Combined ARC⁃MS Study and ReaxFF Molecular Dynamics Simulations on Thermal Decomposition Mechanisms of DNP
2022-05-19LANGuanchaoZHANGLinshengLIUXueyingCHAOHuiLIZhihuaCAODuanlinWANGJianlong
LAN Guan⁃chao,ZHANG Lin⁃sheng,LIU Xue⁃ying,CHAO Hui,LI Zhi⁃hua,CAO Duan⁃lin,WANG Jian⁃long
(1.School of Chemical Engineering and Technology,North University of China,Taiyuan 030051,China;2.Gansu Yin Guang Chemical Industry Group Co.Ltd,Baiyin 730900,China)
Abstract:ReaxFF molecular dynamics(ReaxFF MD)simulations were adopted to identify the main intermediate products,final products and chemical reactions during 3,4-dinitro-1H-pyrazole(DNP)thermal decomposition. Accelerating rate calorimeter(ARC)-mass spectrometer(MS)technique was adopted to study DNP thermal decomposition properties and identify the gaseous products. The simulated results illustrate that C3HO4N4,C3HO3N4,C3HO2N3,C3HNO2,NO2 are the main intermediate products,and H2O,CO2,N2 are the main final products. MS detected main gaseous products are H2O,CO2,N2 as well. According to the simulation results,the produced time and abundance of the products are obtained as well. Among which C3HO3N4 is the first generated intermediate product,and H2O is the first generated final product. C3HO3N4 and N2 are the intermediate and final products with the largest amount,respectively. Additionally,the main chemical reactions in DNP thermal decomposition process are also acquired by molecular dynamics simulations. According to the generation time and abundance of products,the decomposition path of DNP was obtained.
Key words:3,4-dinitro-1H-pyrazole(DNP);ReaxFF MD simulations;ARC-MS technique;thermal decomposition mechanism
1 Introduction
Melt cast explosives,the most widely used explosives in both the military and the civilian area,can be used to fill mortars, grenades, artillery shells,warheads and antipersonnel mines[1]. With the development of weapon systems,modern melt cast explosives have to meet different requirements depending on their applications such as high energetic performance,high density,low sensitivity toward external stimuli and low thermal hazards[2].The carriers with high density can enhance the detonation performances of melt cast explosives,and the carriers with low sensitivity can improve the safety of melt cast explosives. Therefore,it′s necessary to obtain a candidate carrier to meet the requirements of modern munition on energy and safety.
3,4-Dinitro-1H-pyrazole(DNP)is a good candidate of melt cast explosives carrier,whose melting point,friction sensitivity,density and detonation velocity are 87 ℃[1],360 N[2],1.81 g·cm-3[1]and 8.24 km·s-1[1],respectively. The detonation velocity of DNP is higher than that of TNT(6.94 km·s-1)and the mechanical sensitivity of DNP is lower than that of DNAN,indicating that DNP could be a potential energetic material as melt cast explosive carrier to replace TNT and DNAN. It has been proved that DNP could already replace TNT as melt cast explosives in compositions such as Composition B(RDX/TNT)[3].Therefore,DNP has broad application prospects as melt cast explosive carrier. Many researches have reported the synthesis,recrystallization,physicochemical properties and application of DNP[2-6]. However,no researches report the thermal decomposition path and decomposition products of DNP by experiments or simulations. Thermal decomposition mechanisms play important role in studying thermal safety of energetic materials. According to the decomposition path and products,some effective methods can be acquired to prevent the decomposition of energetic materials. It′s difficult to obtain a detailed understanding of energetic materials decomposition process by experiments or simulations,in that the thermal decomposition is complex. Experiments can examine the accuracy of simulations,and simulations can provide some information that can′t be measured by experiments. Combined experiments and simulations,intermediate products,final products and chemical reactions of DNP thermal decomposition can be obtained to analyze the decomposition mechanism.
ReaxFF molecular dynamics(ReaxFF MD)simulations is a good method to study the pyrolysis characteristic of materials[7-11]. ReaxFF MD simulations have developed to one of the main techniques for exploring the reaction of condensed phase using reactive force fields[12-13]. ReaxFF MD simulations have been successfully applied to study the thermal decomposition of many energetic materials,such as TATB(1,3,5-triamino-2,4,6-trinitrobenzene),RDX(trinitro-1,3,5-triazacyclohexane),TKX-50(dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate),HMX(1,3,5,7-tetranitro-1,3,5,7-azacyclo-octane),CL-20(1,3,5-hexanitrohexaazaisowurtzitane) and PETN(pentaerythritol tetranitrate)and so on[12-18].
TG-MS(thermogravimetric-mass spectrometer)is a commonly used method to confirm the gaseous products produced by materials pyrolysis. However,this method is not suitable for studying DNP,in that DNP is evaporated before it decomposed at 100 kPa as the low melting point of DNP. Therefore,in this study,accelerating rate calorimeter(ARC)was used to investigate the decomposition of DNP in the closed system,and the gaseous products were gathered after the decomposition of DNP. MS technique was used to identify the gathered gaseous products.Then,the main intermediate products,main final products and main chemical reactions were analyzed by ReaxFF MD simulations at 2000,2500 K and 3000 K,respectively. Based on the evolution of products and reactions, thermal decomposition mechanisms of DNP are obtained.
2 Experiments and simulations
2.1 Chemical materials and instruments
DNP sample was synthesized in the laboratory.It was purified by recrystallization and measured by High Performance Liquid Chromatography. The purity was greater than 0.995. ARC from NETZSCH Co.,Ltd using a 1/4-inch hastelloy C vessel with a thermocouple clip located on the bottom of the vessel was used. MS spectra(NETZSCH QMS 403D Germany)worked in two scanning functions,including full scanning mode and selected ion recording(MID) mode. The MS signals of all the fragment ions were detected by the former mode and the MID mode tracked MS signals of some specific ions marked accurately like m/z 18 and 28.
2.2 ARC⁃MS measurements
ARC was adopted to investigate DNP thermal decomposition in the closed system. 194.4 mg DNP samples were placed in a spherical hastelloy C vessel with a volume of 10 mL and a mass of 21.5 g,and the standard‘heat-wait-search’procedure was used for the measurement with temperature increments of 5 ℃. At each step,the system was kept adiabatic for a‘wait’period until thermal transients disappeared and then placed in‘search’mode,looking for an exotherm. ARC experiments were started at 50 ℃and the apparatus would repeat the heat-wait-seek model until the temperature reached 350 ℃.
The gaseous products produced by DNP were gathered when the temperature of spherical hastelloy C vessel of ARC decreased to 100 kPa. Then,them/zof the gathered gaseous products were analyzed by MS technique. Full scanning mode of MS technique withm/zbetween 1 and 400 was adopted to detect MS signals of all fragment ions. Based on the experimental results, final gaseous products were identified. Before scanning,pure argon was pumped to MS apparatus for 1 h to remove the air and create an inert atmosphere.
2.3 ReaxFF MD simulations
2.3.1 Simulations details
In this study,the isothermal decompositions of DNP were studied by large-scale atomic/molecular massively parallel simulator (LAMMPS)[19]using ReaxFF-lg reactive force field[12-13]at different temperature. The crystal structure of DNP belongs to monoclinic system with the lattice parameters ofa=9.70 Å,b=12.08 Å,c=9.76 Å,andβ=93.96°[20].A 3×3×3 DNP supercell(Fig. 1,216 molecules,2808 atoms)was used to study the thermal decompositions process of DNP at different temperature. In order to relax the internal stress of DNP,10 ps molecular dynamic simulations were first performed with NVT ensemble at 298 K using ReaxFF-lg force field,and then another 10 ps molecular dynamic simulations were performed with NPT ensemble at 298 K and 100 MPa using ReaxFF-lg force field[12].Then,400 ps,200 ps and 100 ps MD simulations were performed at 2000,2500 K and 3000 K,respectively,with NVT ensemble using ReaxFF-lg force field to study the decomposition properties of DNP.The temperature and pressure were controlled by using the Nosé-Hoover thermostat and barostat,respectively.As the bond orders and charge will be calculated after each reactive molecular dynamic simulation step,small integration time step should be adopted for efficient coverage of the phase space allowing collisions and reaction to occur smoothly[12,21]. In this study,the time step was set to 0.1 fs,and the dynamic trajectory was recorded every 200 fs. These collected simulation values were used to identify the species produced by DNP to provide information for analysis chemical reactions[22].
Fig.1 Crystal structure of DNP
2.3.2 Reaction kinetic parameter analysis
DNP thermal decomposition process includes three reaction steps:initial pyrolysis stage,intermediate pyrolysis stage,and final product evolution stage[23-24]. The initial pyrolysis stage and pyrolysis stage are divided at the time when potential energy(Ep)of DNP reaches the highest(tmax). The relationship between DNP thermal decomposition reaction rate constant and temperature can be described by linear Arrhenius function(Eq.1)[25].

wherek,A,Ea,RandTrepresent rate constant,pre-exponential factor(s−1),activation energy(kJ·mol−1),universal gas constant(8.314 J·mol−1·K−1)and temperature(K),respectively.
The rate constant of DNP initial decomposition stage(k1)can be calculated by Eq. 2 from the beginning of DNP decompositiont0totmax.
whereUtisEpvalue ,kcal·mol−1,U∞is Ep asymptotic value,kcal·mol−1,UtmaxisEpmaximum value,kcal·mol−1. Then,EaandAof DNP intermediate decomposition stage can be obtained by Eq. 1.
The rate constant of final product evolution stage(k3)can be calculated by Eq. 4 from the formation time of the product.

3 Results and discussion
3.1 ARC⁃MS analysis
3.1.1 ARC analysis
In this study,ARC is used to study the decomposition properties of DNP in the closed system,and the gaseous products are gathered after the decomposition at room temperature. The variation of temperature(T)and pressure(p)with time(t)during the whole measurements are displayed in Fig.2,and the temperature change rate(dT/dt)and pressure change rate(dp/dt)with time(t)during DNP decomposition process are depicted in Fig. 3. The measuredT0(initial decomposition temperature),Tf(final decomposition temperature),ΔTad(adiabatic temperature rise),β0(initial temperature rise rate),βm(maximum temperature rise rate),tm(time to maximum temperature rise rate),Tm(temperature of maximum temperature rise rate),p0(initial decomposition pressure),pf(final decomposition pressure)andpm(maximum pressure rise rate)are summarized in Table 1.
It can be concluded from Fig.2 that when the temperature is belowT0the pressure increases slowly. When DNP start to decompose,the temperature and pressure rise rate are gradually increased. Fig.3 shows that two peaks exist on the dT/dtand dP/dtcurves,illustrating that the decomposition of DNP can be divided into two steps. The first step is the decomposition of DNP to produce intermedia products,and the second step is the reaction between the interaction products. Table 1 illustrates that theT0of DNP is higher than that of TNT(2,4,6-trinitrotoluene,200.9 ℃)but lower than that of DNAN(1-methoxy-2,4-dinitro-benzene,232.0 ℃)[26],indicating that the thermal stability of DNP is higher than that of TNT and lower than that of DNAN. ΔTadandβmof DNP are lower than TNT(135.4 ℃,811.9 ℃·min-1)and DNAN(125.9 ℃,14.9 ℃·min-1),andtmof DNP is higher than TNT (883.1 min) and DNAN(898.7 min),illustrating that the thermal hazardous of DNP is lower than TNT and DNAN.In addition,thePmof DNP is lower than TNT(9.74 MPa·min-1)and DNAN(1.14 MPa·min-1),illustrating that the thermal safety of DNP is higher than those of TNT and DNAN.The time to maximum temperature rise rate(tm)of DNP is long,demonstrating that the decomposition rate of DNP is low. Moreover,the initial decomposition pressure(P0)of DNP is 0.302 MPa that is higher than 0.165 MPa(the pressure of air heated toT0),illustrating that some DNP are evaporated before thermal decomposition. In Fig.2,when temperature is below the melting point of DNP(Tm,87 ℃),the pressure rise of the system is contributed by the temperature rise. When the temperature is betweenTmandT0,the evaporation of DNP will contribute to the pressure rise of the system. Therefore,if thermal decomposition properties of DNP are studied in an open system,most of DNP are evaporated and thermal decomposition products can′t be obtained.
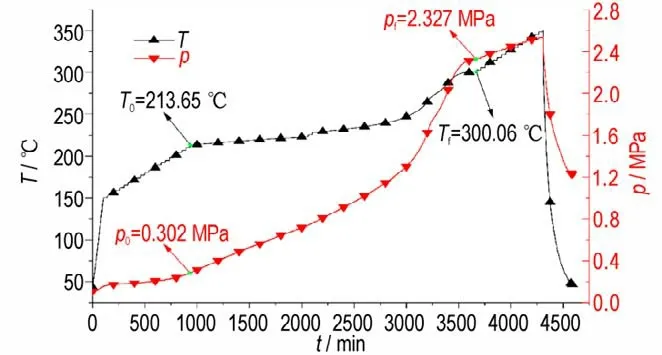
Fig.2 Curves of p-t and T-t during the whole measurements
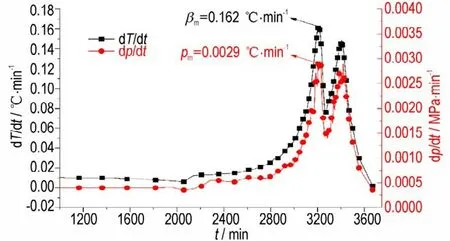
Fig.3 Curves of dT/dt-t and dp/dt-t during the decomposition process of DNP

Table 1 ARC measured thermal decomposition parameters of DNP
3.1.2 MS analysis
MS technique is used to analyze the gathered gaseous products produced by DNP decomposition in ARC. Full scanning mode of MS technique withm/zfrom 1 to 400 is performed to analyze them/zof the gathered gaseous products generated by DNP pyrolysis. The obtained MS spectrum of DNP thermal decomposition products is shown in Fig.4. As no products withm/zgreater than 60 are detected,Fig.4 displays the products withm/zfrom 1 to 60.
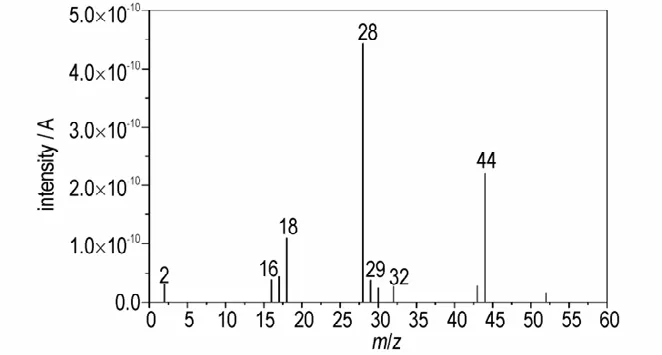
Fig.4 MS spectrum of DNP thermal decomposition products
It can be concluded from Fig.4 that the main MS peaks arem/z=18,28 and 44. The gaseous products are gathered at ambient temperature resulting in that some H2O(m/z=18)are condensed to liquid state and the content of H in DNP molecule is low,which results in the low intensity of H2O. Since DNP only contains C,H,O and N four kinds of atoms,the species withm/zof 28 can be assigned N2or CO or the composite of N2and CO,and the species withm/zof 44 can be assigned to CO2.In addition,some other gaseous products with low concentration are also detected such asm/z=2,16,17,29,30,32 and 43.Among which,m/z=32 can be assigned to O2(originating from air)that may be introduced to the gas cartridge when we gather the gaseous products. The species withm/zof 2 can be assigned to H2,because there are no other species withm/zof 2. The species withm/zof 16 and 17 can be assigned to CH4and NH3,respectively.Typical fragments corresponding to the possible species are list in Table 2. As the gaseous products were gathered and analyzed at room temperature rather than their generation time,resulting in that some gaseous products condensed to condensed state such as H2O and some unstable ionic products react with each other to form condensed or other products such as NO2. In addition,some products may have the samem/zsuch as N2and CO.These phenomena will result in MS experimentally measured gaseous products are less than the real situations. In order to study the real time products,ReaxFF MD simulations are adopted in this study.
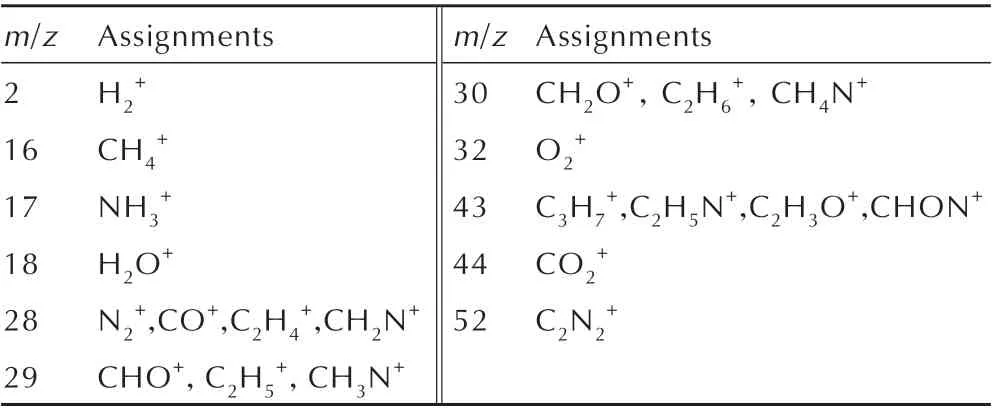
Table 2 Typical fragments corresponding to the possible species
3.2 ReaxFF MD simulations
3.2.1 Validation of the accuracy of ReaxFF
ReaxFF simulated density and cell parameters of DNP are compared with X-ray single-crystal diffraction experimental results to examine the accuracy of ReaxFF in describing DNP. The simulated density and cell parameters of DNP together with experimental data are shown in Table 3. ReaxFF simulated density and cell parameters of DNP show great agreement with the XRD results,demonstrating that ReaxFF simulation is reliable.
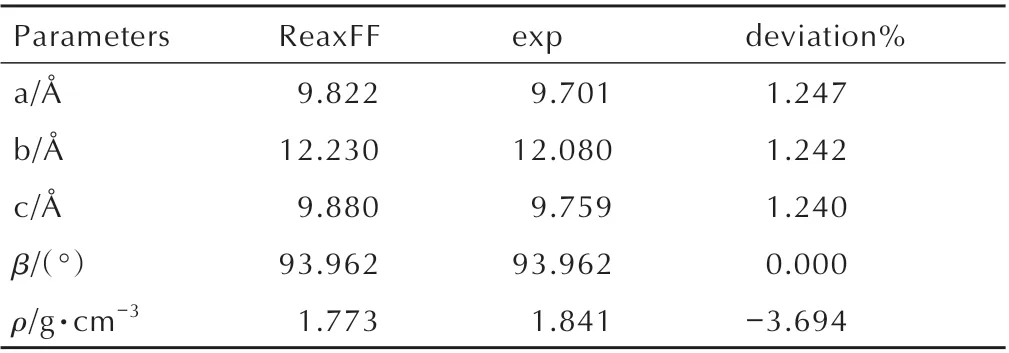
Table 3 Lattice parameters and density of DNP.
3.2.2 Evolution of potential energy and total number of DNP
The evolution ofEpof DNP can be used to determine whether a chemical reaction reaches equilibrium,in thatEpis related to the molecular interactions and relative position[18]. Total number of DNP molecules can be used to evaluate the degree of the thermal decomposition. In this study,within 400 ps,200 ps and 100 ps simulations,theEpof DNP reach equilibrium at 2000 K,2500 K and 3000 K,respectively. The evolutions ofEpand total number of DNP molecules with time at different temperatures are shown in Fig.5.
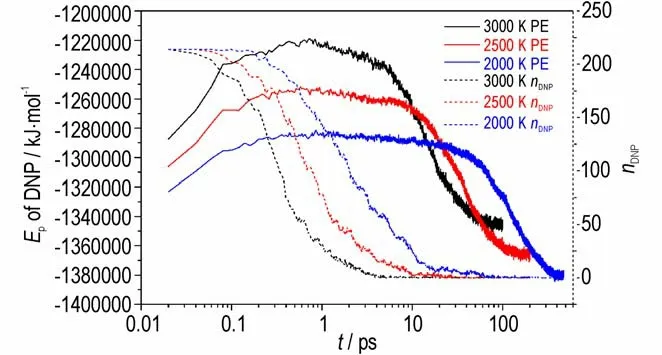
Fig.5 Evolutions of Ep(solid lines)and total number of DNP molecules(dash lines)with time at different temperatures
The simulatedEpcurves illustrate that theEpof DNP at each temperature reaches a maximum value within a short time indicating that DNP will absorb energy before the decomposition. Then,Epgradually decreases,which means that the exothermic decomposition of DNP is in progress. Finally,theEptends to constant demonstrating that all DNP molecules have decomposed and chemical reactions between intermediate products reach equilibrium. It can be concluded from the simulatedEpcurves that at high temperature DNP molecules absorb energy to reach its thermal decomposition activation energy initially,and then DNP molecules start to decompose and release energy until the reaction reaches equilibrium. Moreover,at higher temperature,the maximumEpvalues,theEpdecrease rates and the stableEpvalues are higher,and the time of the chemical reaction reaching equilibrium is shorter,illustrating that high temperature is conducive to DNP decomposition.
The curves of total number of DNP molecule show that higher temperature cause faster decrease of DNP molecule indicating a higher decomposition rate.Generally,the decomposition process of an explosive can be divided into two steps:the first step is the decomposition of explosive molecules to generate intermediate products,and the second step is the reaction between intermediate products to form final products[27]. It can be concluded from Fig.5 that theEpof DNP decrease rapidly when the total number of DNP tend to zero demonstrating that the second step releases more energy than the first step during the DNP decomposition,and the released energy of DNP decomposition is mainly originating from the reaction between intermediate products.
3.2.3 Evolution of intermediate and final products
The simulation results show that a large number of intermediate and final products are produced during the thermal decomposition of DNP. Final simulated configuration of the decomposition products of DNP after 400 ps at 2000 K are displayed in Fig.6.
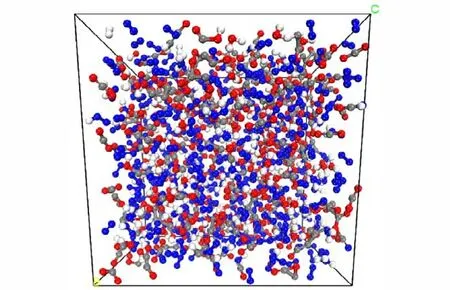
Fig.6 Final products of DNP decomposition
Fig. 6 illustrates that there are no DNP molecules existence after 400 ps ReaxFF MD simulation at 2000 K,and the main intermediate products(C3HO4N4,C3HO3N4and NO2etc.) produced by DNP react with each other formed stable final products after 400 ps simulation at 2000 K. The final products produced by DNP are small molecular products such as N2,H2O,CO2,H2,NH3,C2O2etc. Among which 52.2 percentages(mass fraction)are N2,H2O,CO2illustrating that the main products are N2,H2O,CO2during the decomposition of DNP,which is similar with the experimental results.The decomposition mechanism of DNP can be deduced by the abundance and generation time of intermediate and final products. Fig.7 shows the simulated generation time of main unstable intermediate and stable final products during DNP decomposition at 2000 K. Fig.8 shows the abundance evolution of main unstable intermediate products (C3HO4N4,C3HO3N4,C3HO2N3,C3HNO2,NO2)and final stable products(N2,H2O,CO2)generated by DNP during thermal decomposition at 2000 K.
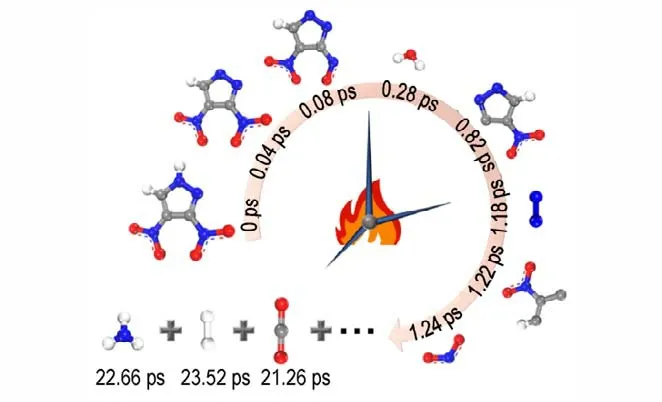
Fig.7 Main intermediates and final products generated by DNP decomposition at 2000 K
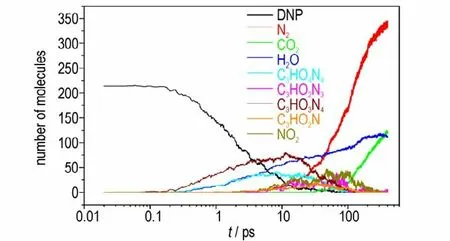
Fig.8 The evolutions of the amounts of the main intermediates and final products generated by DNP decomposition at 2000 K
Fig.7 illustrates that the chronological orders of the appearance of intermediate and final products are C3HO4N4,C3HO3N4,C3HO2N3,C3HO2N,NO2and H2O,N2,CO2,NH3,H2,respectively. The simulated chronological orders can provide important guidance for studying the decomposition path of DNP,which is difficult to obtain by experiment. Fig.8 illustrates that with the reactions going on the abundance of the intermediate products C3HO4N4,C3HO3N4,C3HO2N3,C3HO2N and NO2molecules first increase and then gradually decrease to zero,because these intermediate products react with each other or react with DNP to form stable products. In the initial decomposition stage of DNP,the main intermediate products are C3HO4N4,which are produced by the decomposition of DNP. With the decomposition going on,the main intermediate products change to C3HO2N3,C3HN2and NO2,that are generated by the reactions between intermediate products. After 300 ps simulations, intermediate products decrease to zero,illustrating that intermediate decomposition stage of DNP has completed.H2O molecules are generated at 0.28 ps which is earlier than the appearance of C3HO2N3,C3HN2and NO2,indicating the generation of H2O molecules is undergoing during the whole decomposition process. After 280 ps simulation,the abundance of H2O molecule gradually decreases,demonstrating that at high temperature the activity of H2O molecule is high resulting in the reaction between H2O and other intermediate products in the decomposition of DNP. In the initial decomposition stage of DNP,there are not N2and CO2molecules generated,which illustrates that N2and CO2are produced by the reaction between intermediate products rather than the decomposition of DNP directly.
Experimental detected products are H2,CH4,NH3,H2O,N2and CO2,which have been detected by ReaxFF MD simulations as well. As the abundance of some products is low such as H2,CH4,NH3,Fig.9 displays the main final products(H2O,CO2,N2)simulated by ReaxFF MD simulations. Fig. 9 illustrates that the increasing temperature enhances the abundance of CO2and N2,demonstrating that high temperature is benefit for the production of CO2and N2. H2O can react with other unstable fragments to form stable products,which decrease the abundance of H2O. In addition,the number of total chemical reactions and final products kinds are increased with the increasing temperature,which demonstrates that the higher the temperature is,the more complete the thermal decomposition reaction is.
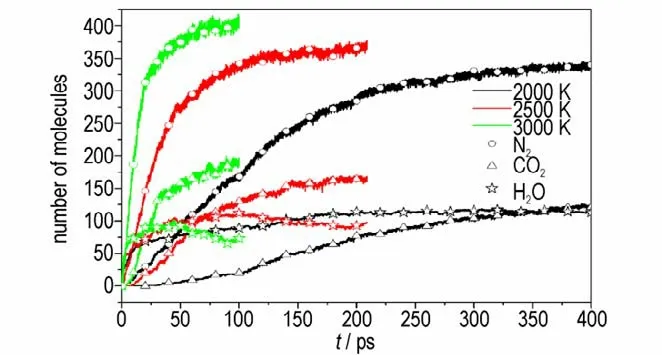
Fig.9 The main final products produced by DNP decomposition at different temperatures
3.2.4 Reaction kinetic parameter analysis
(1)Initial pyrolysis stage
At different simulation temperature,thek1of DNP decomposition are calculated by fitting Eq.2,and the fitting results are showed in Fig.10.The calculatedk1of DNP at 2000 K,2500 K and 3000 K are 0.436 ps-1,1.075 ps-1and 2.259 ps-1,respectively.With the increase of temperature,thek1is increased,illustrating that higher temperature is benefit for enhancing the decomposition rate of the initial decomposition stage of DNP. Then the pre-exponential factor and activation energy of DNP thermal decomposition can be calculated by Eq. 1. The values ofAandEaare 79.38 ps-1and 87.19 kJ·mol-1,respectively.
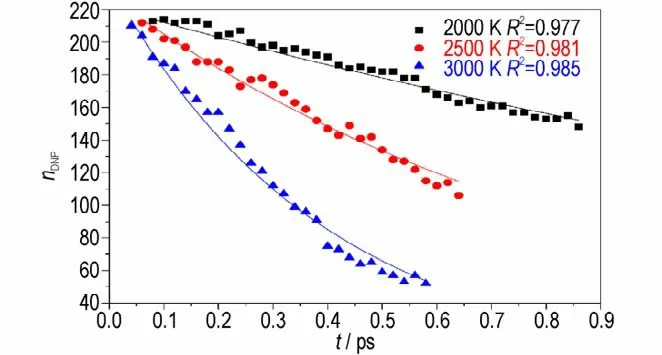
Fig.10 Evolutions of nDNP(point)and their corresponding fitted curves(lines)at different temperatures
(2)Intermediate pyrolysis stage
At different simulation temperature,thek2of DNP are calculated by fitting by Eq. 3,and the fitting results are shown in Fig.11. TheUtmax,U∞,tmaxand calculatedk2are summarized in Table 4.The calculatedk2increase with the increasing temperature,illustrating that high temperature can accelerate the intermediate decomposition stage of DNP. Then the pre-exponential factor and activation energy of DNP decomposition can be obtained by Eq. 1. The values ofAandEaare 4.53 ps-1and 108.24 kJ·mol-1,respectively.
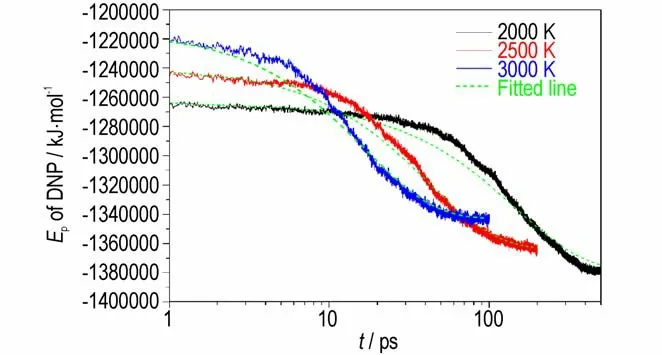
Fig.11 Evolutions of Ep(solid lines)and their corresponding fitted curves(dashed lines)at different temperatures
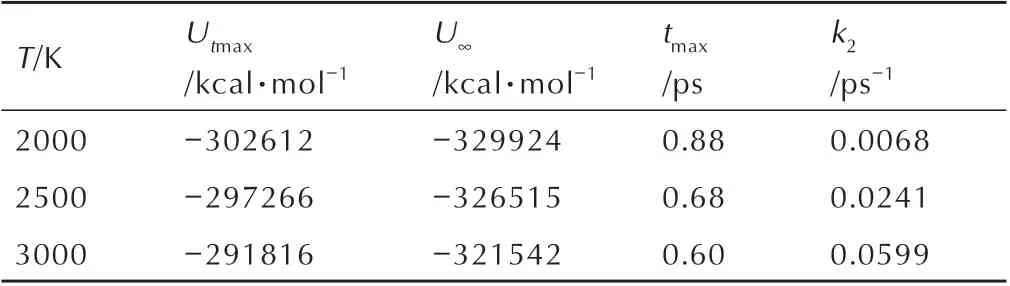
Table 4 Parameters describing the exponential behaviour of the change of Ep with Time.
(3)Final product evolution stage
Based on the experimental and simulated results,it can be concluded that the main final products of DNP decomposition at different temperature are N2,H2O and CO2. At different simulation temperature,thek3of DNP is calculated by fitting by Eq. 4,and the fitting results are shown in Fig.12,and asymptotic amounts extracted per product and rate constants are listed in Table 5. Table 5 illustrates that at higher temperature the asymptotic amounts of N2and CO2are higher,demonstrating that high temperature is benefit for the formation of N2and CO2.However,the asymptotic amount of H2O decreases with the increasing temperature because of the reaction between H2O and other intermediate products at high temperature. The calculatedk3increase with the increasing temperature,illustrating that higher temperature is benefit for the formation of final products during the decomposition of DNP. In addition,at the same temperature the order ofk3of N2,H2O and CO2is H2O >N2>CO2,illustrating the formation rates of H2O is the highest.
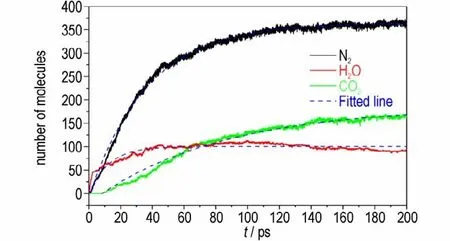
Fig.12 Evolutions of the numbers of N2,H2O and CO2 molecules(solid lines)and their corresponding fitted curves(dash lines)at 2500 K
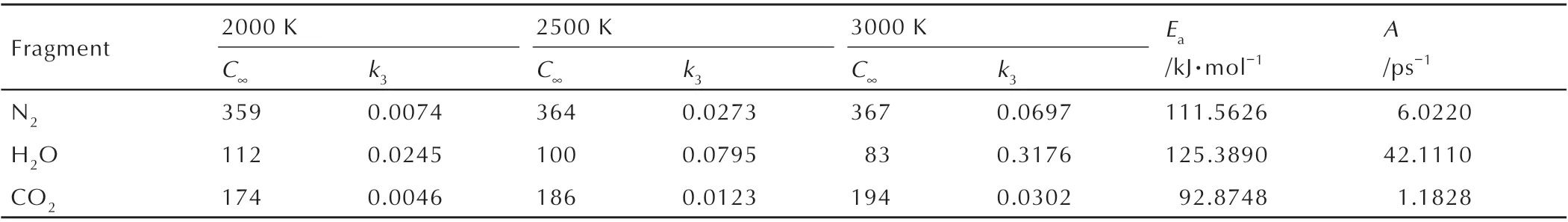
Table 5 Final products properties of DNP at 2500 K
3.3 Thermal decomposition mechanisms of DNP
More than 40000 chemical reactions are detected by ReaxFF MD simulations during the decomposition of DNP at all three temperatures,and with the increase of temperature the number of chemical reactions increases. Most of these reactions are the reaction between intermediate products rather than the reaction of DNP,illustrating that the intermediate decomposition stage is main stage during the decomposition of DNP. Actually,the reaction of initial decomposition stage can provide some important information for analysis the decomposition mechanism of DNP. In this study,ReaxFF simulated main chemical reactions and their reaction frequencies in initial 30 ps MD simulation during DNP decomposition are analyzed to obtain the decomposition path of DNP at different temperatures. The simulated main chemical reactions and their frequencies are summarized in Table 6.
Table 6 illustrates that the total number of DNP(216)is lower than the total frequencies of the reaction C3H2O4N4→ C3HO3N4+ OH at 2000 K and 2500 K,because of the formation of C3H2O4N4by the reaction between unstable intermediate products,and many other the same phenomena exist during the DNP decomposition. At 2000 K and 2500 K the main thermal decomposition path of C3HO3N4is C3HO3N4+ NO →C3HO4N5. When temperature raise to 3000 K,the main decomposition path ofC3HO3N4changes to C3HO3N4+ NO →C3HO4N5.The total reaction frequencies of the above mentioned three paths of C3HO3N4decomposition are decline,demonstrating that some other complex decomposition path of C3HO3N4exist with the increase of temperature. The higher the frequency of the reaction is,the greater the probability of DNP decomposition along this path. At 2000 K and 2500 K the total frequencies of C3H2O4N4→C3HO3N4+ OH are higher than the total number of DNP(216),illustrating that all DNP are almost decayed along this path.In addition,the increasing temperature decreases the frequencies of all selected reactions(the total number of chemical reactions increase),demonstrating that DNP exists some other complex decomposition path at higher temperature.
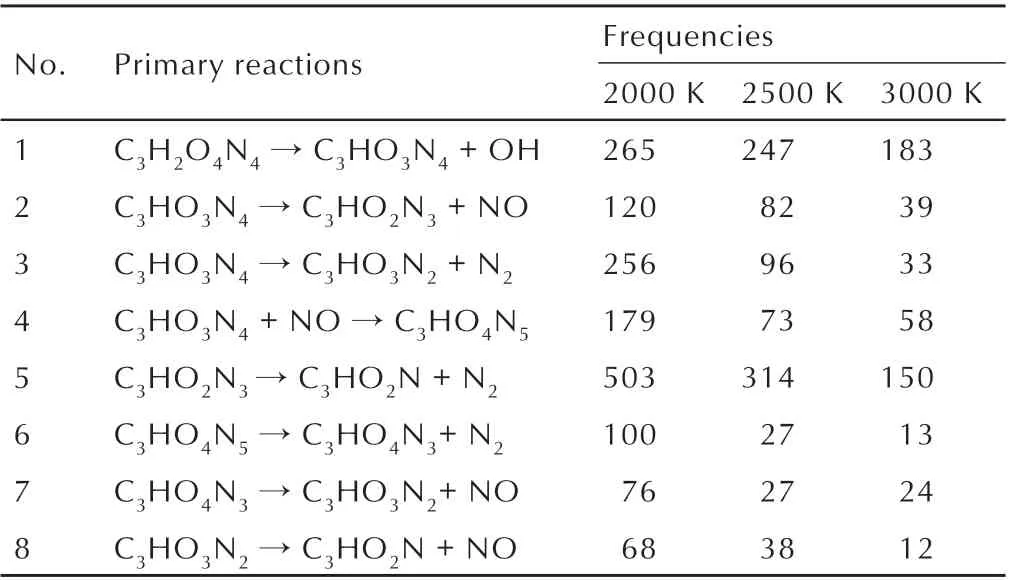
Table 6 Primary reactions and their frequencies
Take thermal decomposition of DNP at 2000 K as an example,at the beginning of the simulation,the main thermal decomposition process of DNP is C3H2O4N4→C3HO3N4+OH. After the formation of C3HO3N4, the decomposition processes become complex,and the main process can be summarized into three paths. The first path is the self-decomposition of C3HO3N4to produce NO,i.e.C3HO3N4→C3HO2N3+NO. The second is the self-decomposition of C3HO3N4to produce N2,i.e.C3HO3N4→C3HO3N2+N2. The third path is the reaction between C3HO3N4and NO,i.e. C3HO3N4+NO→C3HO4N5. At high temperature,C3HO4N5will decompose to C3HO4N3and N2, i. e. C3HO4N5→C3HO4N3+N2. Then C3HO2N3and C3HO3N2will decompose to C3HO2N+N2and C3HO2N+NO,respectively. Finally,C3HO2N will decompose to smaller intermediate and final products. Based on the simulation results,the decomposition mechanisms of DNP can be summarized by Scheme 1.
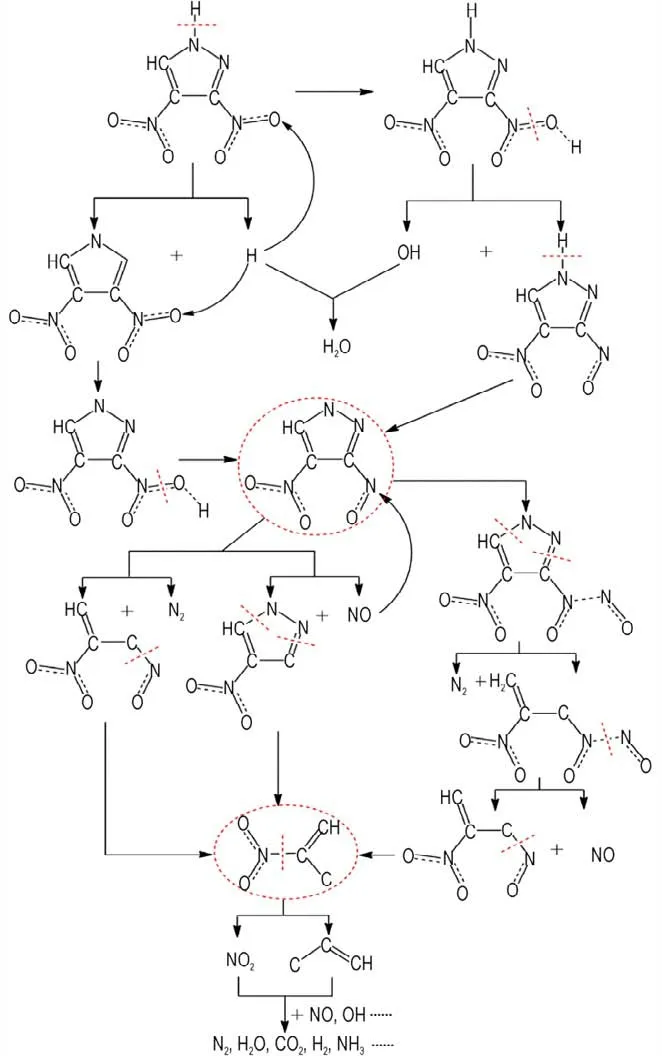
Scheme 1 Thermal decomposition mechanisms of DNP
4 Conclusions
(1)ARC was used to study DNP decomposition properties and to gather the gaseous products,and the gaseous products were identified by MS technique.
(2)ReaxFF MD simulations are performed to investigate the thermal decomposition of DNP at 2000,2500 and 3000 K.
(3)ARC-MS experiment and ReaxFF MD simulation results show that the final products are H2O,N2,CO2,NH3and H2,and the chronological orders of the appearance final products are H2O>N2>CO2>NH3>H2.
(4)ReaxFF MD simulation results show that the main intermediate products are C3HO4N4,C3HO3N4,C3HO2N3,C3HO2N and NO2,and the chronological orders of the appearance of intermediate products are C3HO4N4>C3HO3N4>C3HO2N3>C3HO2N>NO2.
(5)Based on the main intermediate products,final products and key chemical reactions,the decomposition mechanisms of DNP are obtained.
