Volatile metabolites of willows determining host discrimination by adult Plagiodera versicolora
2022-04-17JiahaoLingXiaopingLiGuoYang
Jiahao Ling·Xiaoping Li·Guo Yang·
Tongming Yin1
Abstract Plagiodera versicolora Laicharting is a highly damaging leaf beetle foraging on willow leaves. In willow germplasm collections, observation has shown that Salix suchowensis Cheng was severely foraged by this leaf beetle while Salix triandra L. was damage free or only slightly damaged. Results of olfactometer bioassays show that the headspace volatiles from leaves of S. triandra significantly repelled adult beetles, suggesting that this species produces volatile repellents against P. versicolora. S. suchowensis had no effect on the beetles. Gas chromatography-mass spectrometry was carried out to profile the headspace volatile organic compounds and 23 compounds from leaves of the alternate species in significantly different concentrations were detected. The effects of 20 chemical analogs on host discrimination were examined. Olfactory response to these chemicals showed that o-cymene, a S. suchowensis specific constituent, significantly attracted adult P. versicolora. In contrast, cis-3-hexenyl acetate, a constituent concentrated more in S. triandra than in S. suchowensis, significantly repelled beetles. Mixing o-cymene and cis-3-hexenyl acetate in comparable concentrations as in the volatiles of S. suchowensis demonstrated that the latter could mask the attracting effect of the former, causing a neutral response by adult beetles to leaves of S. suchowensis against clean air. In addition, chemical analogs have the same effect as plants when resembling volatile organic compounds in real samples. Two volatile metabolites were detected triggering host discrimination by one of the most damaging insect pests to host and non-host willows. The two metabolites are of considerable potential for use as olfactory signs in managing the beetles.
Keywords Herbivore insect·Olfactory response·Volatile organic compounds·Cis-3-hexenyl acetate·O-cymene
Introduction
Plagiodera versicoloraLaicharting (willow beetle) feeds exclusively on leaves of species of Salicaceae and is the most damaging herbivore insect onSalix suchowensisCheng as well as on many other willow species (Ishihara and Ohgushi 2008). Willow beetles forage on young leaves and cause bud death (Wade and Breden 1986). Several studies have shown that food preference of the beetles was significantly impacted by many host factors. For instance, secondary metabolites ofSalix integraThunb., chlorogenic acid and 3,5-dicaffeoyl quinic acid, were identified as antifeedants that would inhibit attack by insects and grazing animals, whereas 1,2-dilinolenoyl-3-galactopyranosyl glycerol could stimulate trophic behavior, the position occupied in the food chain, of the beetles (Jassbi 2003). Raupp (1985) reported that leaf toughness affected the morphology, feeding behavior, and ultimately shaped the spatiotemporal distribution ofP. versicolora. Adult willow beetles preferentially feed on young leaves of Salicaceae species (Raupp and Denno 1983), leading to an increase in mortality of willows due to necrosis of apical buds. Leaf age has also been reported to affect the choice of oviposition for female adults (King et al. 1998).
How do insects discriminate their preferred food resource? Conchou et al. (2019) proposed that herbivore selection of plants relies heavily on olfactory clues, although they also have visionary, mechanoreception and gustation mechanisms (Miller and Strickler 1984). Multiple lines of evidence support this hypothesis. For example, host selection by the spittle bug (Mahanarva spectabilisDistant) was affected by several volatile organic compounds (VOCs) emitted from forage grasses (Silva et al. 2019). Whereas the strawberry leaf beetle (Galerucella vittaticollisBaly) used a component in the headspace volatiles of several host plants as olfactory clues (Hori et al. 2006). For the pollen beetleMeligethes aeneusFab., they were more attracted by volatiles from their most preferred host, rapeseed,Brassica napusL. (Ruther and Thiemann 1997). Plant VOCs are important to insects for finding hosts and for discerning non-hosts. Non-host plants succeed in affecting herbivore behavior in two possible ways. First, the VOCs mask those of host plants so that efficiency of host finding is greatly reduced, which was the case for the carrot fly (Uvah and Coaker 1984), several beetle species (McNair et al. 2000; Zhang and Schlyter 2004; Hori et al. 2006; Kerr et al. 2017) and some moth species (Asare-Bediako et al. 2010; Jactel et al. 2011). Secondly, VOCs released from non-host plants contain deterrent chemicals, thus insects are repelled (Nottingham and Hardie 1993) or normal development is disturbed after landing on the plants (Nishida et al. 1990; McNair et al. 2000; Wang et al. 2016).
ForP. versicolora, it was shown that it could determine host status by odor (Yoneya et al. 2009). Through years of empirical observation, we found that infestation by willow beetles was severe on germplasm collections ofSalix suchowensis, while the collections ofSalix triandraL. were pest free. In this study, olfactory response tests were carried out with adultP. versicolorawith leaves ofS. suchowensisandS. triandra. Subsequently, VOCs emitted from the leaves of the alternate species were analyzed by gas chromatography mass spectrometry (GC-MS), and effects on hosts for the VOCs with different concentrations in the two species were examined with chemical analogs. We aimed to identify the VOCs underlying host discrimination for adultP. versicolorain host and non-host willows.
Materials and methods
A large germplasm collection ofS. suchowensisandS. triandramaintained at the Baima Forest Farm in Nanjing, Jiangsu Province. Cuttings propagated with clone “DB134” ofS. triandraand “XY12” ofS. suchowensiswere produced in the greenhouse of the Nanjing Forestry University in February 2018. Leaves were collected from the top first node to the top fifth node on the shoots in June 2018. AdultP. versicolorawere collected from the willow plantation at Baima Forest Farm (118°48′ E, 32°04′ N) and reared in an incubator at 27 °C, 65% ± 10% relative humidity, and under 16 h light, 8 h dark photoperiod. All adults were from the following generations of the initial collection. The duration of development from egg to adult emergence is approximately three weeks. Emerging beetles were provided with poplar leaves for 4-6 days of adult sexual maturation. Before the tests, the adults were starved for 12 h, and both male and females were tested.
Analyzing the headspace volatile organic compounds
Headspace VOCs emitted from leaves ofS. suchowensisandS. triandrawere extracted using solid-phase micro extraction (SPME) in the headspace mode. Separation and identification of the constituents were carried out by GC-MS. For volatile extraction, 2-g fresh leaves were cut into pieces, and placed into a 50 mL vial. The headspace of the vial was extracted with SPME (65 μm PDMS/DVB, Supelco Inc., Bellefonte, PA., USA) for one hour at 50 °C, which was based on a series of preliminary experiments.
The GC-MS (Trace ISQ-LT; Thermo Fisher Scientific Co., Waltham, MA, USA) was equipped with a DB-5 column (30 m × 0.25 mm × 0.25 μm, Agilent J &W) and helium was used as the carrier gas at a column head speed of 1 mL/min. GC was set for splitless injection. The temperature program of the column oven was 40 °C (2-min hold), 6 °C min-1to 250 °C (5-min hold). The MS parameters were set at ionization energy of 70 eV and a scan range of 45-450 U. Six replicates were used to conduct qualitative and quantitative analyses of leaf volatiles of the two willow species.
Olfactometer bioassays
The olfactory responses of adult willow beetles to leaf VOCs were investigated with a glass Y-tube olfactometer (8 cm long × 0.5 cm radius, 75 °Yangle). The stem of the Y-tube was connected to a rubber hose where the test beetles were released. Each arm was connected to a 50 mL air chamber, an airflow (500 mL/min) was introduced into each chamber through activated charcoal and purified-water using an air pump. All tests were performed under ambient temperatures at 25 ± 1 °C and relative humidity of 80 % ± 10 % (Koschier et al. 2017).
Beetles were placed into the stem of the olfactometer, with each arm defined a “finishing line” at 3 cm from the end of the long stem. The selection behavior was recorded by observing if a beetle crossed the finishing line of the corresponding Y-tube glass connecting to the sample vial within 5 min. If a beetle did not cross the finishing line within 5 min, it was recorded as ‘pest did not make a selection’. The blend and control arms were exchanged after every 20 individual insects, and the glass Y-tube was washed in 75% ethanol and oven-dried at 90 °C after testing every 40 insects. The host-selection behavior was estimated by the excess proportion index (EPI) (Hori et al. 2006):

where Nt = number of insects in a treated sample side, and Nc = number of insects in a control sample side. To give a meaningful explanation ofEPI, we defined theEPI0as follows:

where Nc1 = number of insects in one control sample and Nc2 = number of insects in the other control sample. WithEPIsignificantly lower thanEPI0= repel;EPIshowed no significance withEPI0= no preference; andEPIsignificantly higher thanEPI0= attract.
In bioassay 1, the olfactory response of adultP. versicolorato odors of 2-g leaf samples was compared against clean air. For each sample, 40 insects were tested with five replicates for each test. In addition, to demonstrate preference of beetles between the willow species, a fourchoice olfactory bioassay (bioassay 2) was conducted with the two willows and two controls. The olfactometer was designed on the basis of the glass Y-tube olfactometer; the two long arms of the Y-tubes were connected with openings at the connection point. This is similar to bioassay 1.
In bioassay 3, olfactometer bioassays were carried out with chemical analogs of the headspace VOCs. These were purchased from ChengShao Biological Technology Co., Ltd., Shanghai, China and Wuhan Yuanchen Technology Development Co., Ltd., Wuhan, China. The chemicals were dissolved separately in liquid paraffin and used in bioassay 2. In each treatment, the volume of solution applied to filter paper was calculated based on the concentration of the corresponding compound inS. suchowensisorS. triandra. The filter paper with solution was placed in the blend-side of the container, whereas the filter paper with an equal volume of control solution (liquid paraffin) was placed in the control-side of the container. For each chemical analog, 40 insects were tested, with each test having five replicates.
Statistical analyses
Chi-square test was used to evaluate the difference in number of insects choosing between the blend arm and the control arm for data in both bioassays 1 and 3. One-way ANOVA was conducted to identify whether the numbers were significantly different among the two willow species treatments and the two controls in bioassay 2. The difference in relative abundance of plant volatiles was also determined using the Chi-square test. One-way ANOVA was identified whether theEPIvalues were significantly different between the odor sources. All analyses were carried out using SPSS 19.0, SPSS Inc.
Results
Olfactometer bioassays with leaves ofS. suchowensis andS. triandra
The response byP. versicolorato VOCs of willow leaves was tested in bioassays 1 and 2. ForS. triandravs. clean air, the selection rate indicated that the beetles significantly preferred clean air over the airborne substances (Table 1), with anEPIvalue of -0.45 ± 0.18 (Fig. 1a), while theEPI0of bioassay 1 was -0.01 ± 0.17 (Fig. 1a), meaning that the beetles were significantly repelled byS. triandraVOCs (Table 1). ForS. suchowensisvs. clean air, the selection rateshowed no significant difference (Table 1), and theEPIvalue was -0.03 ± 0.19 (Fig. 1a), which was also insignificantly different compared with theEPI0(Fig. 1a). Therefore, somewhat unexpectedly, the olfactometer bioassay showed no preference forS. suchowensisby adultP. versicolora, so that plant odors ofS. suchowensismight not attract the beetles, although it is a host plant in the field.

Table 1 Olfactory response of adult Plagiodera versicolora to willow leaves and clean air in bioassay 1 (Mean ± SD)

Table 2 Olfactory response of adult Plagiodera versicolora to willow leaves of two species and clean air simultaneously in bioassay 2. (Mean ± SD)
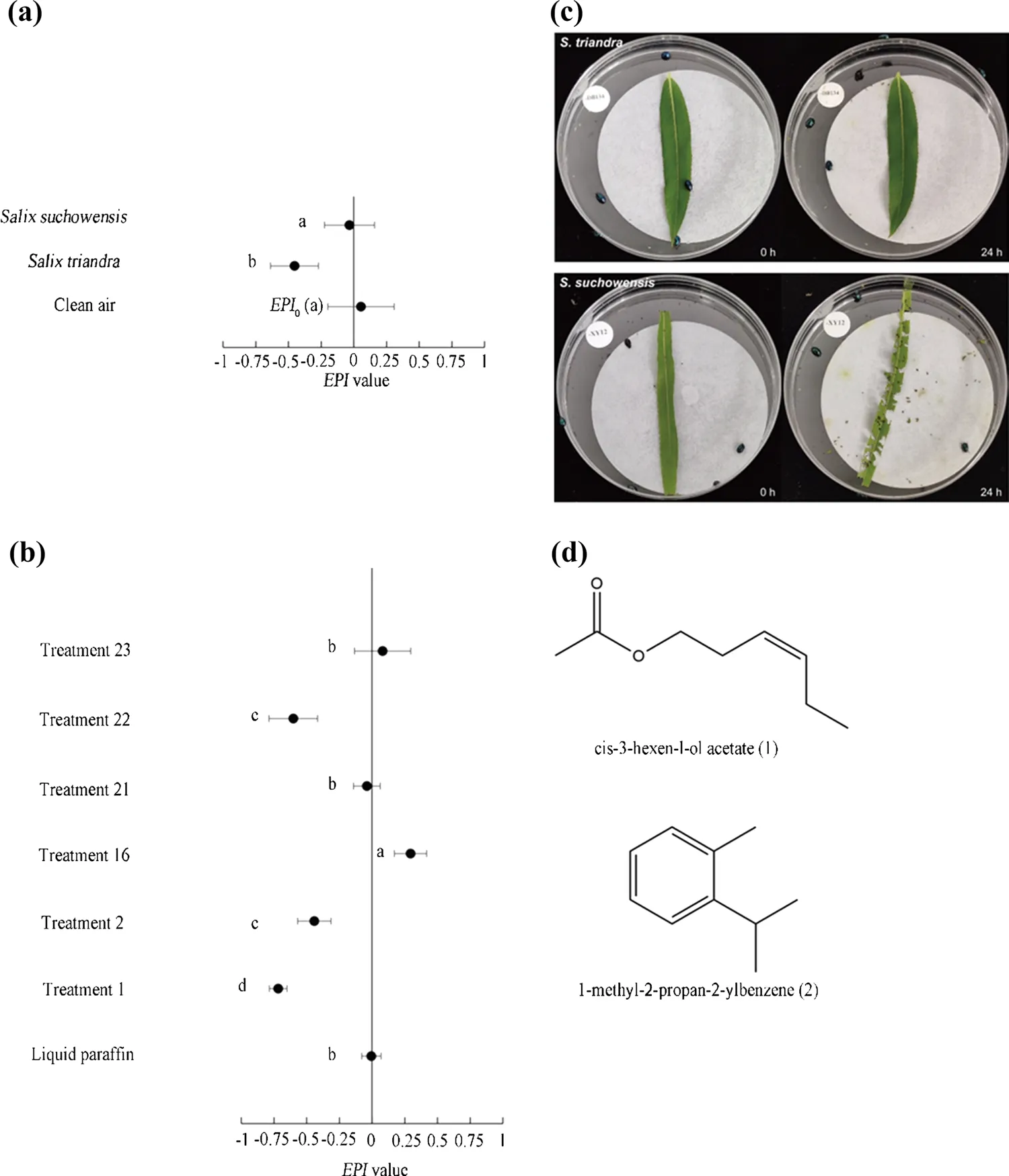
Fig. 1 a Host-selection behavior of Plagiodera versicolora to olfactory cues from leaves of the two willow species. Note: X-axis shows the EPI values. Different letters on the left of the horizontal bars indicate significant difference at P < 0.05 between the corresponding leaf samples and clean air. b Selection behavior of Plagiodera versicolora to olfactory cues in different treatments. Note: X-axis shows the EPI values. Different letters on the left of the horizontal bars indicate significant difference at P < 0.05 between the corresponding treatment and the control. c Damage on leaves of Salix triandra and Salix suchowensis after foraging by adult Plagiodera versicolora for 24 hours. d The molecular structure of the detected volatile repellent and attractant
Subsequently,S. triandra,S. suchowensisand clean air were compared simultaneously, the selection rate indicated preference by the beetles as:S. triandra<S. suchowensis= clean air (Table 2).
Identification of headspace VOCs from leaves ofS. suchowensis andS. triandra
To detect the VOCs triggering host discrimination for adult willow beetles, the headspace VOCs emitted fromS. suchowensisandS. triandraleaves were analyzed by GC-MS. Twenty compounds were identified, 14 present inS. triandraand 11 inS. suchowensis(Table 3). For compounds detected fromS. triandra, nine were species-specific (Table 3) and were abundant ascis-3-hexenyl acetate,cis-3-hexenyl isovalerate, farnesane andcis-3-hexenyl 2-methylbutanoate, while the remaining constituents were trace components. For the compounds detected inS. suchowensis, six were species-specific (Table 3).
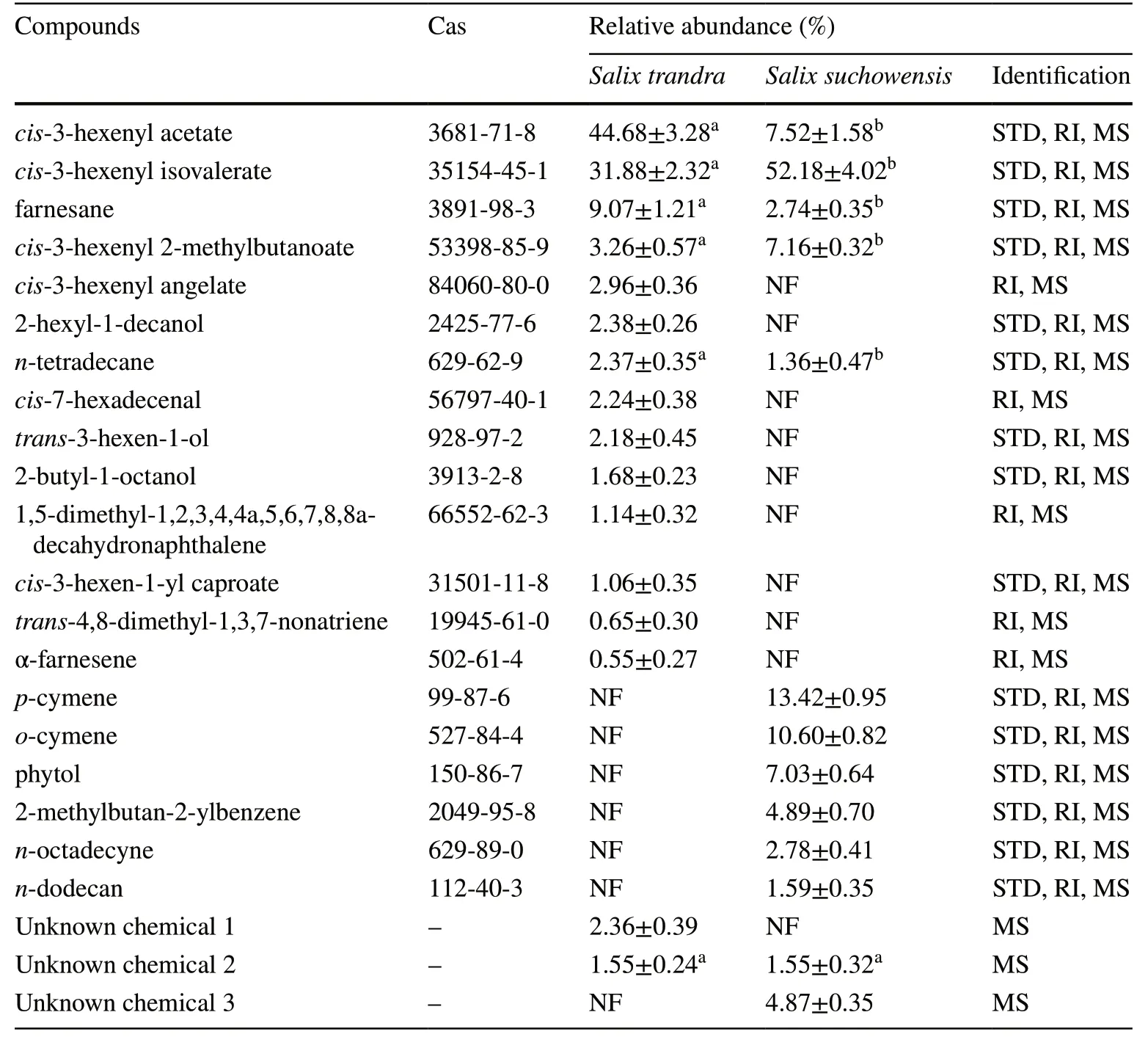
Table 3 Headspace volatiles of Salix suchowensis and Salix triandra detected by SPMEGC-MS analysis
Effects of VOC analogs on finding hosts
To analyze the effects of the emitted compounds on host finding or host identification, 15 commercially available standards were purchased. With these chemicals, 23 treatments were carried out in bioassay 3 and details are shown in Table 4.
Olfactory response to these analogs showed that adultP. versicolorawere significantly repelled in treatment 1 and treatment 2 (Table 5), because both their numbers for choosing were significantly lower than the control. TheEPIvalue was significantly lower (P< 0.001) thanEPI0of bioassay 2 (-0.01 ± 0.07) (Fig. 1b) and also showed beetle repellence in treatments 1 and 2. However, theEPIvalue of treatment 1 was -0.72 ± 0.12 (Fig. 1b), which is significantly lower (P= 0.03) than treatment 2 (-0.44 ± 0.13) (Fig. 1b), indicating that the repelling effect decreases with a decrease incis-3-hexenyl acetate. In contrast to treatments 1 and 2, the beetles exhibited significant preference to treatment 16 (Table 5). TheEPIvalue of treatment 16 was 0.29 ± 0.12 (Fig. 1b), significantly higher (P= 0.02) than theEPI0, demonstrating thato-cymene had significant effects on attracting adultP. versicolora.
In Table 3, VOCs profiling shows thatS. suchowensispossessed both the repellent (cis-3-hexenyl acetate) and the species-specific attractant (o-cymene) but concentration of the repellent was much lower than inS. triandra. We mixed the two treatments forS. suchowensisin treatment 21 (Table 4) and the selection rates vs. control are shown in Table 5. There was no significant difference observed. TheEPIvalue of treatment 21 (-0.04 ± 0.15) is also not significantly different (P= 0.716) from theEPI0of bioassay 2 (-0.01 ± 0.07) (Fig. 1b), indicating the effect of the volatile attractant was masked by the repellent. Moreover, blends of VOCs resembling the compounds in the leaf samples ofS. triandraandS. suchowensiswere prepared as treatments 22 and 23, respectively. The number of beetles attracted to treatment 22 was significantly different compared to those attracted to the solvent (Table 5). TheEPI0was significantly higher than theEPIof treatment 22 (Fig. 1b; Table 5), indicating that the higher repulsive power of the blend was similar to the smells ofS. triandra. However, treatment 23 failed in influencing the selection behavior of the beetles (Table 5; Fig. 1b), indicating that the beetles showed no preference to the blend, which was similar to the odors ofS. suchowensis.

Table 4 Solutions of chemical analogs and treatments in olfactory response tests in bioassay 3

Table 5 Olfactory response of adult Plagiodera versicolora to chemical analogs in different treatments (Mean ± SD)
Discussion
Through years of empirical observation in the field, we found thatS. suchowensiswas a favorable host for the leaf beetle,P. versicolora, whileS. triandrawas a non-host plant. On a macro level, studies have shown that the preference of oligophagous herbivores, especially those feeding on plants of the same genus, were strongly affected by whether the plants were native species or not. Native species were more readily threatened by biotic stresses, while introduced species were more tolerant (Agrawal et al. 2005; Richard et al. 2019). In this study,S. suchowensisis a native species of Jiangsu province in southern China butS. triandragermplasm was collected in Heilongjiang province in northern China. Our study confirmed that adultP. versicolorawas repelled by the chemicals from leaves ofS. triandra, partially explaining why it is a non-host plant. ForS. suchowensis, the olfactory bioassay indicated no preference by adult beetles for this favored host compared with clean air, although it produced volatile attractants. Subsequent analyses showed that the effect of the attractant was masked by the endogenous repellent. Therefore, locating host plants might not be triggered by olfactory signals. Enclosed starved beetles with leaves ofS. triandraandS. suchowensiswere only observed occasionally on the former, while leaves of the latter were consumed rapidly (Fig. 1c). SinceS. triandraproduced more concentratedcis-3-hexenyl acetate thanS. suchowensis, this chemical might act as an antifeedant. However,S. triandramight also synthesize other nonvolatile antifeedants yet to be discovered.
Plant secondary metabolites, including C6-C10 hydrocarbons, aldehydes, alcohols, esters and their acetates, commonly serve as olfactory signals to insects (Matsui 2006). Besides directly attracting or repelling herbivore insects (Mitchell and McCashin 1994; Müller and Hilker 2000; Samantaray and Korada 2016; Castro et al. 2017; Mbitsemunda and Karangwa 2017), they also affected mating and reproduction behavior (Tabashnik 1985; Shelly 2000; Gerofotis et al. 2013; Molnár et al. 2017; Xu et al. 2017). As a typical VOC,cis-3-hexenyl acetate is widely known as an olfactory signal for herbivores (Sun et al. 2020), and also repelled maleAnoplophora glabripennisMotschulsky (Nehme et al. 2009) and femaleCephus cinctusNorton (Piesik et al. 2008) in finding hosts. Other effects ofcis-3-hexenyl acetate were also reported to be an inhibitor of pheromone response of spruce bark beetles (Ips typographiusL.) (Zhang et al. 1999), while it enhanced activities ofCotesia glomerataL. andC. plutellaeKurdjumov, natural enemies of spruce bark beetles and thus reduced damage by the herbivores (Shiojiri et al. 2006). However, this chemical could also act as an attractant for some other insect pests in finding hosts (Schröder et al. 2015; Xin et al. 2017) and mates (Rouyar et al. 2015; Yu et al. 2015).
In this study,cis-3-hexenyl acetate was detected as a repellent (Fig. 1d) and the effect is directly proportionate to concentration: the higher level, the stronger repellency. Accumulated studies have revealed that concentration is a key factor determining the effect of VOCs. For instance, the essential oil of sweet wormwood (Artemisia annuaL.) to two stored product insects:Tribolium castaneumHerbst andCallosobruchus maculatusL. worked at a 1% volume concentration but stronger repellency occurred when increased to 2% volume concentration (Tripathi et al. 2000). Rosemary oil effect on onion thrips (Thrips tabaciLinde.) was positively correlated with concentrations in the range of 0.1% to 10% (Koschier and Sedy 2003). Similar effects were observed for the silverleaf white fly,Bemisia argentifoliiGenn., where repellency increased with increased concentration (Zhang et al. 2004). The reduction in predation by a species of wolf-spider,Pardosa pseudoannulataBoesenberg, occurred with increasing plant essential oil concentration in the range of 100 ppm to 1000 ppm (Farid et al. 2019).
A constituent of plant oils,O-cymene, is recognized as a toxin toSitophilus zeamais,Tribolium confusum(Tapondjou et al. 2005), andBemisia tabaci (Bleeker et al. 2009). However, we found thato-cymene was an attractant (Fig. 1d) to adultP. versicolora. It is also noteworthy thatS. suchowensiscontains both repellent and attractant chemicals. Chemicals with opposite effects on insect behavior have been found in numerous plants such as inOlea europaea (Scarpati et al. 1993),Solanum tuberosum(Dickens 2000) andPastinaca sativa(Carroll and Berenbaum 2002). Research has shown that functional chemicals should be mixed in the right ratio for insects to discern (Bruce and Pickett 2011).
Conclusions
Since the favored host resulted in a neutral response in the olfactory experiment, finding a favorable host by the leaf beetles was not triggered by olfactory signals but likely through visual ones. In contrast, the repellency properties of the non-host willow was unambiguously mediated by olfactory signals. The functional chemicals detected in this study not only improve our understanding of interactions between herbivore insects and plants but also have potential to control willow beetles.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.
杂志排行
Journal of Forestry Research的其它文章
- Molecular characterization and functional analysis of daf‑8 in the pinewood nematode, Bursaphelenchus xylophilus
- Modeling habitat suitability and utilization of the last surviving populations of fallow deer (Dama dama Linnaeus, 1758)
- The identification and pathogenicity of Fusarium oxysporum causing acacia seedling wilt disease
- Growth and decline of arboreal fungi that prey on Bursaphelenchus xylophilus and their predation rate
- Soil ecosystem changes by vegetation on old-field sites over five decades in the Brazilian Atlantic forest
- Response of soil respiration to environmental and photosynthetic factors in different subalpine forest-cover types in a loess alpine hilly region
