The Chinese version of the revised Diabetes Distress Scale for adults with type 2 diabetes: Translation and validation study
2022-05-10YuYunZhngWeiLiYuSheng
Yu-Yun Zhng , Wei Li , Yu Sheng ,*
a School of Nursing, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
b Department of Endocrinology, Peking Union Medical College Hospital, Beijing, China
Keywords:China Diabetes distress Patents Psychometrics Type 2 diabetes
ABSTRACT Objectives: This study aimed to translate the revised 17-item Diabetes Distress Scale (DDS17, 2017) into mandarin (simplified) Chinese and validate the Chinese version of DDS17 (C-DDS17, 2021) among adult patients with type 2 diabetes in China.Methods: A scale translation and cross-sectional validation study was conducted.The DDS17 was translated into mandarin (simplified) Chinese through a five-step process: authorization, forward translation, synthesis, back translation, and amendment.During this session, 59 patients assessed the understandability and readability of the translated scale.From June 7 to September 4, 2021, a crosssectional study that adhered to the COSMIN checklist was conducted with 400 individuals with type 2 diabetes from three Class A tertiary comprehensive hospitals in Beijing, China.The content, construct,convergent, discriminant validity, and reliability (Cronbach’s α coefficient and item-total correlation coefficients) of the C-DDS17 were evaluated.This study was a part of a project registered in the Chinese Clinical Trial Registry (no.ChiCTR2100047071).Results: Among the participants, 33.3% (133/400) of them experienced moderate to high diabetes distress.The content validity indices of the C-DDS17 equaled 1.00.The scale yielded a four-factor structure.The average variances extracted were 0.42-0.57, which was lower than squared correlations.Cronbach’s α coefficient was 0.88 for the overall scale and ranged from 0.76 to 0.81 for sub-scales.Corrected item-total correlation coefficients ranged from 0.42 to 0.61.The eighth item(“Feeling that I am often failing with my diabetes routine”)was better fit to physician distress than regimen distress but had little influence on the validation results.Conclusions: The C-DDS17 is a reliable and valid instrument for assessing diabetes distress in patients with type 2 diabetes.It is a promising instrument for early identification and management of diabetes distress in clinical practice and trials.
What is known?
· The 17-item Diabetes Distress Scale (DDS17) is an assessment tool with good validity, reliability, and feasibility.In 2012, the item order of the original DDS17 (DDS17, 2005) in English was revised, the cutoff points were formally established, and the scoring method was standardized.The scale has been translated to 24 languages.
· The item order and language accuracy of the previous simplified Chinese Diabetes Distress Scale translated in 2010 requires improvement and revision.
What is new?
· Based on the revised DDS17 in English (US, 2017 version), this study provided an updated, more accurate, reliable, and valid Chinese version of the DDS-17(C-DDS17,2021)for patients with type 2 diabetes in China.
· The adaption of the C-DDS17 allows for a better assessment of diabetes distress in clinical practice and trials, which may promote Chinese and transnational studies on diabetes distress of patients.
1.Introduction
Type 2 diabetes (T2D) is a growing burden, with an estimated 416.70 million adult patients worldwide and 110.58 million patients in China[1].It is a chronic metabolic disease characterized by hyperglycemia,which is caused by the inability of pancreatic β cells to secrete enough insulin or the inability of the body to effectively use the secreted insulin [2].T2D influences both physical and mental health.About two-fifths of patients experience mental problems, such as anxiety, depression and diabetes distress [3].Among patients with T2D, diabetes distress (DD) is the most common psychological problem[4].A systematic review found that the overall global prevalence of DD was 36.0%in patients with T2D[5].A study also showed that approximately 42.5%-77.2% of Chinese people with T2D experienced DD [6].DD is a negative emotional response that includes worries, fears, and frustrations caused by the perceived burden from living with and managing diabetes on a daily basis [7,8].DD is associated with low compliance, glycemia, adverse outcomes, and increased costs [3].Moreover,although DD is not a psychiatric symptom,it could progress to depression if not properly managed [2].Therefore, the American Diabetes Association recommends physicians and nurses evaluate DD in disease development and treatment [9].
It is meaningful to use validated tools to assess DD[10].Several disease-specific instruments have been developed to identify and assess DD in recent years, including the 17-item Diabetes Distress Scale (DDS17), the Problem Areas in Diabetes Scale (PAID), the Diabetes Health Profile, and the Diabetes-specific Quality-of-Life Scale and Questionnaire[11].Among all the instruments,the PAID and the DDS17 are most commonly used in patients with diabetes because of their high reliability and validity in assessing diabetesrelated stress specifically [12].Both scales have been translated into various languages (e.g., Chinese, Turkish, Norwegian) and show good psychometric qualities (e.g., Cronbach’s α coefficient ranged from 0.56 to 0.93 for PAID,and ranged from 0.75 to 0.90 for DDS17) in different countries [13].
The DDS17 has the advantage of not only focusing on an overall DD level but also identifying key sources of DD[14].In addition,the Diabetes Distress Scale has separate versions for type 1 diabetes and T2D; however, the PAID only developed a hybrid version [12].Further,the Diabetes Distress Scale has separate versions for adults,partners, adolescents, and parents [15,16].It uses a 6-point Likert scale (developed by Polonsky in 2005 in America) that has been revised afterwards[17].The scale was developed based on previous self-report instruments, resulting in four sub-dimensions with 17 items: five items for emotional burden, four items for physician distress, five items for regimen distress, and three items for interpersonal distress [18,19].For each item, scores range from 1 to 6(1 =not a problem, 2 =a slight problem, 3 =a moderate problem,4 =a somewhat serious problem,5 =a serious problem,or 6 =a very serious problem) [20].Each sub-scale was scored separately by averaging the total score of the sub-scale, while the total score is generated by averaging all item responses [21,22].DD increases as the mean score rises[16].Because the associations between DDS17 scores and behavioral and biological variables occurred with mean scores of no less than 2.00, an average score of 2.00 is used as the cut-off point to define clinically significant DD [22].Precisely, a mean score less than 2.00 indicated “little or no distress,” a mean score of 2.00 to less than 3.00 was considered“moderate distress,”and a mean score equal to or more than 3.00 was considered“high distress” [23].
The DDS17 has been translated and validated in 24 languages using a formal validation process[24].In China,the DDS17 was first translated by Yang and Liu in 2010[16].The Cronbach’s α coefficient of the Chinese DD Scale ranged from 0.84 to 0.95 [6].Although it demonstrated good psychometric properties and has been used in observational and experimental studies for several years, the language accuracy and understandability still had room for improvement.Specifically, the phrases “not know enough,” “end up with serious long-term complications,” and “not take my concerns seriously enough” were translated into phrases in Chinese with meaning “lack of knowledge,” “die of serious long-term complications,” and “not take my concerns seriously,” respectively; while the words “depressed,” “fail,”and “overwhelmed” were translated into “repression,” “go wrong,” and “puzzled,” respectively.Furthermore, the order of items in Yang’s Chinese scale differed from the original scale, which might influence its reliability [24].There was a traditional Chinese version provided by Hong Kong,China in 2011, but it only included 15 items, and the order of the items was different from DDS17,which might“make the order and content of the subscales unreliable” [24].The DDS17 had been revised in 2012 to adjust the order of the items,establish the cut-off points for different levels of distress, and standardize the scoring method for the scale[25].Therefore,an updated Chinese version of the Diabetes Distress Scale (C-DDS17) was needed.
The objectives of the current study were to translate and validate the C-DDS17(2021 version)based on the updated English(US)version of DDS17 (2017) [24].
2.Material and methods
2.1.Design
A scale translation and cross-sectional validation study was conducted.First,the DDS17 was translated to mandarin(simplified)Chinese based on semantic and idiomatic equivalence.Second,the Chinese scale was culturally and conceptually adapted.Third, its application for DD in T2D was validated.The validation work was conducted through an analytical cross-section study that adhered to enhancing the quality and transparency of health research(EQUATOR checklist) based on the consensus-based standards for selecting health status measurement instruments (COSMIN guidelines).
Similar to the original English version and other translated versions,we hypothesized that the C-DDS17 would demonstrate 1)excellent fit for four-factor models in the validity analysis, 2) good content and convergent validity with a poor discriminant validity,and 3) high Cronbach’s α coefficients and item-total correlations.
2.2.Ethical approval
This study was registered in the Chinese Clinical Trial Registry(no.ChiCTR2100047071) and was approved by the Human Biomedical Research Ethics Committee of Peking Union Medical College(no.[2020]03).This study was performed according to the Declaration of Helsinki.Participation and written informed consent was provided by participants before the formal investigation.The C-DDS17 was approved on the Behavioral Diabetes Institute website(https://behavioraldiabetes.org/scales-and-measures/).
2.3.Translation procedure
The translation of the scale included five stages [26,27].1)Authorization:The approval and authorization for translating the DDS17 were obtained from the author of the scale.2) Forward translation: The original English version of the DDS17 workbook was translated into Chinese by two bilingual English-Chinese translators whose mother language is Chinese.One translator is a nurse,and another translator is a linguistics expert with no medical background.3) Synthesis: The two initial drafts in Chinese were collaboratively compared and revised as a synthesis version by four professionals with experience in endocrinology or psychology.4)Back translation: The synthesis version was translated back into English by two independent translators who did not participate in the initial translation nor possess knowledge of the original scale.5)Amendment:The translation validity was evaluated to assess the comparability of the Chinese version, the original English version,and the back-translated version by five professionals.Further, 59 patients were asked to assess the understandability and readability of the scale and provide revision suggestions.Amendments were made until the comparability of the wording, cultural appropriateness,fluency, clarity, and understandability were confirmed.
2.4.Scale evaluation and validation procedure
2.4.1.Study settings
The validation work was conducted from June 7 to September 4,2021 at the outpatient clinics of the endocrinology department in three Class A tertiary comprehensive hospitals in Beijing, China.Class A tertiary comprehensive hospitals are considered the highest-level medical institutions in the mainland of China according to the current regulation, the Measures for the Administration of the Hospital Grade.
2.4.2.Participants
During the cross-cultural adaptation of a scale, the sample size required for a reliable factor analysis is classified as follows:100 =“weak,”200 =“medium,”300 =“good,”500 =“very good,”and 1000 =“perfect” [28].Considering human and material constraints, the sample size of the study was determined to be 400.Participants were recruited through convenience sampling.
Patients diagnosed with T2D by an endocrinologist,according to the WHO 1999 diabetes diagnosis and classification criteria; aged≥18 years; had clear consciousness and understanding ability;could communicate with researchers in writing or verbally; and provided informed consent and voluntarily participated in the study were eligible to participate.The exclusion criteria included severe cognitive impairment, inability to cooperate or act autonomously, inability to express clearly, acute or serious diabetes complications,serious comorbidity,and definite diagnosed mental illness or intake of antipsychotics.Participants who did not complete the investigation were also excluded from the final analysis.
2.4.3.Measurements
Data were collected using a paper-based self-reported questionnaire including the C-DDS17,socio-demographic characteristics(sex,age,income,ethnic group,religion,marital status,educational status, and primary payment of medical costs), and clinical information (duration, complications and treatment of diabetes, family history, BMI, waist-to-hip ratio (WHR), and drinking and smoking status).Investigations were conducted face-to-face between patients and the same researcher.All patients were asked to complete the questionnaires independently,if possible.If they could not(e.g.,because of visual problems,low education level,or other reasons),the researchers objectively stated questions and options with unified instructions and completed the questionnaire for the patients on their behalf.
2.4.4.Data analyses
Data were analyzed using SPSS Version 24 (IBM, Armonk, NY,USA)and Mplus version 8.3(Muth′en&Muth′en,Stoner Avenue,LA,USA).Statistical significance was set at P <0.05.
Socio-demographic and clinical characteristics were described by descriptive analysis.Continuous variables that follow a normal distribution were represented by means and standard deviations(SD), while medians and 25th and 75th percentiles (P25-P75) represented other variables.Count and percentage values were calculated for categorical variables.
Concerning validity,we evaluated the degree of content validity,construct validity, convergent validity, and discriminant validity.The content validity index (CVI) was used to check whether the scale subjectively addresses the concept it intends to measure[27].Experts were asked to assess each item by scoring between 1 and 4(4 =appropriate,3 =needs minor revision,2 =needs major revision,1 =not appropriate) [29].Item-level CVI (I-CVI) equals the percentage of experts who selected four or three for the specific item;scale-level CVI (S-CVI) was calculated by averaging I-CVIs of all items [30].S-CVI >0.90 and I-CVI >0.78 suggested sufficiency content validity levels [31].The expert group included two endocrinologists, two nursing specialists, and one psychologist.
As for construct validity, both an exploratory factor analysis(EFA) and a confirmatory factor analysis (CFA) were conducted to examine and confirm the latent structure of the C-DDS17.EFA extrapolates the latent factors responsible for shared variance among items, while CFA objectively compares prior theory-based models to definite latent structures [32].
Furthermore, Bartlett’s and Kaiser-Meyer-Olkin (KMO) tests were used to evaluate the suitability for factor analysis.A KMO value lower than 0.50 is unacceptable, whereas a value between 0.80 and 0.90 is considered good, and a value higher than 0.90 is considered great for factor analysis [33].After confirming the fitness for factor analysis, the preferred factor structure was adjudged by factor loadings through principal component analysis and Kaiser normalization.Factor loadings ≥0.40 were considered sufficient to include factors [28].Specifically, EFA models ranging from one to four were estimated with oblique GEOMIN rotation,and CFA was used to test the competing models.To assess whether the models established were suitable for the data, the absolute objective goodness-of-fit indices were considered using the following criteria: an χ2/df value no more than 3.00 indicates a perfect fit, and a value between 3.00 and 5.00 indicates a good fit[28].A comparative fit index (CFI) ≥0.95, Tucker Lewis index(TLI) ≥ 0.95, root mean square error of approximation(RMSEA) ≤ 0.08, standardized root mean squared residual(SRMR) ≤ 0.08, and weighted root mean squared residual(WRMR) ≤1.00 were considered acceptable [32].The relative goodness-of-fit of the estimated models was evaluated using information criteria indices including the Akaike Information Criteria(AIC),Bayesian Information Criteria(BIC),and sample size-adjusted BIC (SSA-BIC).Models with lower information criteria were considered relatively better fits to the data [32].
Convergent validity is the true correlation degree between two construct measures that should be related theoretically.It can be assessed by inter-scale correlations and average variance extracted(AVE), which should exceed 0.50 [34].Discriminant validity refers to the extent to which two theoretically unrelated measures are truly unrelated [34].It occurs when the squared correlations (SCs)between paired constructs are lower than the AVE of the individual constructs [35].
Regarding reliability, internal consistency was examined using Cronbach’s α coefficient and corrected item-total correlation coefficients.Considered sufficient reliability coefficients should be as close to 1.00 as possible.In particular, a Cronbach’s α coefficient value less than 0.40 is considered unreliable,a value between 0.40 and 0.59 is less reliable, between 0.60 and 0.79 is reliable, and between 0.80 and 1.00 is extremely reliable [28].Item-total correlation is the correlation between an individual item and the total score without that item, which is acceptable with a value no less than 0.40, as estimated by Pearson’s correlation coefficient [36].
3.Results
3.1.Characteristics of the study sample
The data from 400 participants with T2D were analyzed.Table 1 presents the composition of the participants.Participants’ clinical characteristics are shown in Table 2.The median duration for T2D equaled 6.09 years(P25-P75:1.00-14.57 years),and the mean was 8.44 years.The median BMI was 25.22 kg/m2(P25-P75:23.18-27.75 kg/m2),and the mean BMI was 25.76 kg/m2.The mean WHR was 0.93(SD =0.07).Most participants tended to be general and central obese under the Asian criteria (BMI ≥25.00 kg/m2is defined as general obesity and WHR ≥0.80 is defined as central obesity[37]).Most participants had comorbidities(n =342,85.5%);however, only about one-third reported complications (n =137,34.3%).Almost half (n =205, 51.3%) were mainly treated at home,did not consume alcohol(n =222,55.5%).More than half accepted both lifestyle and medication interventions(n =273,68.3%),had a family history of T2D(n =262,65.5%),and never smoked(n =250,62.5%).
3.2.C-DDS17 scores
The median of the C-DDS17 total and sub-scale scores of the sample are shown in Table 3.The median total score of C-DDS17 was 1.53 (P25-P75: 1.18-2.18).The emotional burden and regimen distress dimensions had the highest score (1.60) and the interpersonal distress dimension had the lowest score(1.00).As depicted by percentage of severity, 33.3% (133/400) of the participants experienced moderate to high DD:24.0%moderate DD and 9.3%high DD.The prevalence of emotional burden was 43.0%(172/400),followed by regimen distress(39.0%,156/400),physician distress(26.0%,104/400), and interpersonal distress (19.3%, 77/400).
3.3.Validity
3.3.1.Content validity
The back-translated version showed overall semantic similarities to the terms adopted in the original scale.Further,the content validity of the C-DDS17 was established after evaluation by five experts.The experts remarked that the C-DDS17 had a high degree of relevance and representativeness as assessed by CVI (SCVI =1.00, I-CVI =1.00).Hence, the C-DDS17 was adequately adapted to Chinese and had a satisfactory content validity as a rating scale for DD in T2D patients.
3.3.2.Construct validity
KMO coefficient of the C-DDS17 was 0.88, the χ2value was 2703.57 based on Bartlett’s Test of Sphericity analysis and the df was 136.The test results were significant (P <0.001).The results showed that the sample size for the scale was sufficient and suitable for factor analysis.
The results of EFA of the C-DDS17 data are summarized in Table 4.χ2/df, CFI, TLI, and RMSEA estimates suggested that the one- and two-factor models were a poor fit to the C-DDS17 data,while the EFA three-factor and four-factor models were strong fits to the data: χ2/df, CFI, TLI, RMSEA, and SRMR all fell in the excellent-fit range.The AIC, BIC, and aBIC favored a four-factor model over the three-factor model.
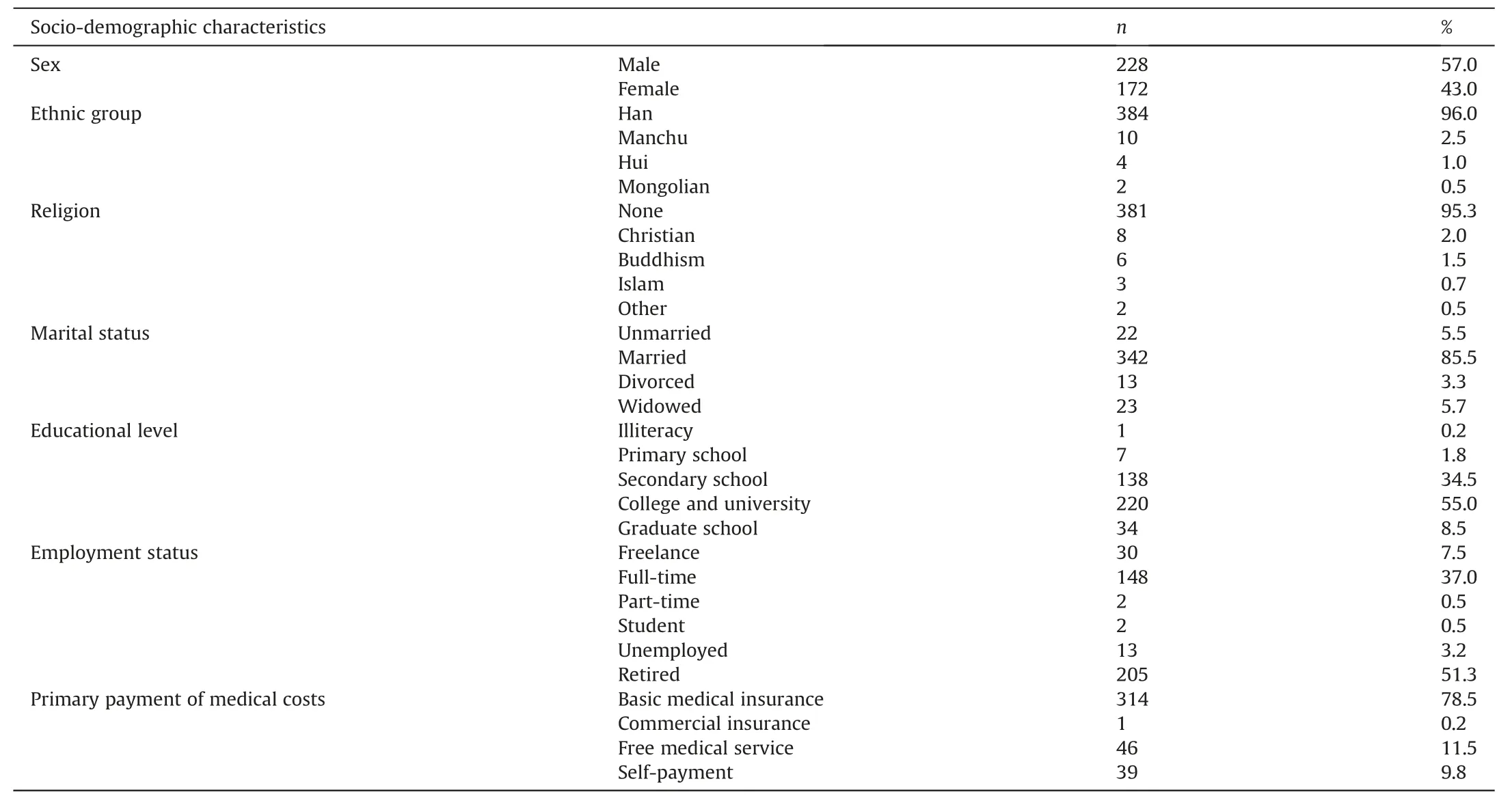
Table 1Participants’ socio-demographic characteristics (n =400).
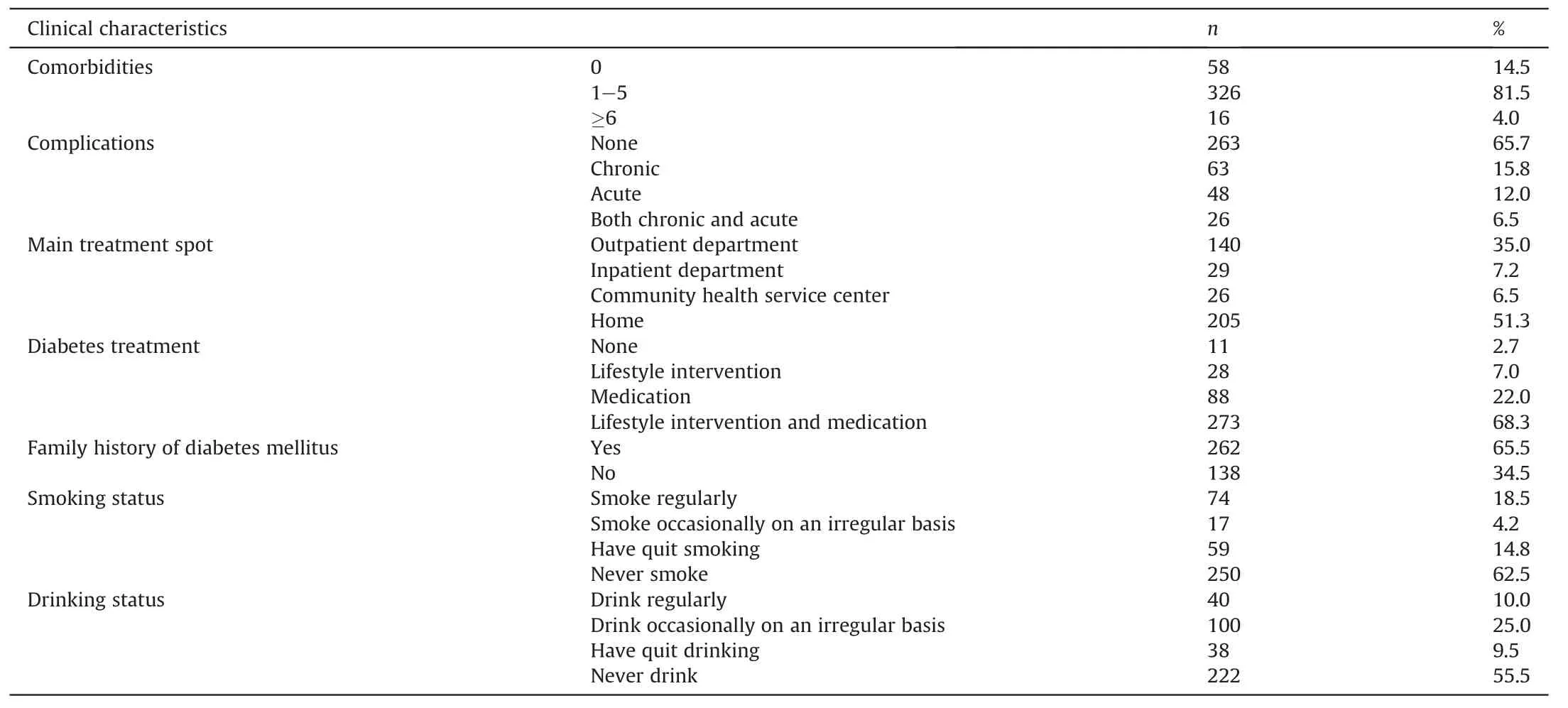
Table 2Participants’ clinical characteristics (n =400).
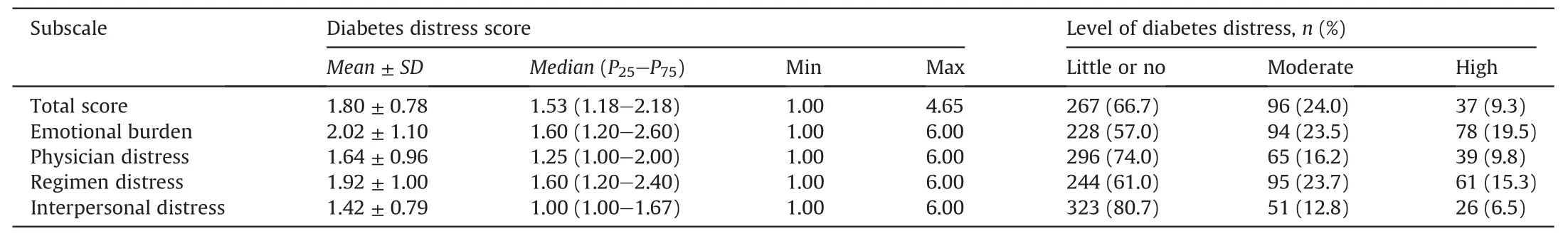
Table 3Scores and levels of the C-DDS17 among adult patients with type 2 diabetes (n =400).
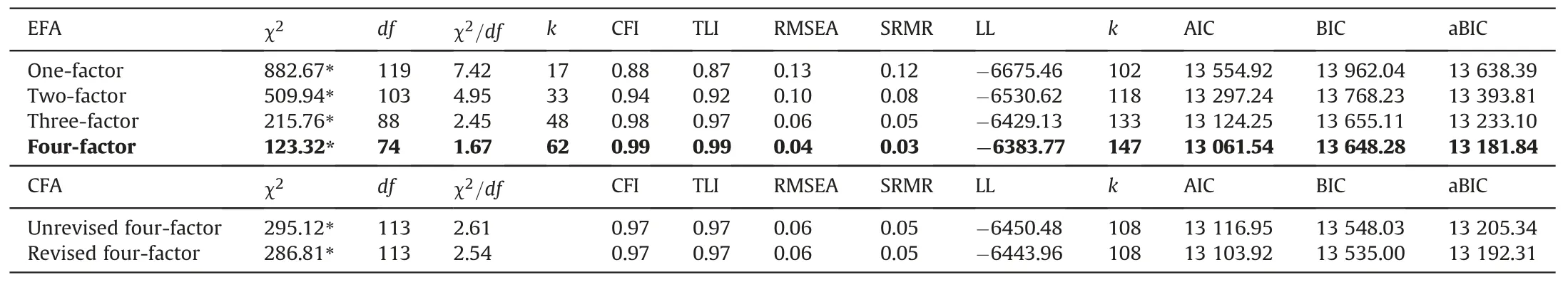
Table 4Factor analysis goodness-of-fit statistics for the EFA and CFA.
As shown in EFA model,the four sub-dimensions accounted for 61.6% of the total variance.Since this exceeds 52.0%, the scale met the construct validity criterion [33].Specific items could be replaced by a new factor.More specifically, Factor 1 comprises items 1, 4, 7, 10, and 14, with factor loadings ranging 0.58-0.77.Factor 2 could replace items 2, 5, 11, and 15 with factor loadings ranging 0.52-0.86.Factor 3 could replace items 3,6,12,and 16 with factor loadings ranging 0.56-0.78.The last factor could replace items 9,13,and 17 with factor loadings ranging 0.73-0.76.All factorloadings are listed in Appendices.The results correspond to the emotional burden, physician distress, regimen distress, and interpersonal distress sub-dimensions raised in the original scale(except for the eighth item).The eighth item (“Feeling that I am often failing with my diabetes routine”) loaded on physician distress with a factor loading of 0.48 instead of regimen distress with a factor loading of 0.34.
The original item classification of sub-dimensions were χ2=295.12, df =113 (P <0.001), χ2/df =2.61, RMSEA =0.06,SRMR =0.05,CFI =0.97,and TLI =0.97 and the fit index of CFA was based on this classification (Table 4).After classifying the eighth item into the physician distress dimension, the revised CFA model showed a slightly better fit to the scale.The revised and unrevised models both had an acceptable fit.The results showed that the 17-item,4-dimensional C-DDS17 was a valid measurement instrument compatible with the original scale.
3.3.3.Convergent validity
The AVE estimates were 0.50, 0.56, 0.42, 0.57 for the original four sub-dimensions, respectively.After changing item 8 to the physician distress dimension, the AVE estimates for physician distress and regimen distress changed to 0.50 and 0.50.The findings showed that the convergent reliability of the C-DDS17 was more acceptable after revising the regimen distress and physician distress dimensions.
3.3.4.Discriminant validity
The results showed moderate to high correlations between the total score and the four sub-scales (Spearman’s r =0.60-0.87,P <0.001)in both the unrevised and revised scales,which indicated the effectiveness and sufficiency for each sub-scale to measure DD.Further,the SCs ranged 0.42-0.63 between sub-dimensions,which were higher than the AVEs.Therefore,the four domains of the scale showed low discriminant validity,which was suitable for the scale to measure the same variable.The SCs are listed in Table 5.
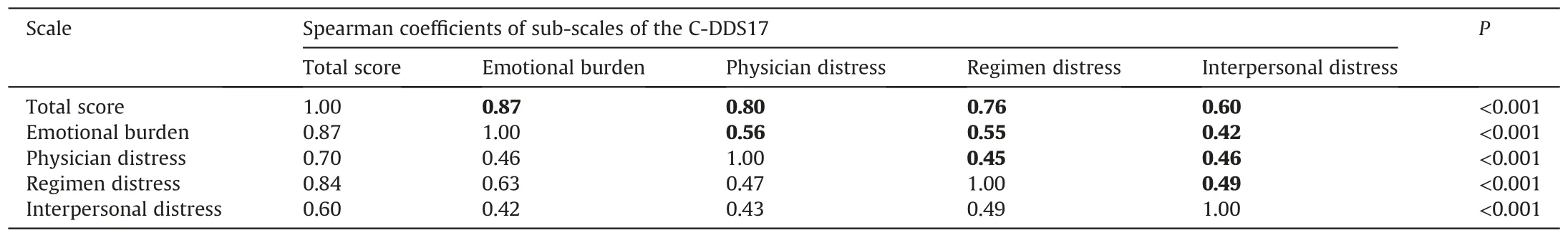
Table 5Squared correlations among variables.
3.4.Reliability
3.4.1.Cronbach’s α coefficient
The Cronbach’s α coefficients of the total C-DDS17 was 0.88 and“Cronbach’s α coefficients if item deleted” were lower than the overall scale,demonstrating great internal consistency of the scale.The internal consistencies of the sub-scales,as shown in Appendix B, were also acceptable.Cronbach’s α coefficients of emotional burden, physician distress, regimen distress, and interpersonal distress sub-dimensions were 0.81, 0.76, 0.78, and 0.76, respectively.After changing the eighth item into the physician distress sub-dimension, the Cronbach’s α coefficients increased to 0.77 for the physician distress dimension and 0.78 for the regimen distress dimension.
3.4.2.Corrected item-total correlation
The dimensionality of the C-DDS17 was assessed using corrected item-total correlations.The results ranged 0.42-0.61: all passed the acceptable cut-off point of 0.40.All correlations are shown in Appendix C.
4.Discussion
4.1.The C-DDS17 is a reliable patient-rated scale for assessing DD in China
The current study systematically translated the DDS17 into Chinese and evaluated the psychometric properties of the C-DDS17.
The mean DDS17 score in this study was lower as compared to America (2.20 ± 1.00), Australia (1.90 ± 0.80), Malay (1.99 ± 0.77),and Turkey (2.50 ± 0.90) [38-41].In addition, there were more men in this study than in the studies conducted in America(34.8%),Australia (52.0%), Malay (48.1%), Turkey (41.5%), Thailand (31.8%),and Indonesia(43.5%)[38-43].The participants of this study were also younger than those in America (59.90 ± 11.20), Australia(58.20 ± 8.80), Thailand (69.00 ± 7.25), and Indonesia(60.14 ± 9.52), but slightly older than those in the studies from Malaysia (55.20 ± 9.74) and Turkey (55.29 ± 10.00) [38-43].The mean T2D duration was shorter than in America (14.00 ± 9.30),Australia(8.90±7.00),and Thailand(10.30±7.96),but longer than in Malaysia(4.90±4.39)[38-40,44].The mean BMI was lower than in the studies in America (33.10 ± 7.70 kg/m2) and Turkey(32.20 ± 12.00 kg/m2) [40,41].In conclusion, the lower DD in this study as compared to prior studies could be owing to the increased prevalence of male participants, the shorter T2D duration, and/or the lower body weight,which was consistent with the correlations between diabetes distress and demographic characteristics in previous studies [5].
In general, the translated version demonstrated excellent psychometric properties and was consistent with the original English scale and other translated versions.The four-factor model offered excellent fit to the data and explained 61.6% variance of the scale.The factors correspond to the four domains reflecting emotional burden, physician distress, regimen distress and interpersonal distress identified in the original DDS17 study [19].The preferred factor structures were also comparable across DDS17 data obtained from other translation versions, including Yang’s Chinese version and the German,Danish,and Norwegian versions,supporting good cross-cultural consistency [13].
All DDS17 items were retained in the factor analysis (all exceeded 0.40),with no cross-loaded items.The distribution of the items over factors was similar to the original scale, except for the eighth item (“Feeling that I am often failing with my diabetes routine”),which loaded on physician distress instead of regimen distress.This might be explained by cultural differences because physicians are considered to play a more dominant role in diabetes routines, and diabetes routines place more reliance on numerical targets in China than in America [45].Similarly, one question was classified in a different sub-dimension from the original scale in the validation analyses of other language versions[41].The Norwegian version of the DDS17 found that the seventh item(“Feeling that I will end up with serious long-term complications, no matter what I do”) was allocated to regimen distress instead of the emotional burden subscale [46].The Malay version also showed that the seventh item(“Feeling that I will end up with serious long-term complications,no matter what I do”) and the fifteenth item (“Feeling that I don’t have a doctor whom I can see regularly enough about my diabetes”)were allocated to regimen distress instead of the physician distress or emotional burden sub-scales,while the third item (“Not feeling confident in my day-to-day ability to manage diabetes”) was allocated to emotional burden instead of regimen distress [38].The reasons include the patients’ perception of regimens, doctors, and different cultural backgrounds.Nevertheless, the instrument still enables the assessment of sub-domains of DD.
Regarding convergent and discriminant validity, this study found significant positive correlations between the total score and the four sub-domains of the scale,as well as between the four subdimensions.The results were higher than in the Malaysian English version [11].The moderate to high correlations confirmed the convergent validity of the scale.These results indicated that the Chinese version was a valid version for DD evaluation in Chinese populations.
The C-DDS17 demonstrated excellent reliability in terms of Cronbach’s α coefficients and item-total correlations.The Cronbach’s α coefficients were similar to the Arabic, Turkish, and Indonesian versions,of which the indexes ranged 0.87-0.82 for the total scale and 0.78-0.88 for the sub-dimensions [36,41-43].However, it was slightly lower than that of the original English version used among different ethnic groups, of which the Cronbach’s α coefficient equaled 0.93 for the total score and 0.88-0.90 for individual sub-scales [47,48].Further, item-total correlations passed the acceptable cut-off point of 0.40.These results indicate that the C-DDS17 is a reliable patient-rated scale for assessing DD and its four domains in the mainland of China.
4.2.Strengths and limitations
This study has the following strengths.The validation process followed a stringent methodology; amendments of the C-DDS17 were completed according to the translation validity evaluated by experts, which increased the language accuracy and understandability.Moreover, the sample size was larger than other scale translation studies, and the study was multi-centered, which provides more robust evidence supporting the validity and reliability of C-DDS17 in patients with T2D in China.
However, this study has some limitations as well.Our participants were recruited using convenience sampling from Class A tertiary comprehensive hospitals in Beijing.This might have biased the sample toward participants in high-grade hospitals and with a high degree of cooperation,which affects the generalizability of our results to patients in other levels of hospitals and other areas of China.Nevertheless, the sample can still represent most patients with T2D in China to some extent because the age and sex distributions were consistent with that of a large Chinese cohort study of T2D[49].For better representation of patients with diabetes,future studies should include patients from different areas and diverse medical institutions.A random sample could also be considered to improve the generalizability of the findings.Furthermore, because criterion validity was not evaluated in this study, a comparison study between C-DDS17 and other scales is necessary for future studies to confirm the criterion validity of the scale.
Another limitation is that the EFA results showed different factor classification for the eighth item.This might be partially explained by cultural differences[41].However,the CFA and reliability results did not show significant differences between the unrevised and revised models.Further,statistical results cannot provide adequate evidence for revision of sub-scales for a mature scale.To maintain comparability across culture, future usage and analysis of the CDDS17 should still follow the construct of the original instrument.Additionally, it may be useful to conduct more studies in a larger Chinese T2D population.Owing to its cross-sectional study,some of the psychometric properties, such as sensitivity to change and predictive validity, were not explored in this study.Thus, a longitudinal study should be conducted to increase the accuracy of CDDS17 for dynamically evaluating DD and investigating the interventions’ effectiveness.Easy identification of patients with DD could facilitate future research in deriving early interventions.Future studies could also explore the physio-psychological mechanisms of the scale domains.
5.Conclusion
The findings obtained in this study were mostly consistent with the original scale.The results of the EFA and CFA confirmed the four-factor structure of the scale.Moreover, the scale had acceptable Cronbach’s α coefficient s and item-total correlations.These results demonstrate that the C-DDS17 could be an easily applied,psychometrically robust,reliable,and valid instrument for Chinese patients with T2D.It is a good instrument for early identification and management of DD in clinical practice and clinical trials.
Funding
No external funding was utilized.
Ethics approval statement
This study was registered in the Chinese Clinical Trial Registry(no.ChiCTR2100047071) and was approved by the Human Biomedical Research Ethics Committee,School of Nursing,Chinese Academy of Medical Sciences&Peking Union Medical College(no.[2020]03).This study was performed according to the Declaration of Helsinki.Participation was voluntary and written informed consent was provided by participants before the formal investigation.The C-DDS17 was already published on the Behavioral Diabetes Institute website (https://behavioraldiabetes.org/scales-andmeasures/).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.The data are not publicly available now because of privacy and ethical restrictions.They will be accessible after the entire study program is completed.
Credit authorship contribution statement
Yu-Yun Zhang:Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing - original draft.Wei Li:Methodology, Resources,Supervision, Writing - review and editing.Yu Sheng:Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing - review and editing.
Declaration of competing interest
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors gratefully thank the authors of DDS17,Dr.Polonsky and his colleagues,for their permission to translate and validate the scale.The authors express heartfelt gratitude to all experts involved in the translation and reliability evaluation procedures, including Fan Ping and Ling-Ling Xu From Peking Union Medical College hospital, Jing Li and Zhen-Ling Ma from Peking Union Medical College, Qun Wang from Peking University Third Hospital, Fang Zhao from China-Japan Friendship Hospital and Jian-Jing Kuang from the University of Pennsylvania.The authors also gratefully thank all participants and medical staff of the Department of Endocrinology of the Peking Union Medical College Hospital,China-Japan Friendship Hospital and Peking University Third Hospital for their support during data collection.Finally, the authors thank the School of Nursing,Chinese Academy of Medical Sciences& Peking Union Medical College for supporting this study.
Appendices.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2022.03.002.
杂志排行
International Journal of Nursing Sciences的其它文章
- Effects of mindfulness meditation on trait mindfulness, perceived stress,emotion regulation,and quality of life in hemodialysis patients:A randomized controlled trial
- Application of rational emotive behavior therapy in patients with colorectal cancer undergoing adjuvant chemotherapy
- The effect of slow deep breathing relaxation exercise on pain levels during and post chest tube removal after coronary artery bypass graft surgery
- The association between frailty of older stroke patients during hospitalization and one-year all-cause mortality:A multicenter survey in China
- Translation and piloting of the Chinese Mandarin version of an intensive care-specific pressure injury risk assessment tool (the COMHON Index)
- Adaptation and validation of pediatric peripheral intravenous catheter insertion and care practices audit tools
