic pathway (Riddle et al. 1981; Vowels and Thomas 1992). Favourable environmental factors, such as the presence of food, stimulatedaf-7expression in amphid chemosensory neurons ASI (Schackwitz et al. 1996). DAF-7 activates DAF-8 to block the DAF-3 and induce dauer formation by direct protein–protein interaction between DAF-8 and DAF-3. When DAF-7 becomes deficient continuously under unfavourable growth conditions, DAF-8 activity falls below a threshold, allowing DAF-3 to switch the reproductive development cycle to dauer diapause by repressing the transcription ofdaf-7anddaf-8(Park et al. 2010). Interestingly, most orthologues involved in dauer larva formation inC. eleganswere also identified inB. xylophilus(Kikuchi et al. 2011), indicating the pathway for dauer formation is conserved. Only one alternative dauer stage called DL3 is found inC. elegans. However, two dauer stages, DL3 and DL4, are known inB. xylophilus. Gene expression data inB. xylophilusshowed that both dauer stages are transcriptionally very distinct from the propagative stages. Functions of genes that are highly expressed in DL3 are enriched in proteolysis, cysteine-type peptidase activity and oxidoreduction process, whereas functions of genes highly expressed in DL4 are enriched in G-protein coupled receptor signalling pathway (Tanaka et al. 2019).
InC. elegans, there is crosstalk between TGFβ and insulin/IGF signalling (IIS) pathways associated with dauer diapause and longevity; e.g., thedaf-8mutant has an increased life span (Shaw et al. 2007). DAF-8 can form a complex with the nuclear hormone receptor NHR-69 to repress the transcription ofexp-2, a potassium channel gene and promote neuronal insulin-like peptide DAF-28 secretion (Park et al. 2012). DAF-8 also controls animal locomotion by negatively regulating the abundance of an AMPA-type glutamate receptor GLR-1 at neuronal synapses in the ventral nerve cord (VNC) (Burbea et al. 2002; McGehee et al. 2015). In the adultC. elegans, DAF-8 downregulateslag-2expression in the distal tip cells (DTCs), thus promoting germ line meiosis (Park et al. 2010). Mutation indaf-8not only causes dauerconstitutive phenotypes, but also causes an egg-laying defect (Trent et al. 1983). However, though DAF-8 has versatile biological roles inC. elegans, little is known about the roles and molecular features ofdaf-8in the phytoparasitic nematodeB. xylophilus.
Compared to conditions forC. elegans,B. xylophilushas totally different living conditions. In our molecular characterization and functional analysis ofdaf-8inB. xylophilus(Bx-daf-8), we used reverse transcription quantitative realtime PCR (RT-qPCR), GFP-mediated promoter analysis and RNA interference (RNAi) to better understand the molecular and developmental biology ofB. xylophilusto develop new strategies to control this notorious I,nvasive nematode.
Materials and methods
Nematode materials
Bursaphelenchus xylophilusNXY61 strain was originally isolated from wiltedPinus massonianain Zhejiang Province of China and was maintained on a mycelial mat ofBotrytis cinereaon potato dextrose agar plates (Zhu et al. 2016).C. elegansN2 strain (from Dr. Xiao Liu, Tsinghua University, China) was cultured on plates of nematode growth medium seeded withEscherichia coliOP50 at 20°C in the dark.
Bx‑daf‑8 cloning
Total RNA ofB. xylophiluswas extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The cDNA was synthesized and purified using a PrimeScript RT Reagent Kit with gDNA Eraser (Takara Bio, Shiga, Japan). A primer pair was designed to amplify the cDNA ofdaf-8gene inB. xylophilus(WormBase ParaSite accession number BXY_0464200.1) (Table 1). The thermocycling conditions were 94 °C for 5 min; 35 cycles of 94°C for 30s, 60°C for 30 s, 72 °C for 1 min; and 72 °C for 5 min. The amplified products were cloned into the pGEM-T Easy vector (Promega, Madison, USA) for sequencing.
Bioinformatic analysis of Bx‑daf‑8
TheBx-daf-8gene structure was deduced from the BXY_0464200.1 gene information obtained from the WormBase Parasite genome project PRJEA64437. The Bx-DAF8 amino acid sequence used in sequence analyses was derived from theBx-daf-8coding sequence from NCBI (GenBank accession JX855256). Other DAF-8 and other DAF protein sequences were also retrieved from NCBI. Multiple sequence alignments were carried out with ClustalW (Thompson et al. 1994), and phylogenetic analysis was performed with MEGA 7.0 using the neighbor-joining method (Kumar et al. 2016). The Bx-DAF-8 three-dimensional structure was predicted by homology modelling using the SWISS-MODEL computer program (https:// swiss model. expasy. org/). Peptide signals and transmembrane domains of Bx-DAF-8 were predicted using the classical predictors of SignalP (http:// www. cbs. dtu. dk/ servi ces/ Signa lP-3. 0/) and TMHMM (http:// www. cbs. dtu. dk/ servi ces/ TMHMM/), respectively. The physicochemical properties of Bx-DAF-8, including molecular mass, amino acid composition, isoelectric point and instability, were analyzed by using ProtParam (https:// web. expasy. org/ protp aram/).
RT‑qPCR
Bx-daf-8expression levels were examined by qRT-PCR withtbb-2gene (Tubulin beta-2 chain, GenBank accession MT769316) as the reference gene. Primers for the target and reference genes were designed with Primer Premier 5 software (Premier Biosoft International, San Francisco, CA, USA) (Table 1). Synchronized embryos (early embryo at 6h, late embryo at 12h), juveniles (J2, J3 and J4) and adults (male and female) were collected as previously reported (Tang et al. 2020). Total RNA was extracted and cDNA synthesized as above described. RT-qPCR were performed with TaKaRa SYBR®Premix Ex Taq™ II (TliRNaseH Plus ROX pus) (Takara Bio) on Mx3000P qPCR System (Agilent, Santa Clara, CA, USA), in which amplification, detection and analysis steps were combined (Zhou et al. 2018). RT-qPCR was carried out with three replicates from three independent experiments.
Construction of B. xylophilus daf‑8 promoter and transgenic lines
By comparingB. xylophilusgenomic and EST libraries in WormBase (WormBase ParaSite accession prjea64437),Bx-daf-8promoter (Bx-daf-8p) was identified. Specific primers with 15-base extensions homologous to pCFJ90-Pmy-2::Cherry::unc-54utr end were designed for cloning the promoter (Table 1). Genomic DNA was extracted from the mixed stages nematode with QIAamp® DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. ThenBx-daf-8pwas amplified by PCR.Bx-daf-8pwas cloned upstream of the reporter gene in the mCherry (red) expression vector pCFJ90 and injected intoC. elegans. mCherry expression was regulated byBx-daf-8p.The myosin heavy chain genemyo-2is expressed exclusively in pharyngeal muscles (Okkema and Fire 1994; Okkema et al. 1997). Hence Pmyo-2::GFP was used as a marker to indicate the location of pharynx development in the nematode. TransgenicC. eleganswas constructed as described in previous reports (Vicente et al. 2015; Zhou et al. 2021a). pCFJ90-Bx-daf-8p::Cherry::unc-54utr was constructed and injected (80ng μL−1) into hermaphrodites ofC. eleganswith pCFJ90-Pmyo-2::GFP::unc-54utr (5ng μL−1) as a coinjection marker.The temporal and spatial expression patterns of pCFJ90-Bx-daf-8p::Cherry::unc-54utr were then examined in the transgenic lines (F2 transgenic progenies) using a Leica DM 2500 fluorescence microscope (Leica, Wetzlar, Germany).
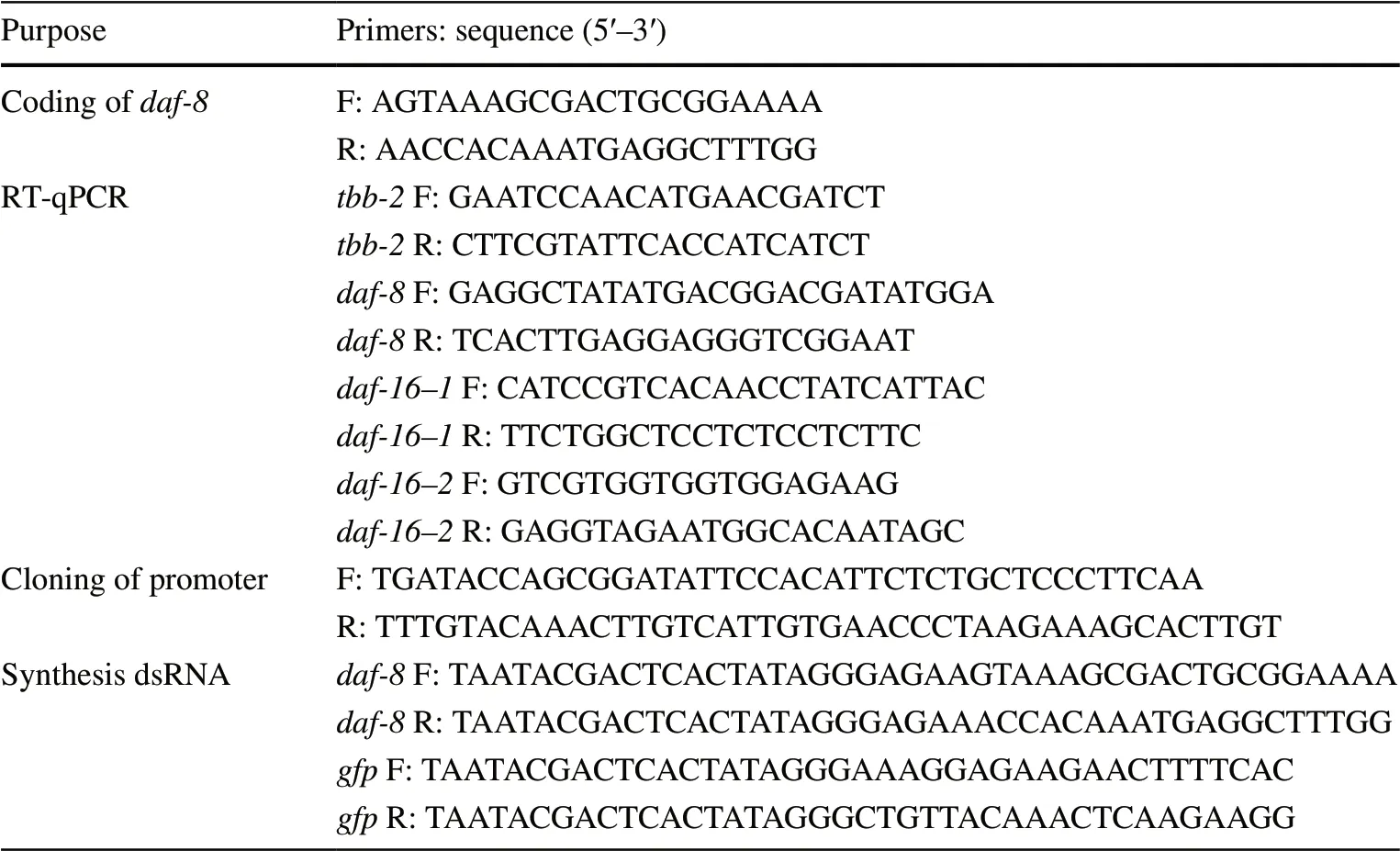
Table 1 Primers used in the present study. F, forward; R, reverse
RNAi
The PCR product correspondent to the cDNA of partial coding region ofBx-daf-8gene, obtained as described above, was used for synthesis of dsRNA with MEGAscript® T7 High Yield Transcription Kit (Ambion, Austin, TX, USA), according to the manufacturer’s instructions. Green fluorescent protein gene (gfp) was unitized as a non-endogenous control. The fragment ofgfpwas amplified from pHKT2 vector (Tomlin et al. 2004), and its dsRNA was synthesized with gene-specific primers (Table 1) as described above. To induce RNAi inB. xylophilusembryos, synchronized embryos (early embryos at 6h and advanced embryos at 12h) were soaked inBx-daf-8dsRNA (0.8 µg mL−1) with the soaking buffer (0.05% gelatin, 5.5mM KH2PO4, 2.1 mM NaCl, 4.7 mM NH4Cl, 3 mM spermidine) for 24h at 25 °C in the dark (Zhou et al. 2021b). As controls, nematodes were soaked in the soaking buffer without dsRNA and in soaking buffer with non-endogenousgfpdsRNA (0.8 µg mL−1). Then morphological phenotypes were examined and photographed with a Nikon NI-SS microscope (Nikon, Japan) and attached camera. RNAi efficacy was determined using qRT-PCR withtbb-2as an endogenous reference gene, and the expression of two downstream genes of the IIS pathway (Bx-daf-16-1andBx-daf-16-2) (Narasimhan et al. 2011) were also quantified (Table 1).
Statistical analyses
Excel 2016 (Microsoft Corp., Redmond, WA, USA) and SPSS version 22.0 (IBM, Armonk, NY, USA) were used to analyse the qRT-PCR data. Independent samplet-tests were used to compare the expression level of genes and egg hatching rates. Differences were considered significant whenp< 0.05. All experiments were conducted with at least biological triplicate.
Results
Sequence analysis of Bx‑daf‑8
Sequencing results from partial coding region ofBx-daf-8revealed a fragment of 962 bp, identical to previously published data (GenBank JX855256.1; WormBase Parasite BXY_0464200.1).Bx-daf-8(BXY_0464200.1) is located on the scaffold01198 from PRJEA64437 genome project, which spans 2.29 kb (from 21 896 to 24 186) on the genome and consists of six exons and five introns (Fig.1a). It has an open reading frame of 1626 bp, encoding 541 amino acids. Two conserved MAD homology domains (MH) of Smads, MH1 and MH2, were detected in the Bx-DAF-8 predicted amino acid sequence; MH1 was localized between amino acids 45 and 149, and MH2 between amino acids 347 and 465 (Fig.1b). The phylogenetic analysis of the DAF proteins showed that Bx-DAF-8 formed a well-supported cluster with those of other nematodes (Fig.1c). Its three-dimensional structure was predicted to have the crystal structure of Smad (PDB ID: 1khx.1.A) as a template by the online program SWISS-MODEL (Fig.1d). The QMQE value was 0.28 for the template, while the QSQE value was 0.30. The Ramachandran plot structural assessment of model quality by MolProbity (Chen et al. 2010) gave a significantly high score of 2.56 (MolProbity) and Favoured value of 95.96% (Ramachandran). No peptide signal or transmembrane domain was predicted for Bx-DAF-8. The molecular formula of Bx-DAF-8 was predicted to be C2674H4166N756O814S38with an estimated molecular mass of 61.15 kDa and a theoretical pI of 8.51. The computed instability index (II) of 52.58 indicated that the protein is unstable.
Quantitative analysis of Bx‑daf‑8
In the RT-qPCR to analyzeBx-daf-8expression in early (6 h) and late (12 h) embryos, juveniles (J2, J3 and J4) and male and female adults,Bx-daf-8was detected at 6 h after egg-laying when the embryo was in the ~200-cell stage in early embryogenesis (Fig.2). Expression ofBx-daf-8was gradually increased as embryogenesis progressed, peaked in the J2, the critical stage for entering the dauer stage. A dramatic drop inBx-daf-8expression was found at J3, and the expression remained this low through the rest of its life. In addition, significant differential expression levels were observed between males and females (Fig.2).
Temporal and spatial expression of Bx‑daf‑8
To mimic theBx-daf-8dynamic expression pattern inB. xylophilus, the promoter region ofBx-daf-8gene (Bx-daf-8p) was cloned into a mCherry expression vector and then injected intoC. elegans. Expression patterns ofBx-daf-8pdiffered during embryonic and postembryonic development. During early embryogenesis,Bx-daf-8pactivity was distributed in specific areas of theC. elegansembryo, and promoter activity was found close to the pharynx developmental area (Fig.3A1–A4). DuringC. eleganslate moderately conserved amino acid residues. c Unrooted phylogenetic tree based on amino acid sequences of DAF proteins constructed with the neighbour-joining method. Percentage bootstrap support (1000 bootstrap replicates) is given on the branches. Scale bar indicates evolutionary distance (0.2 substitutions per position). d Three dimensional structure of Bx-DAF-8 obtained via SWISS-MODEL with Smad (PDB ID: 1khx.1.A) as a template. Red:α-helices; green:β-sheets embryogenesis,Bx-daf-8promoter activity was weakly distributed throughout the whole body with strongBxdaf-8activity between the anterior and posterior pharynx in the nerve ring area (Fig.3B1–B4).
Fig. 1 Sequence analysis of B. xylophilus daf-8 (Bx-daf-8). a Bxdaf-8 structure was determined using SWISS-MODEL. Relative positions and respective sizes (bp) of exons are indicated as boxes and introns as lines. b Multiple amino acid sequence alignments for Bx-DAF-8 with DAF-8 in other nematodes using the ClustalW program (B. xylophilus AFY98833.1, Caenorhabditis elegans NP_4922321.1, Caenorhabditis remanei XP_003112140.1, and Caenorhabditis briggsae XP_002640041.1). Asterisk (*): invariant amino acid residues; colon (:): highly conserved amino acid residues; period (.):

Fig. 2 Real-time quantitative PCR analysis of daf-8 expression levels in Bursaphelenchus xylophilus at different developmental stages. Data were normalized with tbb-2 as the endogenous reference gene. Error bars show standard deviations. The asterisks (*) indicated statistically significant differences between two groups (p < 0.05)
Bx-daf-8pactivity in head neurons was consistent throughout all postembryonic stages inC. elegans(Fig.4). However, the initial weakBx-daf-8pactivity present throughout body became more and more restricted to the head and tail ganglions and intestine (Fig.4b), vulva and ventral nervous cord (VNC; Fig.4d, e), and excretory cells of adult hermaphrodites (Fig.4e).

Fig. 3 Temporal and spatial expression of mCherry (red) under control of Bursaphelenchus xylophylus daf-8 promoter during Caenorhabditis elegans embryonic development. The reporter gene GFP was driven by C. elegans promoter myo-2 as a marker for pharynx development. Fluorescence (A1–A3, B1–B3) and differential interference contrast (DIC) optics are superimposed in A4 and B4 images of F2 transgenic embryos that carried both Bx-daf-8::Cherry and Pmyo-2::GFP transgenes and were raised at 20°C. a Bx-daf-8::Cherry was expressed during early development stage and was unevenly expressed in the embryo (A1). Bx-daf-8p::Cherry and Pmyo-2::GFP were not coexpressed (A3, A4). b Bx-daf-8::Cherry was expressed between anterior and posterior pharynx at threefold stage during late embryo development (B1, B3). In all cases, images are representative of 50 examined embryos. Scale bar = 1 μm
Analysis of Bx‑daf‑8 RNAi
To explore the role ofdaf-8duringB. xylophilusdevelopment, RNAi was employed to knockdownBx-daf-8expression in the early (6h) and late (12h) embryos. Severe abnormal embryonic phenotypes were found withdaf-8RNAi treatment (Fig.5a, b), whereas in control groups, the embryos developed into wild-type J2 (Fig.5c). Afterdaf-8RNAi treatment, the egg hatching rate for the 6- (25.3%) and 12-h (38.7%) embryos was also significantly lower than the control rate of 96.2% (Fig.5d). The hatched J2 developed into adults normally; no third-stage dauer larvae were observed. From the RT-qPCR to measure the efficiency ofdaf-8RNAi in treated J2 and compare the expression levels withBx-daf-8in the control (no dsRNA), the soaking delivery method caused a decline of 66% inBx-daf-8expression in treated J2, while downstream genesdaf-16-1andda-f16-2in the IIS pathway were 50% lower (Fig.5e). Hatching rates and expression levels of target genes in thegfpdsRNA-treatedB. xylophiluswere similar to those in the untreated controls.
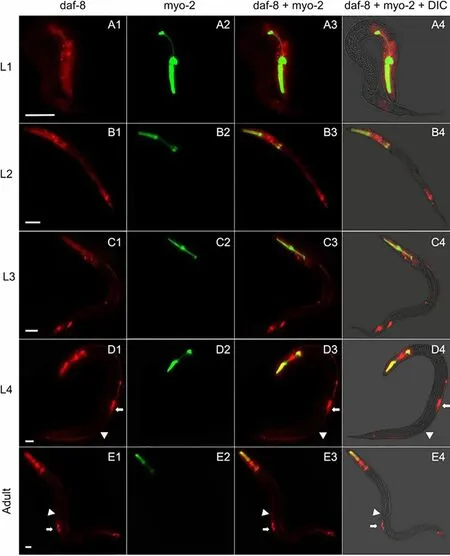
Fig. 4 Temporal and spatial expression of mCherry (red) under control of Bursaphelenchus xylophylus daf-8 promoter during C. elegans postembryonic development. Fluorescence and differential interference contrast (DIC) optics and superimposed images of F2 transgenic C. elegans that carried both Bx-daf-8::Cherry and Pmyo-2::GFP transgenes in L1 (a), L2 (b), L3 (c), and L4 (d) stages and in adult hermaphrodite (e). White arrow: vulva; white arrowhead: ventral nervous cord. In each age group, 50 nematodes were examined. Scale bar = 2μm

Fig. 5 Effect of Bx-daf-8 dsRNA on daf-8, -16–1,-16–2 expression on Bursaphelenchus xylophilus embryos and hatching after eggs were soaked for 24 h in Bx-daf-8 dsRNA at 25°C. Abnormal embryos at 6 (a) and 12h (b). Untreated control embryos developed into secondstage juveniles (c). d Percentage of eggs that hatched after soaking in dsRNA. Early embryos (6h) and late embryos (12h) were soaked in dsRNA at 25°C for 24 h in the dark, control group soaked in RNAi buffer without dsRNA. e mRNA expression of Bx-daf-8, and two downstream genes Bx-daf16-1 and Bx-daf16-2 in B. xylophilus J2 after soaking RNAi daf-8 and control for 24h. Scale bar = 20μm. Asterisks (*) indicated p < 0.05
Discussion
Although the morphology, pathology, population genetics, and infection cycle ofB. xylophilushave been extensively studied (Mamiya and Kiyohara 1972; Kikuchi et al. 2011; Zhao et al. 2016; Zhou et al. 2017; Wu et al. 2019), little is known about its reproduction and development at the molecular level. Because DAF-8 is crucial for larval development and dauer formation in the model organismC. elegans(Trent et al. 1983; Park et al. 2012; McGehee et al. 2015), we investigated its amino acid sequence and features, spatiotemporal expression, and biological functions inB. xylophilus. The results provide direct evidence thatBx-daf-8is important for embryogenesis and may act as a master regulator ofdaf-16inB. xylophilus.
Two well-conserved domains in the Smads, the N-terminal MH1 domain and the C-terminal MH2 domain, were detected in Bx-DAF-8. MH1 has a highly conserved 11 residue β hairpin which binds to the sequence GNCN in the major groove of DNA, are known to be important for activating transcription of target genes (Shi et al. 1998). Unlike MH1, MH2 does not bind DNA, but mediates interaction with all kinds of proteins and confers specificity and selectivity to Smad function and also is critical for mediating interactions in Smad oligomers (Wu et al. 2001). No transmembrane region or signal peptide was predicted, indicating Bx-DAF-8 is located in the cytoplasmic matrix or in organelles (Nielsen et al. 2019). This result is consistent with DAF-8 as one of the receptor-regulated Smads, which are signal mediators of the TGFβ signalling pathway, and present in the cytoplasmic matrix (Schilling et al. 2006). The phylogenetic analysis of DAF proteins revealed that Bx-DAF-8 formed a well-founded cluster with those of other nematodes, suggestingBx-daf-8might play the conversed role in dauer formation, mitosis and longevity as seen that inC. elegans(Trent et al. 1983; Shaw et al. 2007; McGehee et al. 2015).
In the RT-qPCR analysis ofBx-daf-8expression in transgenicC. elegans,Bx-daf-8expression gradually increased during the embryonic stages, and peaked in the J2, then dramatically dropped in J3, then remained low.B. xylophilus daf-8::Cherry expression patterns in the transgenicC. eleganswere similar to the RT-qPCR results. High levels of embryonicBx-daf-8expression imply thatBx-daf-8may be crucial role for embryogenesis. The highestBx-daf-8expression in J2 inB. xylophiluswas consistent with highest expression of DAF-8 in L1 inC. elegans(Park et al. 2010). Both stages are the earliest pre-dauer entry sites for the respective nematode species. Since loss-of-function mutations indaf-8lead to constitutive developmental arrest at the dauer larval stage inC. elegans(Trent et al. 1983), a high level of expression might be required for the propagative life cycle during J2 stage inB. xylophilus.
Bursaphelenchus xylophilus daf-8::Cherry expression is very similar to endogenousdaf-8expression inC. elegans. However, theB. xylophilus daf-8::Cherry was found in the 200-cell embryonic stage which is earlier than the pre-comma stage for expression ofC. elegansendogenousdaf-8(Park et al. 2010). Similar to the endogenousdaf-8, the expression ofB. xylophilus daf-8::Cherry was detected in the intestine, VNC, subsets of head and tail neurons and excretory cells. The same expression patterns ofdaf-8suggest conserved functions of the gene in the two species. However,B. xylophilus daf-8::Cherry was not expressed in the gonadal distal tip cells (DTCs), but in the vulva. Our results suggest thatdaf-8might not be involved in germ line development in DTCs, but mutation indaf-8might cause a more severe egg-laying defect inB. xylophilus(Kimble and White 1981).
The RNAi results proved thatBx-daf-8is essential forB. xylophilusembryogenesis. DownregulatingBx-daf-8expression showed the lethality to embryos, and egg hatching rates were significantly lower than in the control, as observed indaf-8 C. elegansmutants (Park et al. 2010). Down regulatingBx-daf-8expression did not lead to the occurrence of dauer larvae inB. xylophilus. However, a loss-of-function mutationdaf-8does lead to constitutive developmental arrest at the dauer larvae stage inC. elegans(Golden and Riddle 1984). This result suggests thatBx-daf-8by itself is not sufficient for the developmental switch to the dispersal life cycle inB. xylophilus, or it may have redundant regulatory elements, such as DAF-14 (Inoue and James 2000). Since the crosstalk between the TGFβ and the IIS pathways are tightly associated with dauer diapause and longevity (Shaw et al. 2007), we examined the effect ofBx-daf-8onBx-daf-16, a key downstream gene in the IIS pathway. The RNAi results showed that downregulation ofBx-daf-8supressed the expression of theBx-daf-16-1andBx-daf-16-2isoforms. We therefore propose thatBx-daf-8acts as an upstream regulator ofBx-daf-16during the crosstalk between the TGFβ and the IIS signalling pathways and either directly or indirectly controlsBx-daf-16expression.
Conclusions
Strategies that inhibit population growth ofB. xylophilusor block the spread of the invasive nematode will greatly contribute to the control of pine wilt disease. Interfering with normal ontogenesis is a direct way to constrain population growth, while supressing dauer formation is a direct way to prevent epidemics of pine wilt nematode. Our RNAi results revealed thatBx-daf-8was indispensable for embryogenesis and is thus a potential target for suppressing population growth ofB. xylophilus. However, knockdown ofBx-daf-8did not lead to constitutive developmental arrest at the dauer larvae stage, indicating that the dauer entry process inB. xylophilusis more complicated than inC. elegans. Moreover, we propose that there is crosstalk between TGFβ and IIS pathway at the upstream ofBx-daf-16and thatdaf-8may act as a master regulator ofdaf-16inB. xylophilus.
Author’s contributionsJinghan Wang and Huan Hong contributed equally to this study.
杂志排行
Journal of Forestry Research的其它文章
- Modeling habitat suitability and utilization of the last surviving populations of fallow deer (Dama dama Linnaeus, 1758)
- The identification and pathogenicity of Fusarium oxysporum causing acacia seedling wilt disease
- Growth and decline of arboreal fungi that prey on Bursaphelenchus xylophilus and their predation rate
- Volatile metabolites of willows determining host discrimination by adult Plagiodera versicolora
- Soil ecosystem changes by vegetation on old-field sites over five decades in the Brazilian Atlantic forest
- Response of soil respiration to environmental and photosynthetic factors in different subalpine forest-cover types in a loess alpine hilly region
