Comparison of potential potassium leaching associated with organic and inorganic potassium sources in different arable soils in China
2022-05-11DianjunLUYanhongDONGXiaoqinCHENHuoyanWANGandJianminZHOU
Dianjun LUYanhong DONGXiaoqin CHENHuoyan WANG*and Jianmin ZHOU
1 State Key Laborator y of Soil and Sustainable Agriculture,Institute of Soil Science,Chinese Academy of Sciences,Nanjing 210008(China)
2 University of Chinese Academy of Sciences,Beijing 100049(China)
3 Soil and Fertilizer Station of Shandong Province,Jinan 250100(China)
ABSTRACT Potassium(K)leaching is detrimental to the maintenance of sustainable arable soil K fertility,especially in low-K fixation soils.It is not known whether the application of inorganic fertilizers with lower K mobility or crop straw can reduce potential K leaching in low-K fixation arable soils.The potential K leaching of 14 representative arable soils with different K fixation capacities in China was evaluated with or without the addition of K under two rainfall intensities(90 and 225 mm),and then potential K leaching was assessed in relation to five K sources(KCl,K2SO4,KH2PO4,maize(Zea mays L.)straw,and rice(Or yza sativa L.)straw).Without K addition,K leaching mainly occurred in sandy soils at 90 mm of rainfall and in soils with greater organic matter at 225 mm of rainfall.With K addition,the leaching percentage of exogenous K ranged from 0.6%to 11.6%at 90 mm of rainfall and 1.2%to 21.2%at 225 mm of rainfall.The greatest K leaching occurred in soils with fewer K-bearing minerals and lower pH at both rainfall intensities.In most cases,KH2PO4,which has lower K mobility,markedly reduced K leaching in both high-and low-K leaching soils at the two rainfall intensities.Maize and rice straw reduced K leaching only in soils with high K leaching,regardless of rainfall amount,whereas more K was leached in soils with lower Kleaching at high rainfall intensity.In conclusion,KH2PO4 and straw should be preferred for reducing K leaching in low-K fixation arable soils.
Key Words: crop straw,K-bearing mineral,K fixation,K source,soil type
INTRODUCTION
Potassium(K)has been accepted as an essential nutrient in crop production for many decades(Cakmak,2010;Römheld and Kirkby,2010;Zörbet al.,2014).Plant-available K in soil is significantly constrained by two main processes:K fixation into a non-exchangeable form and K leaching out of arable soil(Reeset al.,2013;Islamet al.,2017).Potassium leaching is detrimental for sustainable arable soil K fertility,especially in soils with poor K fixation capacity(Nguyen and Marschner,2013).Alfaroet al.(2004)reported that K leaching could be more than 70 kg K ha-1in sandy soils under high rainfall intensity,and this rate is sometimes even higher than the K input rate.Such heavy leaching could further aggravate K deficiency situations in many regions(Dobermannet al.,1996;Pathaket al.,2010;Islamet al.,2015),especially in developing countries,threatening crop yield and nutrient-use efficiency.
Potassium exists in the soil in four forms:water-soluble,exchangeable,non-exchangeable,and structural forms(Sparks,1987;Schneideret al.,2013).Water-soluble K is readily subject to leaching.The distribution of various K pools in the soil depends on a number of soil physical and chemical properties(Goli-Kalanpaet al.,2008;Ghiri and Abtahi,2012),such as cation exchange capacity,soil pH,and the quantity and size of particles,as well as the amount of Kbearing minerals.The concentration of H+in soil solutions plays an important role in exchanging K from clay minerals(Wanget al.,2011).Therefore,optimization of soil pH may be an alternative way of enhancing K release.Soils rich in vermiculites and micas have higher K fixation capacities than montmorillonites and kaolinites(Srinivasaraoet al.,2006;Igweet al.,2008;Basak and Biswas,2009).
Optimal K management practices,especially identification of optimal fertilizer rates and placement(Blakeet al.,1999;Yanget al.,2004),could effectively decrease nutrient leaching(Waddell and Weil,2006;Benoitet al.,2014).Nonetheless,the effect of fertilizer source,notably the different impacts of organic and inorganic K fertilizer sources on K leaching,has received relatively little attention.In theory,inorganic K sources should be capable of affecting K leaching due to the activity of accompanying ions(Heidari and Jalali,2016).For example,Cl-is normally freer to move in soil solutions than other multivalent ions,such as H2PO-4,because H2PO-4forms precipitates with many cations and temporally accumulates as an organic-or microorganismbound form.Xie(2000)reported that the degree of Kfixation for different sources of K fertilizer was as follows:KH2PO4>K2SO4>KCl.Hence,the question arises as to whether using less mobile K fertilizer sources,such as KH2PO4,would reduce K leaching in soils with different K fixation capacities compared with the common K fertilizer source KCl.
Crop straws are important organic K sources in agriculture(Gosling and Shepherd,2005;Panaullahet al.,2006;Luet al.,2017).Potassium in crop straw is highly mobile at both the cellular and tissue levels(Marschner,1995).Hence,K can easily be released from decaying straw by rain(Rosolemet al.,2005).Rosolemet al.(2005)reported that K leaching could reach 60 kg K ha-1and was correlated with K concentration in straw.Despite the potential for K leaching,a larger quantity of K in crop straw could be retained temporarily in residues,avoiding fixation by clay minerals,than chemical K fertilizers.However,it is still unknown whether crop straw would perform better at reducing K leaching than chemical K fertilizers in soils with different K fixation capacities.
In this study,we collected 14 agricultural soils with a wide range of K-bearing minerals in China.The 14 soils could represent the different capacities of K fixation or leaching in the country.The research objective was to evaluate the potential Kleaching for exogenous Kfertilizers in the 14 soils under different rainfall intensities and to determine the K leaching losses in relation to inorganic and straw Ksources in differing K leaching soils under different rainfall intensities.
MATERIALS AND METHODS
Arable soils
The soils tested were collected from:Changsha County(S1)in Hunan Province,Yingtan County(S2)in Jiangxi Province,Qichun(S3)and Qianjiang(S4)counties in Hubei Province,Wuhu City(S5)in Anhui Province,Hailun County(S6)in Honglongjiang Province,Fengqiu County(S7)in Henan Province,Guangde(S8)and Huaining(S9)counties in Anhui Province,Laiyang County(S10)in Shandong Province,Changshu(S11)and Yancheng(S12)counties in Jiangsu Province,Chongqing(S13),and Hengshui County(S14)in Hebei Province.
These soils were collected from the 0—20 cm plowed layer and the site characteristics including soil type,elevation,climate,and cropping system are shown in Table I.The 14 soils were sampled from cultivated cereal crop fields fertilized with typical local fertilization practices.The subsamples were air-dried and ground to pass through a 2-mm sieve to determine soil physical and chemical properties(Table II).The soil pH was determined in a 1:2.5(weight:volume)soil to water suspension.The soil organic matter was measured using the Walkley-Black dichromate oxidation method(Lu,1999).Total K was determined after melting by NaOH,and available K and slow available K were measured using the 1 mol L-1NH4OAc method and the 1 mol L-1boiling HNO3method(Lu,1999),respectively.The sand,silt,and clay fractions in the samples were determined by the hydrometer method(Bouyoucos,1962).The mineralogy of the clay fraction(<2μm)was evaluated by X-ray diffraction using an X’Pert-Pro X-ray diffractometer(Philips,The Netherlands)with CuKαradiation(40 kV,40 mA)and a graphite filter(Bouyoucos,1962),from 3.0°to 60.0°with a scan speed of 4.0°min-1.
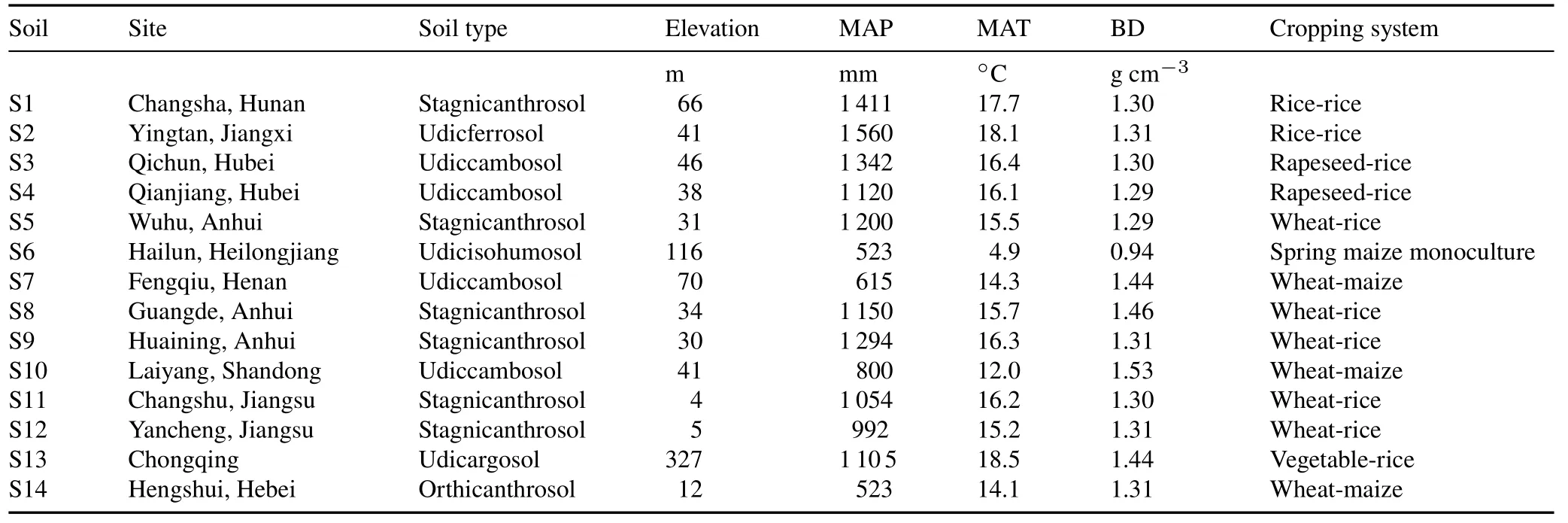
TABLE I Site characteristics including soil type,elevation,mean annual precipitation(MAP)and temperature(MAT),bulk density(BD),and cropping system of the 14 agricultural soils tested in China
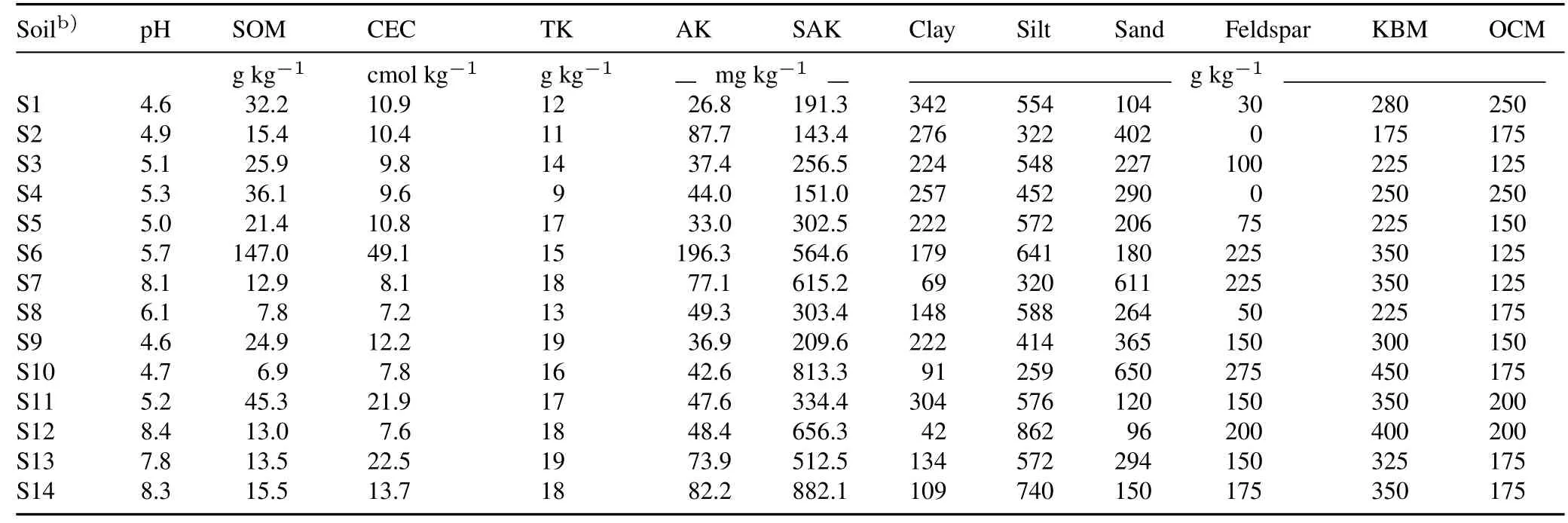
TABLE II Physical and chemical properties a)of the 14 agricultural soils tested in China
Potassium distribution among different K pools
Subsample(2.5 g)of each soil was weighed and placed in a 50-mL centrifuge tube,and 2.5 mL of 5 mol L-1KCl solution was added to the soil.As a control(without addition of K),2.5 mL of distilled water was added to the soil.Each treatment was replicated four times.All the soils were incubated through three dry-wet alternations(12 d)to equilibrate the reaction of K with the soil at 25°C.The K fertilizer form was KCl.The untreated and K-added soil were then air-dried in a no-dust room and again milled to pass through a 2-mm sieve.The air-dried soil was shaken with 25 mL distilled water for 30 min.After centrifugation(5 590×g)and filtration,the water-soluble K in the filtered fluid was determined with a flame photometer.The soil after centrifugation was shaken(120 r min-1)with 25 mL 1 mol L-1ammonium acetate for 30 min in a reciprocal oscillator,and centrifuged(5 590×g)to collect the liquid supernatant.The liquid supernatant was used to determine the exchangeable K.Finally,the fixed percentage of exogenous K was calculated as 100 minus the percentage of exogenous K in water-soluble and exchangeable K.The potentially leachable K for exogenous K fertilizer was calculated by the difference in K leaching(water-soluble and exchangeable K)between untreated and K-added treatments divided by the total K input amount.
Potassium leaching withor without K fertilizer
The soil column method was used to study the Kleaching for different soils.Each arable soil(298 g)with or without K fertilizer(addition of 60 mg K)was mixed and added to a polyvinyl chloride(PVC)tube,25 cm long with an internal diameter of 5.4 cm.The K source was applied as KCl.The height of the soil packed into the PVC tubes was measured and corresponded to the bulk density listed in Table I.A funnel was connected to the bottom of the PVC tube to collect the leachate.A 200-mesh nylon net was placed at the bottom of the PVC tube before the addition of soil to avoid soil leaching out of the tube.After the addition of soil,a 200-mesh nylon net was placed on the top of the PVC tube to avoid disturbing the surface soil when adding the water.Distilled water was added to the soil to reach 90%of soil water saturation by weighing the PVC tube,and all the soils were incubated through three dry-wet alternations(12 d)at 25°C.After incubation,the soil column was continuously leached with distilled water at a constant ratio using an infusion bottle.Two amounts of distilled water,200 and 500 mL,were used to model the minimum(90 mm,S3)and maximum(225 mm,S2)average monthly rainfall amounts in June for the 14 soils,because most rainfall is concentrated in the period from June to August in China.To ensure consistent leaching intensity,a 2-cm water level above the soil was maintained for all soils.The leaching volume was recorded,and K concentration was determined using a flame photometer.Each treatment was replicated four times.
Potassium leaching for five K sources
Three of the 14 soils were selected to determine the effects of five different K sources on K leaching.These three soils were S2(high K leaching capacity),S7(medium K leaching capacity),and S14(low K leaching capacity).Except the control(without K fertilizer),five K sources consisting of KCl,K2SO4,KH2PO4,maize straw(20.5 g K kg-1),and rice straw(18.4 g K kg-1)were evenly mixed with the soils.The application rate of K(60 mg)was the same for each K source,and the corresponding rates of the five K fertilizer sources were 0.11 g for KCl,0.13 g for K2SO4,0.21 g for KH2PO4,2.91 g for maize straw,and 3.24 g for rice straw.Maize and rice straws were ground and passed through a 10-mesh sieve.Each treatment was replicated four times.The leaching process under the two rainfall intensities was the same as that described above.
Statistical analysis
For all the experiments,the differences in K leaching for different K sources in each soil at the same rainfall amount were analyzed separately using one-way analysis of variance.Statistical analysis was performed using the SPSS version 16(SPSS Inc.,USA).
RESULTS
Exogenous K distribution among different K pools
There were considerable variations in the distribution of exogenous K in different soils.For S1,S3,and S9,applications of K fertilizer distributed higher amounts of water-soluble K(Fig.1).In contrast,significantly lower levels of water-soluble K occurred in S13 and S14.For S6,S9,and S2,the distribution in exchangeable K of exogenous K accounted for about 70%,63%,and 62%of total K fertilizer,respectively,and was significantly higher than that in S13,S8,and S12(Fig.1).Available K(water-soluble plus exchangeable K)was significantly higher in S1,S9,and S3,and was relatively lower in S13 and S14(P<0.05).Correspondingly,the fixed K diverged from the trend of available K distribution,with the maximum fixed amount in S13 and the minimum in S1(Fig.1).
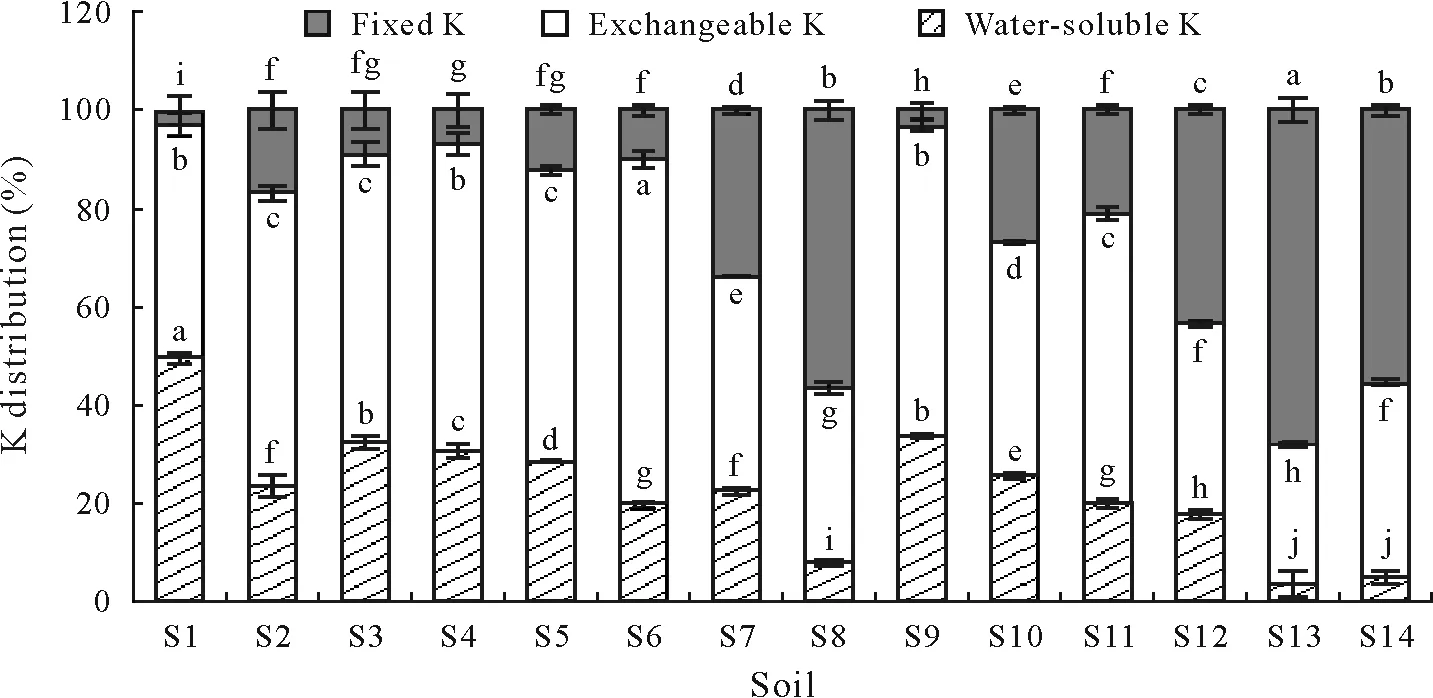
Fig.1 Distribution of exogenous K fertilizer among water-soluble,exchangeable,and fixed K in the 14 agricultural soils tested in China.Vertical bars represent standard errors of of the means(n=4).Means with the same letter(s)for each K fraction among different soils are not significantly different at P<0.05.
Potassium leaching with or without K addition
For untreated soil receiving 90 mm of rainfall,the highest amount of K leaching was observed in S2,followed by S7 and S10 in sandy soils(Fig.2a).A higher amount of K leaching in S2 was attributed to higher K concentrations in the leachate,whereas a higher leachate volume was found in S7 and S10(Table III).The lowest K leaching amount was observed in S14,mainly because of lower K concentrations in the leachate(Table III).When the rainfall intensity increased to 225 mm,it was notable that K leaching in S6 increased the most sharply(approximately 15-fold)(Fig.2a),owing to significant increases in both leachate volume and K concentration in the leachate(Table III).Pooling all soils,a significant positive correlation was observed between the amount of K leaching and sand content in the soil particle composition for untreated soil at 90 mm of rainfall.In contrast,available K concentration,cation exchange capacity(CEC),soil organic matter(SOM),and saturated water content were positively correlated with K leaching at 225 mm of rainfall(Table IV).

Fig.2 K leaching without(a)or with(b)addition of K fertilizer under two rainfall intensities(90 and 225 mm)in the 14 agricultural soils tested in China.Vertical bars represent standard errors of the means(n=4).Means with the same letter(s)for each rainfall intensity among different soils are not significantly different at P<0.05.

TABLE III Leachate volume and K concentration(Kcon)without or with addition of K fertilizer and percentage of leaching of exogenous K fertilizer under two rainfall intensities(90 and 225 mm)in the 14 agricultural soils tested in China
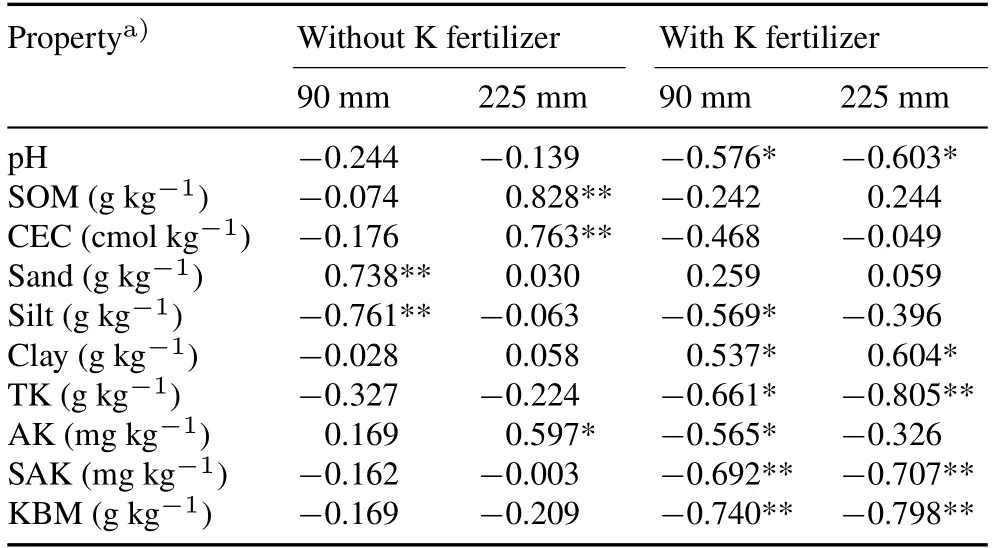
TABLE IV Correlation coefficients between K leaching and various physical and chemical properties of soil without or with addition of K fertilizer under two rainfall intensities(90 and 225 mm)
For the soils with addition of K fertilizer at both 95 and 225 mm of rainfall,the significant highest amount of K leaching occurred in S2 and S1(Fig.2b).Medium levels of K leaching occurred in S9,S4,S8,S3,S7,S5,and S10(Fig.2b).The lowest amount of K leaching was observed in S14,S13,S6,S11,and S12(Fig.2b).The amount of K leaching in S13 was 93%lower than that in S2.The main reason was that K concentration in the leachate of S13 decreased by 94%(4.8vs.75.2 mg L-1)compared with that in S2(Table III).The calculated percentage of apparent leaching of K fertilizer was 11%in S2 and 0.6%in S13 at 90 mm of rainfall,and 18%and 1.2%in the two soils,respectively,at 225 mm of rainfall(Table III).At both rainfall intensities,the amount of Kleaching with Kfertilizer addition was negatively correlated with soil pH,silt particle content,and the amount of K-bearing minerals(Table IV).
Potassium leaching associatedwith K sources
Soil S2 had a high K leaching capacity,and addition of K2SO4did not significantly decrease the K leaching compared with addition of KCl at both rainfall intensities(Fig.3a,b).It is notable that addition of KH2PO4significantly decreased K leaching at 90 and 225 mm of rainfall(Fig.3a),compared with additions of KCl and K2SO4due to decreased K concentration in leachate(Table V).Maize straw and rice straw significantly decreased the K leaching by 19%and 15%,respectively,compared with KCl at 90 mm of rainfall(Fig.3a)and by 12%and 11%,respectively,at 225 mm of rainfall(Fig.3b).The leachate volume and K concentration in the leachate for additions of maize straw and rice straw were significantly lower than those for addition of KCl,especially at 225 mm of rainfall(Table IV).The reduction in leachate volume for addition of crop straw may be attributed to the increase in soil saturated water content(Fig.4).
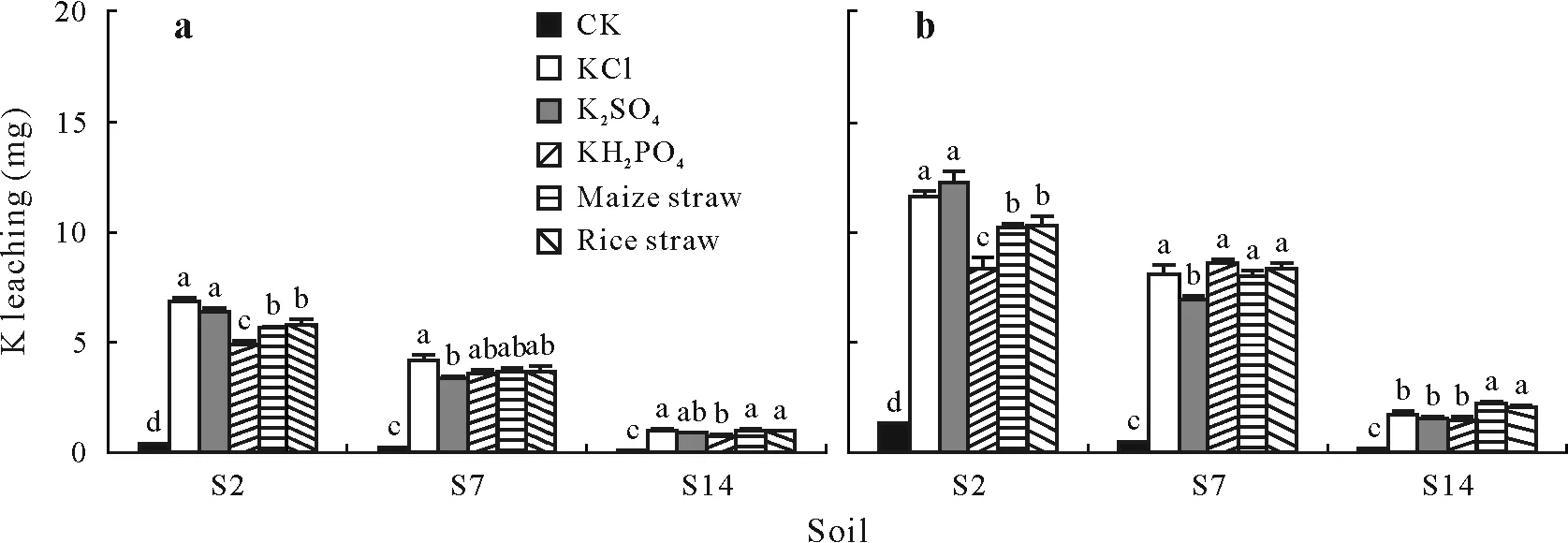
Fig.3 K leaching as affected by five K resources at 90(a)and 225 mm(b)of rainfall in the soils collected from Yingtan County(S2),Fengqiu County(S7),and Hengshui County(S14),which had high,medium,and low K leaching capacities,respectively.Vertical bars represent standard errors of the means(n=4).Means with the same letter(s)among K sources for each soil and rainfall intensity are not significantly different at P<0.05.CK=control without K fertilizer.

Fig.4 Soil saturated water content as affected by five Kresources.Vertical bars represent standard errors of the means(n=4).Means with the same letter among K sources for each soil are not significantly different at P<0.05.CK=control without K fertilizer.
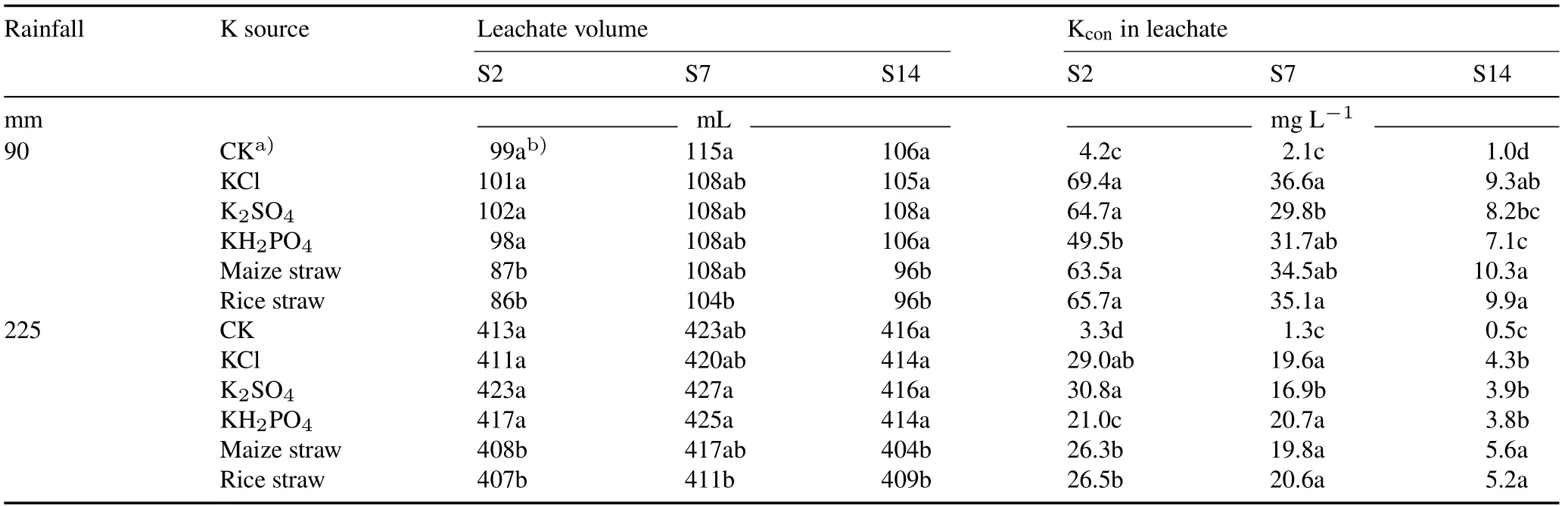
TABLE V Leachate volume and K concentration(Kcon)in leachate as affected by five K resources under two rainfall intensities(90 and 225 mm)in the soils collected from Yingtan County(S2),Fengqiu County(S7),and Hengshui County(S14),which had high,medium,and low K leaching capacitives,respectively
Soil S7 had medium K leaching capacity,and compared with KCl,K2SO4application resulted in a significant reduction in K leaching at both 90 mm and 225 mm of rainfall(Fig.3a,b).However,KH2PO4,maize straw,and rice straw showed reduced K leaching,but without significant differences at both rainfall intensities.The leachate volume and K concentration in the leachate were not significantly affected by crop straw compared with those by KCl regardless of the rainfall amount in the soil.
Soil S14 had a low K leaching capacity at 90 mm of rainfall,and only KH2PO4application significantly decreased the amount of K leaching compared with KCl(Fig.3a),due to decreased K concentration in the leachate(Table V).When the rainfall amount increased to 225 mm,maize straw and rice straw leached 26%and 18%more K compared with KCl(Fig.3b).However,K2SO4and KH2PO4leached similar amounts of K as KCl.In this soil,straw application significantly increased K concentration in leachate despite the lower leachate volume(Table V).
DISCUSSION
Key factors affecting K leaching
In this study,the variation in K leaching was lower in soils without K fertilizer addition than in those with K addition,mainly because of lower available K levels(except in S6).Under low rainfall intensity,significantly greater K leaching mainly occurred in the light-textured soils,such as S7 and S10(Fig.2a).Furthermore,higher K leaching amounts at this rainfall intensity were mainly observed for soils with high available K,such as S6 and S2(Fig.1a).It was likely because the leached K form was mainly soil solution K under low rainfall intensity,whereas exchangeable K was more related to high rainfall intensity.In this situation,the short-term leaching process was generally favored in coarse sandy soil(Wihardjakaet al.,1999;Jalali and Rowell,2003;Nyamangaraet al.,2003).High available K concentration facilitates Kleaching because of consistent Krelease(Kayser and Isselstein,2005;Simonssonet al.,2007).
With K applications,higher K leaching mainly occurred in soils with fewer K-bearing minerals such as the S1,S2,S3,and S4 soils at both rainfall intensities,compared to S11,S13,and S14 soils with more K-bearing minerals(Fig.2b).The main reason was that soils with high amounts of Kbearing minerals fixed more K fertilizer as non-exchangeable forms to avoid partial K leaching(Table II).This result was even applicable to the light-textured soils used in this study;S10 and S7 did not leach high rates of K fertilizer owing to certain amounts of K-bearing minerals(Table II).Hence,light-textured soils may not necessarily result in high K leaching with K fertilizer addition,and the leaching status of K fertilizer in these soils also depends on the type and quantity of K-bearing minerals,in agreement with Rosolemet al.(2005).These authors also concluded that soil texture might not be important in determining K leaching loss when rainfall and K rate increased.
The types of K-bearing minerals are also different for soils with different K leaching or fixation capacities.As reported previously(Donget al.,2014),K-bearing minerals in soils like S1 and S2 mainly possess greater amounts of 1:1 type clay minerals,such as kaolinite,whereas 2:1 type minerals,such as vermiculite,montmorillonite,and mica,are more common in soils like S14,S13,and S11.The 2:1 type clay minerals generally possess more room for fixation of K(Ghiri and Abtahi,2012).The occurrence of inferior K-bearing clay minerals with few amounts in S1 and S2 was mainly attributed to a high degree of weathering in these two soils.The hot temperatures and humid climate accelerate the hydrolysis of primary minerals.Important cations,such as K,Ca,and Mg,are displaced by Al or Fe,and finally leached with water(Bronick and Lal,2005).Hence,these soils were also characterized by lower pH.
Further evidence for the importance of K-bearing minerals in K leaching was that high CEC and SOM levels in soils,such as S6,also could not prevent an increase in K leaching at higher rainfall intensity.This result indicated that the bond strength of K at the edge of exchangeable sites with clay minerals was much weaker than the K fixed in the interlayer of clay minerals,resulting in a greater release of K from exchangeable K than non-exchangeable K(Zörbet al.,2014).Therefore,K fixation seemed more important for reducing K leaching and conserving soil K fertility than K adsorption(exchangeable K).
Potassium leaching as affected by K sources
For the three chemical K fertilizers,we validated the hypothesis that,in most cases,KH2PO4effectively decreased Kleaching compared with common KCl in soils with different K fixation capacities(Fig.3a,b).This may be because K+lacks the companying anion to move into soil solution,while H2PO-4is fixed by calcium in the calcareous soil of S14(high K fixation capacity)and by Al in the acid soil of S2(low K fixation capacity).Many studies have shown that the availability of P is considerably constrained,and thus,its movement in soil is limited(Schachtmanet al.,1998).Duet al.(2006)reported that K mobility and transformation in an acid soil close to a fertilizer site was significantly affected by P when the P and K fertilizers were applied simultaneously.
Compared with KCl,the straw application reduced K leaching only in soils with a lower K fixation capacity(Fig.3a,b).In contrast,in soils with high K fixation capacity(S14),crop straw caused greater K leaching compared with KCl,especially at high rainfall intensity(Fig.3b).The inconsistent result was observed in S2,where crop straw played a temporary“storage”role in retaining proportional K and reducing K leaching compared with KCl.Meanwhile,straw became an active K“pool”in S14,whereas greater KCl fertilizer was easily fixed into non-exchangeable K,thereby resulting in a greater K leaching with straw.Despite the inconsistent effect of straw in K leaching,straw significantly improved soil water-holding capacity and decreased leachate volume for all soils,especially at low rainfall intensity,which certainly played a role in decreasing the K leaching rate.This finding may be explained by the increased sorption capacity and reduced soil bulk density with the application of straw(Lal and Stewart,1995;Zhaoet al.,2014).
Straw significantly caused greater K leaching compared with KCl in soils with greater K fixation capacity;however,this does not mean that chemical fertilizers should be applied more in field.On one hand,straw could still return a larger quantity of K into these soils because K leaching in these soils was lower for all K sources compared with the soils with high K leaching capacity.This is key in developing a balanced approach to K.On the other hand,the long-term return of straw played an obvious role in improving physical,chemical,and biological soil properties(Hatiet al.,2008;Bakhtet al.,2009).This is particularly important for soils with low fertility and K fixation capacity,such as S1,S2,and S3.However,in practice,many farmers in China remove crop straw from the field for other purposes(Liuet al.,2010).Straw removal has resulted in significant losses of plant necessary nutrients for maintaining sustainable soil fertility(Luet al.,2017).Hence,there is a tremendous need for policies supporting outreach and education regarding the benefits of straw return for achieving sustainable soil K fertility,especially in soils with low K fixation capacity.
CONCLUSIONS
Fertilization significantly increased K leaching.High K leaching with exogenous K fertilizer primarily occurred in soils with fewer K-bearing minerals and lower pH regardless of rainfall intensity.High soil organic matter resulted in high K leaching at high rainfall intensities,indicating that the real mechanism of maintaining K in soil was K fixation.Reducing K mobility in soil by the application of KH2PO4could significantly decrease K leaching for soils with high and low K leaching capacities in most cases.Crop residues significantly leached greater K in soils with lower K fixation capacity than those with high K fixation capacity.This finding indicates that crop straw,acting as a temporary K storage,could also act as an active K pool depending on soil K fixation capacity.
ACKNOWLEDGEMENT
This research was supported by the National Key Research and Development Program of China(No.2018YFD-0200901)and the National Natural Science Foundation for Young Scientists of China(No.41907075).
杂志排行
Pedosphere的其它文章
- Ultraviolet B radiation-mediated stress ethylene emission from rice plants is regulated by 1-aminocyclopropane-1-carboxylate deaminase-producing bacteria
- Drying-rewetting rather than sieving stimulates soil respiration
- Methanotrophy-driven accumulation of organic carbon in four paddy soils of Bangladesh
- Complementary effect of zoo compost with mineral nitrogen fertilisation increases wheat yield and nutrition in a low-nutrient soil
- A conceptual framework and an empirical test of complementarity and facilitation with respect to phosphorous uptake by plant species mixtures
- Evaluation of immobilizing agents as soil quality conditioners in addition to their metal(loid)immobilizing effect
