Mixed Metal-Organic Frameworks with Supercapacitor Performance Constructed by Succinic Acid
2022-05-09NIUBaiTongFENGYanHuiLIAOHuiLingGUOHongXuYINGShaoMing
NIU Bai⁃TongFENG Yan⁃HuiLIAO Hui⁃LingGUO Hong⁃Xu*,YING Shao⁃Ming
(1College of Chemistry,Chemical Engineering and Environment,Minnan Normal University,Zhangzhou,Fujian 363000,China)
(2Fujian Provincial Key Laboratory of Featured Materials in Biochemical Industry,Ningde Normal University,Ningde,Fujian 352100,China)
Abstract:A mixed metal⁃organic framework(MOF(Ni,Co))was synthesized through a simple one⁃pot solvothermal method,and was characterized through X⁃ray diffraction,FT⁃IR,scanning electron microscope,X⁃ray photoelectron spectroscopy,and N2adsorption⁃desorption.The performance of the samples as supercapacitor electrode materials was further studied for the first time.The unique nano⁃flower⁃like structure of MOF(Ni1.2Co0.8)provides more electro⁃active sites and a shorter pathway for electron transfer and electrolyte diffusion,resulting in an excellent electro⁃chemical performance with a high specific capacitance of 850 F·g-1at 1 A·g-1.At the same time,this work shows that the MOF(Ni)electrode material doped with an appropriate amount of Co can facilitate the electron/ion transpor⁃tation of the electrode,reduce the contact resistance between active material and electrolyte,increase the electrical conductivity,and enhance the electrochemical performance.
Keywords:supercapacitor;solvothermal method;metal⁃organic frameworks;nano⁃flower
The development and utilization of clean energy have become a mainstream research topic in the world.However,environmentally friendly energy sources such as wind,solar,geothermal,tidal,and biomass energy are intermittent and unstable.Therefore,it is particu⁃larly important to develop efficient devices for storing and managing these unstable energies.Supercapacitors(SCs)have attracted the interest of scientists due to their excellent properties such as fast charge⁃discharge capability,ultra⁃high power density,excellent Coulom⁃bic efficiency,and long service life[1⁃4].
In recent years,metal⁃organic frameworks(MOFs)have been extensively developed as electrode materials for SCs[5⁃8].MOFs are composed of organic linkers and metal ions by strong chemical bonds[9].Due to the large specific surface area,special pore structure,and rich active sites of MOFs,they have aroused more and more concentration among plentiful researchers[10].Neverthe⁃less,from an overall perspective,low electrical conduc⁃tivity and hindered ion entrance usually limit the prac⁃tical applications of many of the MOFs for supercapaci⁃tor applications[11⁃12].Fortunately,by using appropriate linker and metal cations and tuning the resultant struc⁃tures,it is possible to facilitate the ion insertion/extrac⁃tion inside the MOFs likewise enhancing the transport pathways for electrons[13].
In this work,we report a facile one⁃pot solvother⁃mal synthesis of mixed metal MOF(MOF(Ni,Co))and its application as a supercapacitor electrode.The effect of the atomic ratio of Ni to Co in MOFs on the electro⁃chemical properties was investigated,indicating that the MOF(Ni,Co)nano⁃flower exhibited a maximum specific capacitance of 850 F·g-1at 1 A·g-1with an atomic ratio of Ni to Co in MOF(Ni,Co)of 3∶2.The experiment results reveal that the MOF(Ni1.2Co0.8)supercapacitor preserves its excellent specific capaci⁃tance in alkaline aqueous electrolytes compared to the previously reported works in this area,in addition to exceptional stability.Thus,this work demonstrates that the MOF(Ni1.2Co0.8)electrode could find potential appli⁃cations for supercapacitive purposes.
1 Experimental
1.1 Material and characterizations
All chemical reagents were of analytical grade and utilized as received without further purification.All reagents were bought from Xilong Chemical Reagent Co.,Ltd.(China).
The powder X ⁃ray diffraction(XRD)was per⁃formed on a Rigaku D/MAX⁃RB(Japan)using Cu Kα radiation(λ=0.154 06 nm)at 40 kV and 40 mA,and the XRD patterns were recorded in a 2θ range from 5°to 60°.FT⁃IR was measured on a NICOLET iS 10IR(USA)Fourier transform infrared spectrometer.The morphologies of MOF(Ni,Co)were characterized on a Hitachi SU8010 field⁃emission scanning electron microscopy(SEM,Japan)and the operating voltage was 5 kV.X⁃ray photoelectron spectroscopy(XPS)was per⁃formed by ESCALAB 250Xi(USA)for further elemen⁃tal analysis,and Al Kα radiation was used as the exci⁃tation source.Belsorp⁃MAX(USA)was used to conduct the nitrogen adsorption⁃desorption isotherms.
1.2 Preparation of MOF(Ni,Co)nanomaterials
Briefly,the synthesis method was as follows.Solution Ⅰ :Ni(NO3)2·6H2O and Co(NO3)2·6H2O were mixed thoroughly in 40 mL of ethanol and deionized water(50∶50,V/V)until they were fully dissolved.Solu⁃tion Ⅱ:succinic acid(0.35 g,3 mmol)and KOH(0.22 g,4 mmol)were mixed thoroughly in 20 mL of ethanol and deionized water(50:50,V/V)until it was fully dis⁃solved.Solution Ⅱ was added dropwise into solution Ⅰ to get a mixture,and the mixture was then trans⁃ferred to a 100 mL Teflon⁃lined stainless steel auto⁃clave and heated under 120℃for 12 h.After cooling,the products were washed with ethanol several times via centrifugation and dried at 80℃ for 12 h in a vacu⁃um oven.The mole ratios of Ni(NO3)2·6H2O to Co(NO3)2·6H2O were 1.2∶0.8,0.8∶1.2,and the MOF(Ni,Co)were named MOF(Ni1.2Co0.8) and MOF(Ni0.8Co1.2),respectively.Similarly,the pristine MOF(Ni)precursor solution was obtained using the same method without adding Co(NO3)2·6H2O,and the pristine MOF(Ni)was prepared.
1.3 Electrochemical measurements
The working electrode was fabricated through a slurry⁃forging procedure by smearing the sample on nickel foam(NF,1 cm×1 cm).Specifically,active mate⁃rial(MOF(Ni,Co)),polyvinylidene difluoride(PVDF),and acetylene black were ground together in a mass proportion of 8∶1∶1,and then ethanol was dropped in the powder to form slurry conditions.Next,the slurry was equably plastered on NF and dried at 60℃for 12 h.The dried NF was stress⁃treated(10 MPa,about 30 s)before use for electrochemical experiments.
The electrochemical characterization of MOF(Ni,Co)was performed in a three⁃electrode system with 3 mol·L-1KOH as the electrolyte.A saturated calomel electrode(SCE)and platinum plate were used as the reference and the counter electrodes,respectively.The electrochemical tests were performed at room tempera⁃ture.The cyclic voltammetry(CV),galvanostatic charge⁃discharge(GCD),and electrochemical impedance spec⁃troscopy(EIS)measurements were performed on a CS 2350H electrochemical workstation(CorrTest,Wuhan).
2 Results and discussion
2.1 Structure and morphology analysis
The XRD patterns of MOF(Ni),MOF(Ni1.2Co0.8),MOF(Ni0.8Co1.2),and standard cards are shown in Fig.1a.The diffraction peaks centered at 8.4°,10.2°,11.6°,14.3°,20.9°,23.1°,27.5°,34.9°,and 37.8°can be ascribed to the(210),(213),(006),(107),(426),(51 3),(40 10),(61 11)and(838)diffraction planes of[Ni7(C4H4O4)6(OH)2(H2O)2]·2H2O(CCDC No.169140),respectively[14].The sharp peaks in the patterns indi⁃cate that the composites are well crystallized.The XRD peaks of MOF(Ni,Co)did not change significantly,indicating that the implantation of Co metal ions do not affect the crystal phase of the framework.Fig.1b show FT⁃IR spectra of MOF(Ni),MOF(Ni1.2Co0.8),and MOF(Ni0.8Co1.2).The peaks at 1 632 and 1 556 cm-1are attributed to the asymmetric stretching vibration of—COO— coordinated to metal ion by a bidentate mode,while the peaks at 1 440 and 1 412 cm-1are due to its symmetric stretching vibration[15].The peak at 1 250⁃1 140 cm-1can be attributed to C—C stretching vibration(skeleton vibration),and the peak at 3 500⁃3 200 cm-1corresponds to hydrogen bond in H2O mole⁃cules.Overall,the successful synthesis of MOF(Ni)and MOF(Ni,Co)by the hydrothermal method is confirmed.
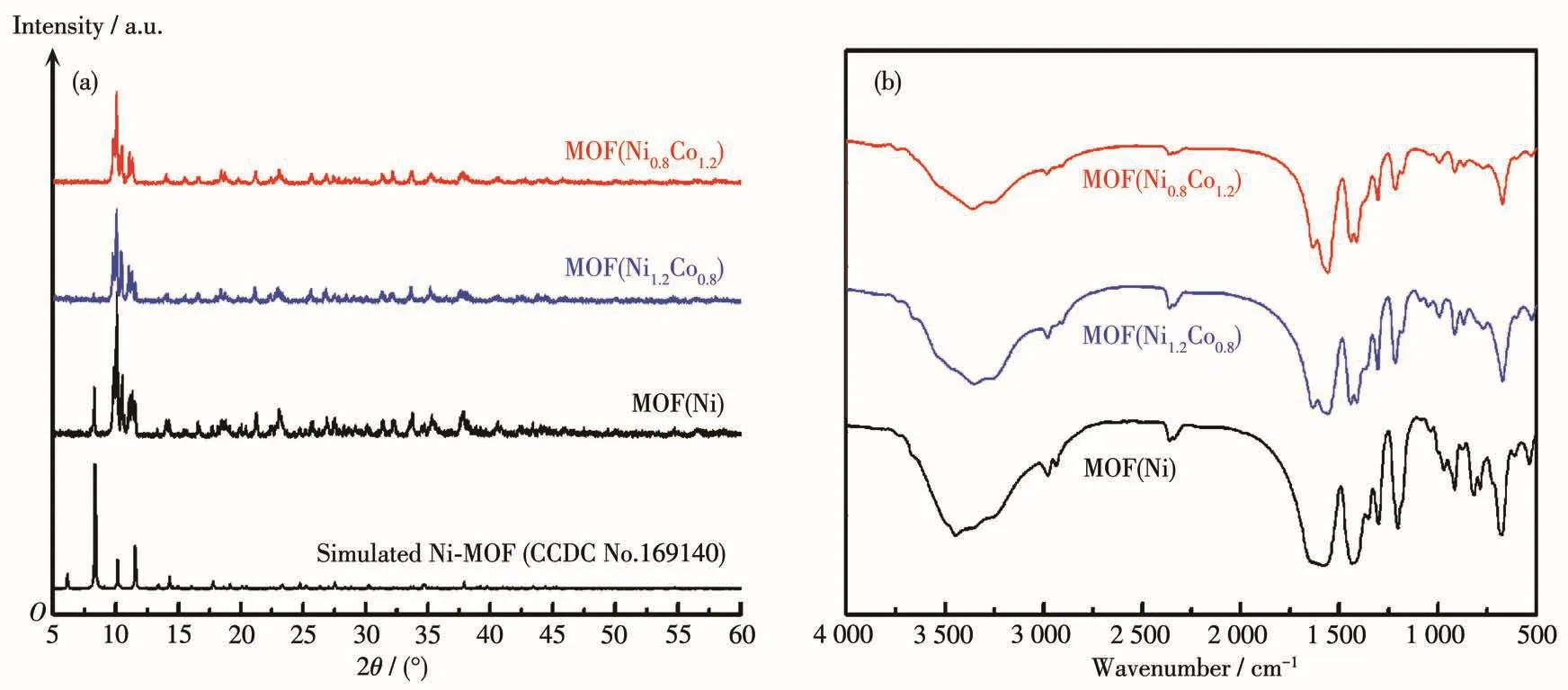
Fig.1 (a)XRD patterns and(b)FT⁃IR spectra of MOF(Ni),MOF(Ni1.2Co0.8),and MOF(Ni0.8Co1.2)
The coordination model of metal ions and the 3D stacking diagram for MOF(Ni)are illustrated in Fig.2.As shown in Fig.2a,each Ni atom is coordinated to six oxygen atoms from carboxylate groups of the ligands.As shown in Fig.2b,the honeycomb structure with tunnel holes is beneficial to the transport of ions and thus greatly improves the electric storage capacity.
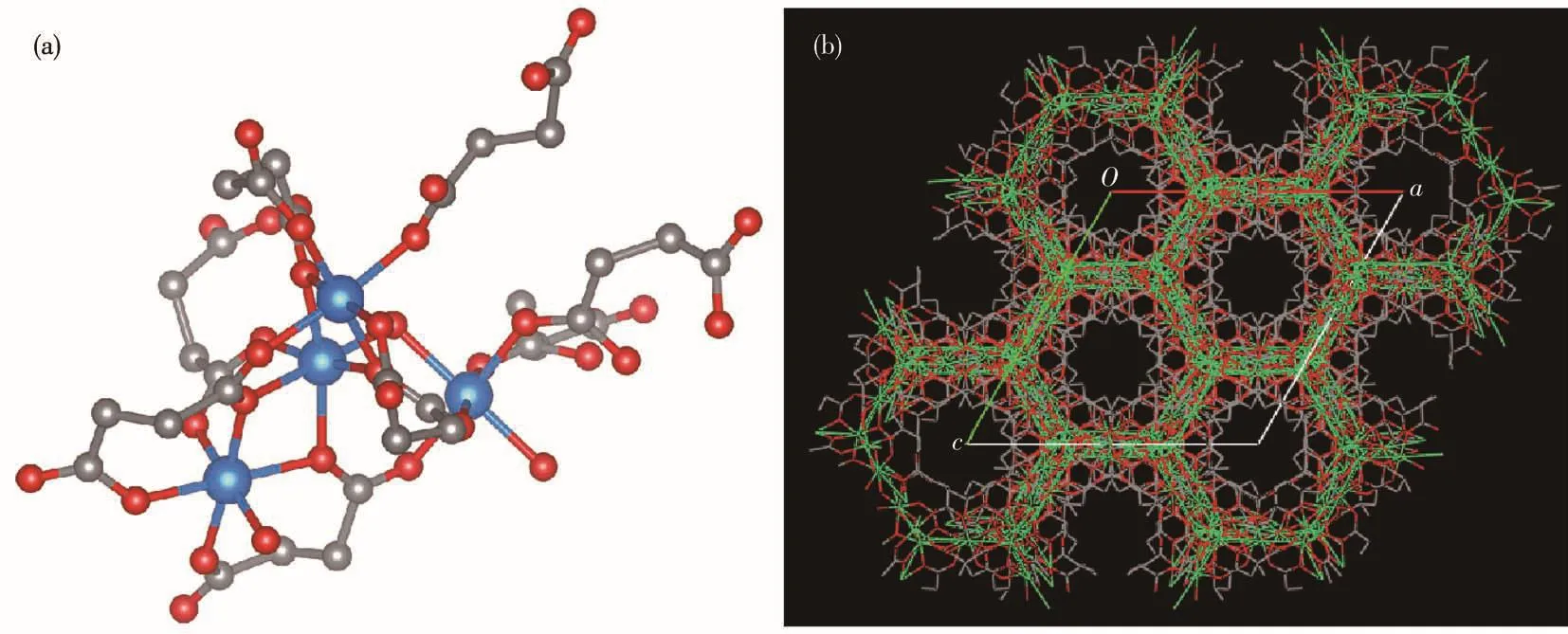
Fig.2 (a)Crystal structure of MOF(Ni)and(b)view of MOF(Ni)structure viewed along the c⁃axis(based on CCDC data)
The morphology of MOF(Ni),MOF(Ni1.2Co0.8),and MOF(Ni0.8Co1.2)nanomaterials was subsequently cap⁃tured(Fig.3a⁃3c).In MOF(Ni),a large number of irreg⁃ular block solids was observed(Fig.3a).As shown in Fig.3b,adding an appropriate amount of Co2+ions to the initial solution can get large⁃scale nanosheets to form flower⁃shaped microspheres,and there are large gaps between the nanosheet.As shown in Fig.3c,when the Co2+content in the reaction system was further increased, the morphology was still large⁃scale nanosheet,but the flower⁃shaped microspheres formed by the accumulation were more compact.Therefore,the addition of Co2+in the initial solution can significantly affect the morphology of MOFs.

Fig.3 SEM images of(a)MOF(Ni),(b)MOF(Ni1.2Co0.8),and(c)MOF(Ni0.8Co1.2)
The chemical bonding state of MOF(Ni1.2Co0.8)was characterized using XPS,and the results are shown in Fig.4.The survey scan spectrum of C1s,O1s,Ni2p,and Co2pcan be observed(Fig.4a).The carbon environ⁃ment had three different binding sites C—C,C—O—C,and O—C=O with corresponding binding energies at 284.5,285.2,and 288.7 eV,respectively(Fig.4b)[16].Fig.4c displays the O1sregion,which shows three peaks at 531.1,531.8,and 532.6 eV due to metal—O,—OH,and—C—O[17],respectively.The peaks in the high⁃resolution Ni2pXPS spectrum of MOF(Ni1.2Co0.8)at 856.2 and 873.8 eV(Fig.4d)can be ascribed to Ni2p3/2and Ni2p1/2,indicating that Ni mainly exists as Ni2+in MOF(Ni1.2Co0.8).Additionally,the two broad peaks at 861.4 and 879.6 eV are identified as shake⁃up satellites(“Sat.”)of Ni2p3/2and Ni2p1/2,respectively[18].Similarly,Fig.4e shows that the peaks located at around 781.5 and 797.4 eV are indexed to Co2p3/2and Co2p1/2,and the other two peaks centered at 786.0 and 802.5 eV correspond to shake⁃up satellites,which are characteristic bands of Co2+[19].The atomic fraction(%)of the elements in MOF(Ni,Co)from XPS analysis is shown in Table 1.It can be seen that the actual atomic ratio of Ni to Co in MOF(Ni1.2Co0.8)was 1.86∶1.There⁃fore,XPS results verify the formation of the nickel and cobalt succinate phases.
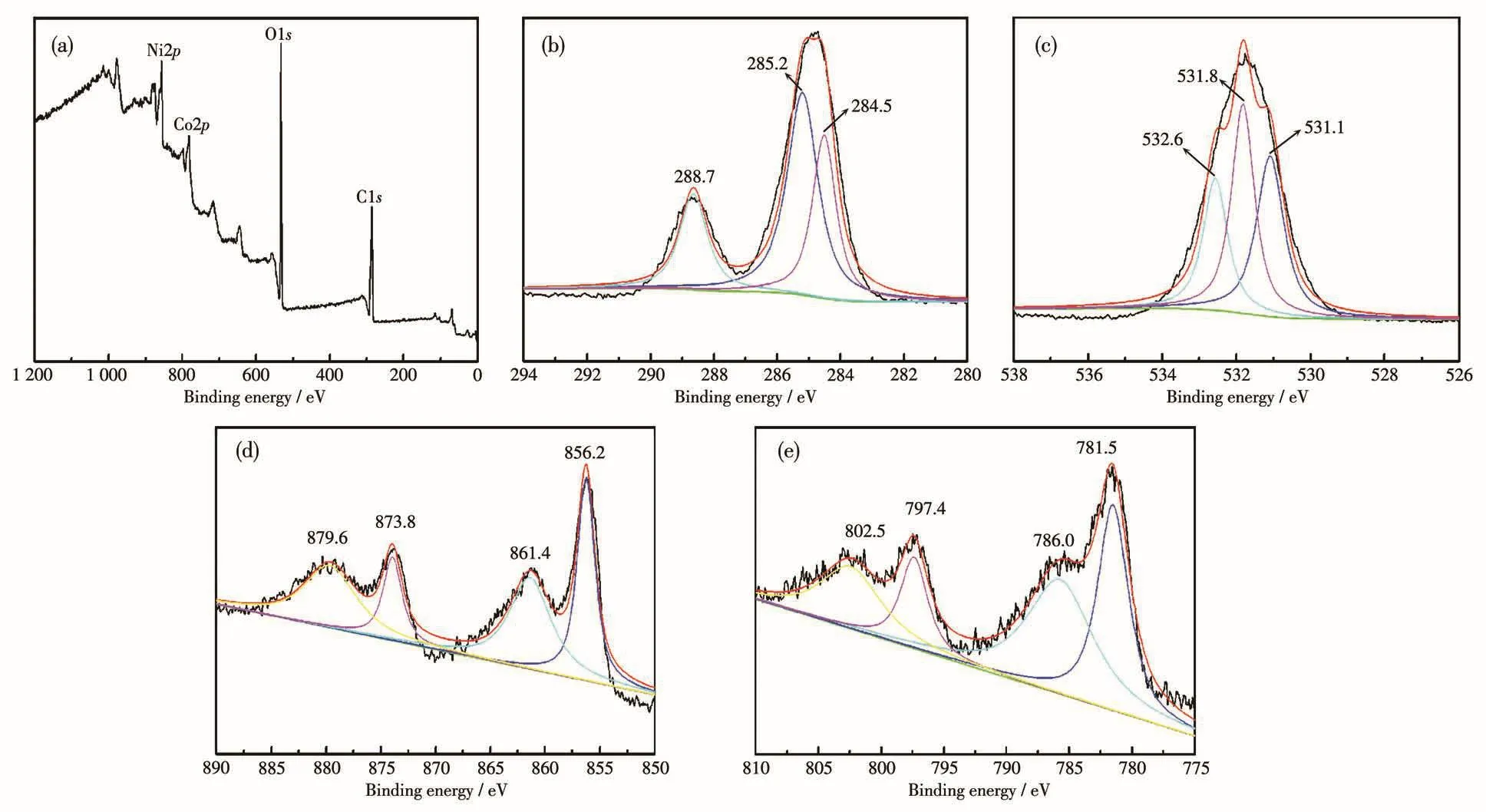
Fig.4 XPS spectra of MOF(Ni1.2Co0.8):(a)survey scan,(b)C1s,(c)O1s,(d)Ni2p,and(e)Co2p
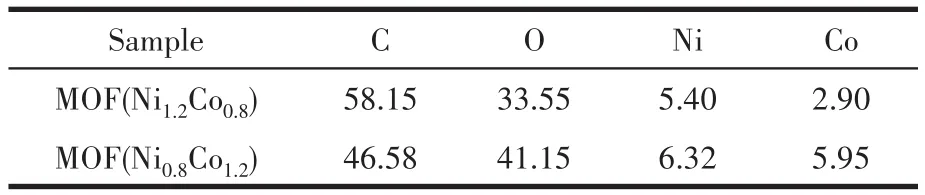
Table 1 Atomic fraction of MOF(Ni,Co)from XPS analysis%
The nitrogen adsorption⁃desorption isotherms of MOF(Ni1.2Co0.8)determined at 77 K and the correspond⁃ing Barret⁃Joyner⁃Halenda(BJH)pore size distribution analysis are shown in Fig.5.The specific surface area,pore size,and pore volume were calculated to be 159 m2·g-1,8.72 nm,and 0.258 cm3·g-1,respectively.Fig.5a shows that MOF(Ni1.2Co0.8)displayed Ⅳ⁃type isotherms with an H3 hysteresis loop,which is typical behavior of mesoporous structure[20].From the pore size distribution in Fig.5b,it can be seen that the pore size of the sample was in a range of 3⁃6 nm,which shows that it has a uniform mesoporosity,providing a channel for ion transmission.
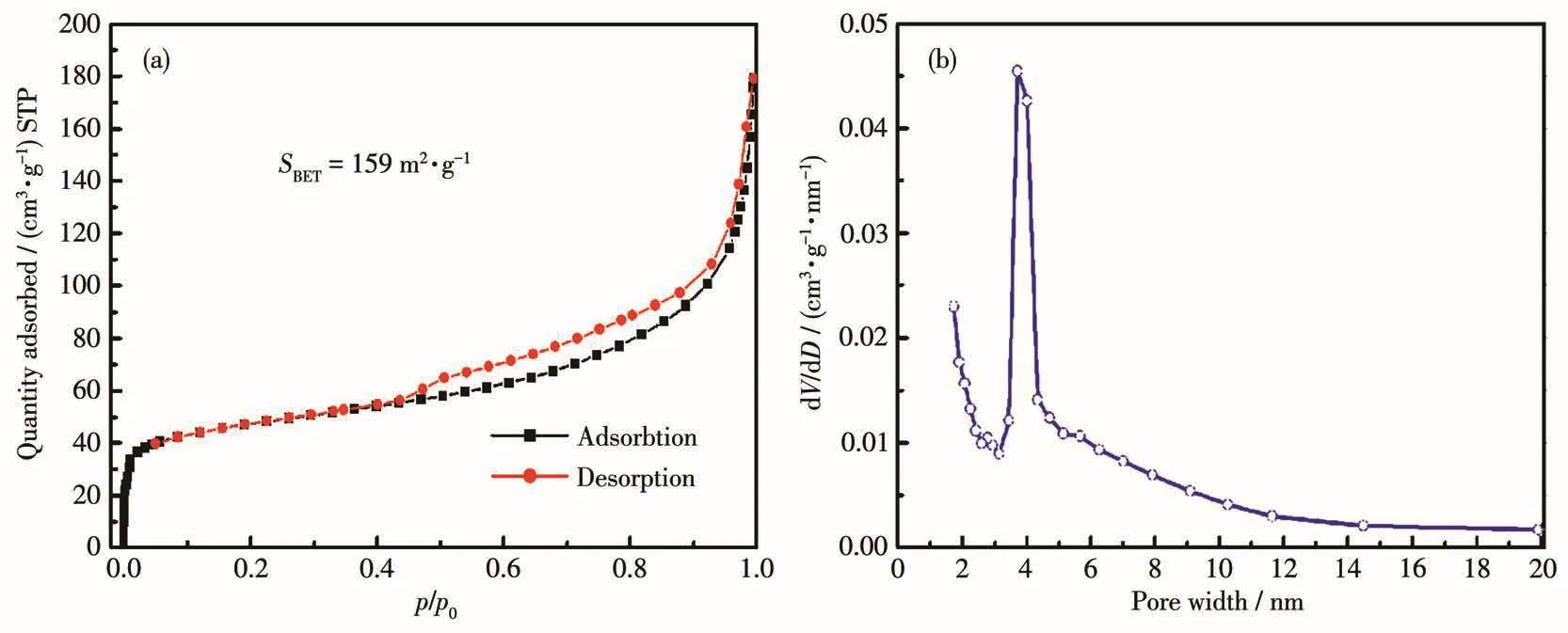
Fig.5 (a)Nitrogen adsorption⁃desorption isotherms and(b)pore size distribution of MOF(Ni1.2Co0.8)
2.2 Electrochemical performance
To study the electrochemical property,CV behav⁃iors of as⁃prepared MOF electrodes were investigated at different scan rates(5,10,20,50,100 mV·s-1)in 3 mol·L-1KOH electrolytes using a three⁃electrodes test system.Fig.6a presents the CV curves of the MOF(Ni1.2Co0.8)electrode at different scan rates in a poten⁃tial range of-0.15⁃0.55 V(vs SCE).Meanwhile,when the scan rate increased,the cathodic and anodic peaks slightly shifted toward positive and negative voltages,respectively,indicating a very low overpotential that is because of the very low charge transfer resistance.This effect is explained by electric polarization and the resistance of the electrodes,which cause irreversible reactions at very high scan rates[21].So,the CV curve lost its regular shape at a higher scan rate.As shown in Fig.6b,under the condition of a scanning rate of 5 mV·s-1,it can be seen that the three electrode materials had a pair of prominent redox peaks,and the curve symme⁃try was good,indicating that they have ideal pseudo⁃ca⁃pacitance electricity.The MOF(Ni1.2Co0.8)electrode showed larger CV areas than other electrodes,indicat⁃ing that the synergistic effect of Ni and Co ions leads to a higher specific capacitance.Fig.6c shows the GCD curves of the MOF electrode within a potential window of-0.15⁃0.39 V(vs SCE)at different current densities.The corresponding specific capacitance of the MOF(Ni1.2Co0.8)electrode at 1 A·g-1was calculated to be 850 F·g-1.The plot of specific capacitance vs current density for the three kinds of electrode materials is shown in Fig.6d.When the current density was in⁃creased by 10 times,the specific capacitances of the three electrode materials were preserved by 36.3%,81.2%,and 70.5%,respectively.MOF(Ni1.2Co0.8)exhib⁃ited excellent specific capacitance.Moreover,com⁃pared with the previous reports of MOF⁃based superca⁃pacitor electrode materials(Table 2),MOF(Ni1.2Co0.8)also exhibited excellent capacitive performance,which is attributed to ultrathin nanosheet structure.The ultra⁃thin MOF(Ni1.2Co0.8)nanosheets provide abundant elec⁃troactive sites for the Faradaic redox reaction and a short pathway for electron transfer and electrolyte ions diffusion.
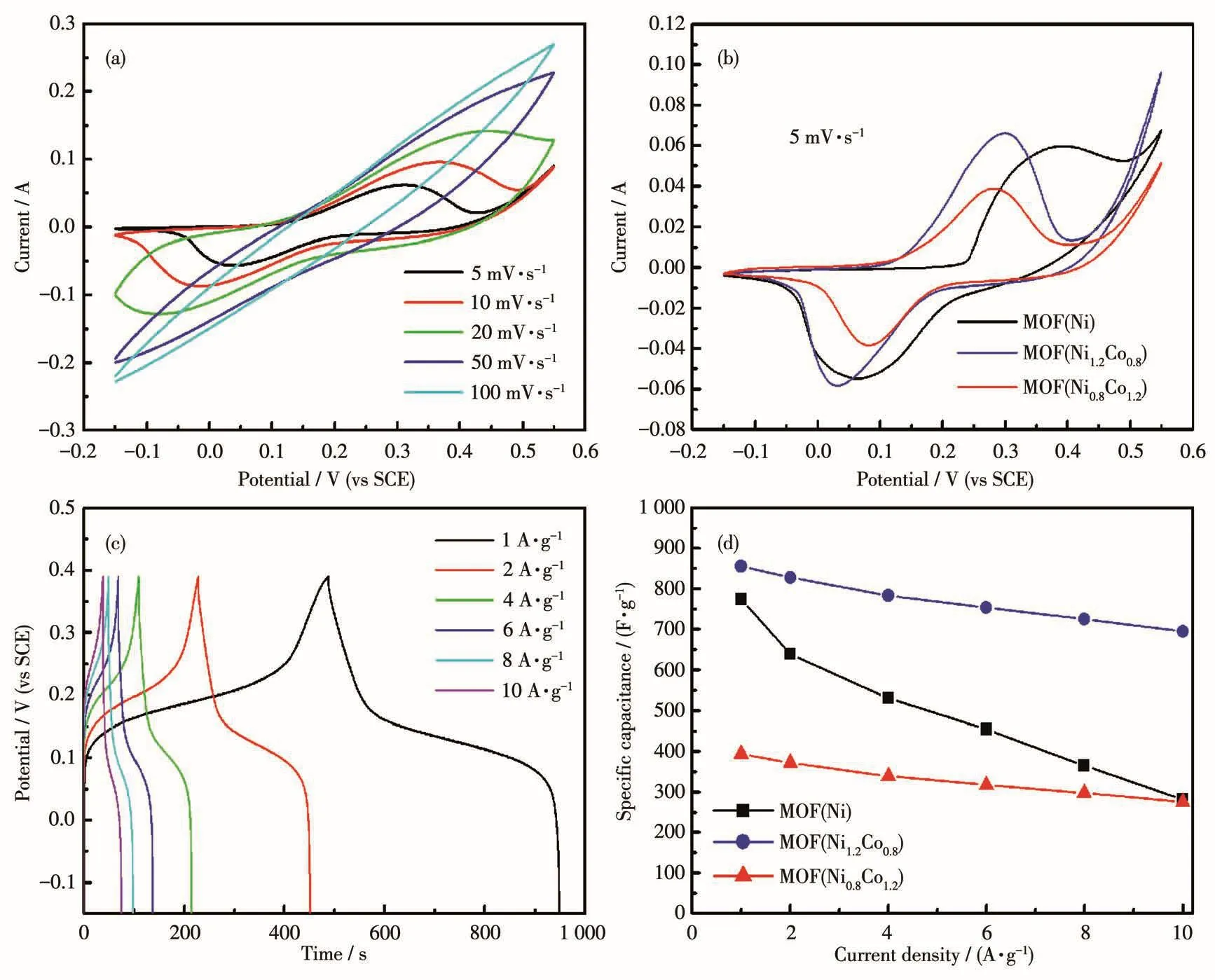
Fig.6 Electrochemical performance of MOF(Ni1.2Co0.8):(a)CV curves at different scan rates;(b)CV curves at 5 mV·s-1;(c)GCD curves of MOF(Ni1.2Co0.8)at different densities;(d)specific capacitance at different current densities
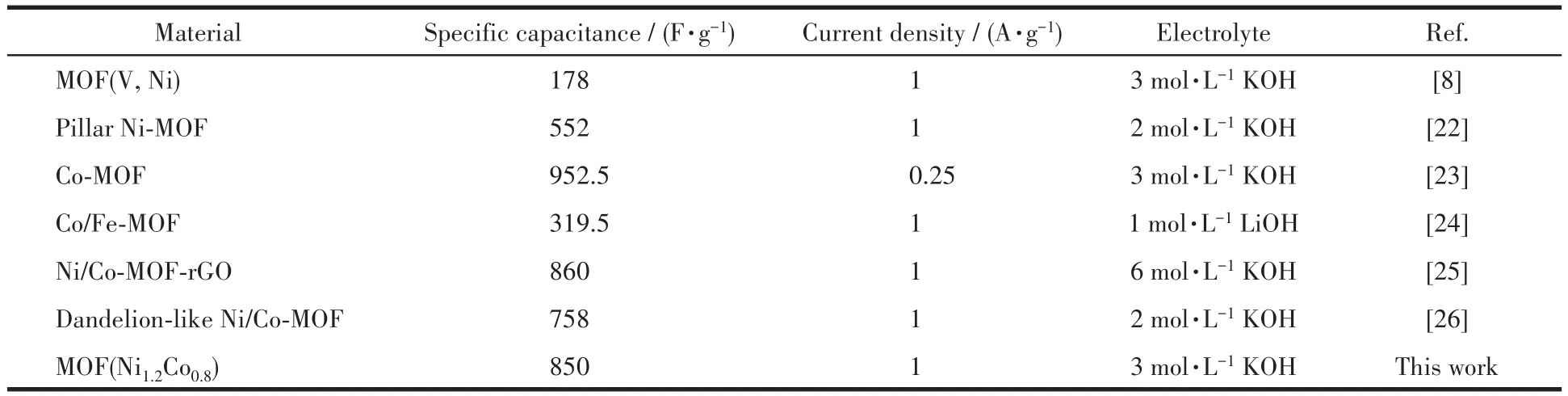
Table 2 Specific capacitance of MOF-based electrode materials
The electroconductivity of different samples was also investigated by EIS.As shown in Fig.7,the radius of the circle in the high⁃frequency region for MOF(Ni1.2Co0.8)and MOF(Ni0.8Co1.2)was smaller than that of MOF(Ni),which means that they have a smaller charge transfer impedance.The inset is the corresponding equivalent circuit diagram of the electrode material of MOF(Ni1.2Co0.8),and its equivalent series resistance was about 0.7 Ω.At the same time,it also shows that the MOF(Ni1.2Co0.8)electrode material is a host material for high electrolyte access,penetration,and ion diffu⁃sion,which is conducive to the rapid storage and release of energy and has good electrical conductivity.
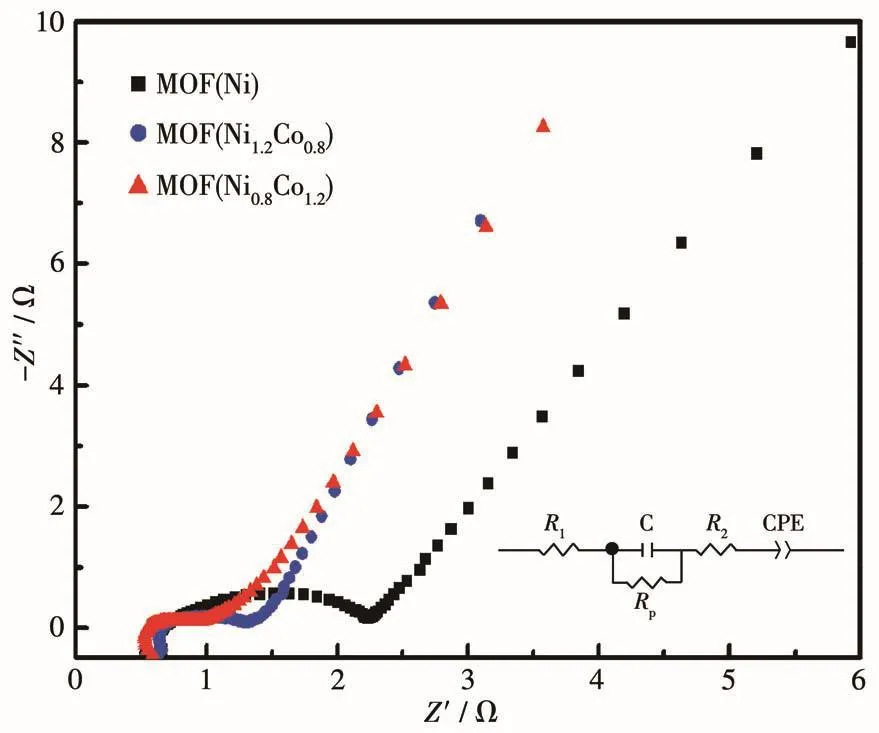
Fig.7 EIS plots of MOF(Ni),MOF(Ni1.2Co0.8),and MOF(Ni0.8Co1.2)in 3 mol·L-1KOH electrolyte
The cycling stability of the MOF(Ni),MOF(Ni1.2Co0.8),and MOF(Ni0.8Co1.2)electrode was tested by GCD at 4 A·g-1.As shown in Fig.8a,compared with the change of specific capacitance of MOF(Ni)and MOF(Ni0.8Co1.2),the MOF(Ni1.2Co0.8) electrode kept stable during 1 000 cycles of charging and discharging,but the specific capacitance decreased to 84.6% of the initial value.CV curves of MOF(Ni1.2Co0.8)at the start,after 500 cycles and after 1 000 cycles are shown in Fig.8b.It can be seen that after 500 and 1 000 cycles,the oxidation peak and reduction peak of the electrode did not change significantly,indicating that the structure of MOF(Ni1.2Co0.8)does not change significant⁃ly after the cycle.It can be seen that the addition of an appropriate amount of Co2+also greatly improves the cycle performance of MOF materials as supercapaci⁃tors.

Fig.8 (a)Cycling stability of MOF(Ni),MOF(Ni1.2Co0.8),and MOF(Ni0.8Co1.2)at 4 A·g-1and(b)CV curves of MOF(Ni1.2Co0.8)at the start,after 500 and 1 000 cycles
3 Conclusions
In summary,a mixed metal⁃organic framework,that is MOF(Ni,Co),was synthesized through a simple one⁃pot solvothermal method.The detailed character⁃izations through XRD,FT⁃IR,SEM,XPS,and N2adsorption⁃desorptiopn were then performed.The potential application of nano⁃flower⁃like MOF(Ni1.2Co0.8)for supercapacitor electrode material was also explored.Remarkably,the electrochemical results show that MOF(Ni1.2Co0.8)nano⁃flower presents a good supercapacitor electrode material,in which a high specific capacitance of 850 F·g-1at 1 A·g-1as well as good cycling stability and rate capability were achieved.These results highlight the promising appli⁃cations of MOF(Ni1.2Co0.8) nano⁃flower as high⁃performance supercapacitor electrode materials.
杂志排行
无机化学学报的其它文章
- 双功能Pd/ZrHP催化剂的制备及其在苯酚选择性加氢反应中的应用
- 硫/多孔碳纳米管复合材料的制备及电化学性能
- R6G@γ-CD-MOFs复合物的制备及其在Fe3+离子传感中的应用
- Effects of Preformed Pt Nanoparticles on Structure of Platinum Nanowire Cathode for Proton Exchange Membrane Fuel Cells
- Effects of Cr,Sn,Co Doping on Electronic and Optical Properties of Layered Two-Dimensional Material MoSi2N4
- Bi9P2O18Cl:Phase Transition and Hydrogen Production by Photocatalytic Water-Splitting
