Solvothermal synthesis of TiO2@MIL-101(Cr) for efficient photocatalytic fuel denitrification
2022-05-04LUYiLIANGRuowenYANGuiyangLIANGZhiyuHUWeinengXIAYuzhouHUANGRenkun
LU Yi ,LIANG Ruo-wen,3 ,YAN Gui-yang ,LIANG Zhi-yu,3 ,HU Wei-neng ,XIA Yu-zhou ,HUANG Ren-kun,3,*
(1. Province University Key Laboratory of Green Energy and Environment Catalysis, Ningde Normal University, Ningde 352100, China;2. State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou 350002,China;3. Fujian Provincial Key Laboratory of Featured Materials in Biochemical Industry,Ningde Normal University, Ningde 352100, China)
Abstract: Solvothermal synthesis technique is an effective method to create composite materials. In this paper, a series of TiO2@MIL-101(Cr) were prepared by the solvothermal method for photocatalytic denitrification of pyridine in fuel under visible light irradiation. The products were characterized by XRD, FT-IR, SEM, TEM, BET, DRS and ESR. The result shows that 20%TiO2@MIL-101(Cr) has high catalytic activity, the pyridine removal efficiency reaches values as high as 70% after irradiation for 240 min. Finally, we obtained the possible mechanism of photocatalytic denitrification according to the HPLCMS spectrometry results analysis.
Key words:photocatalytic;fuel;denitrification;MIL-101(Cr);TiO2
Crude gasoline is a necessity for human survival.And with the improvement of oil exploitation technology[1], more oil can be exploited and used.However, there are many kinds of nitrogen-containing compounds (NCCs) in fuel, such as pyridine derivatives and pyrrole derivatives[2−4]. These NCCs will be released into the atmosphere in the form of nitrogen oxides by burning[5]. It will seriously damage our air environment and our health[6]. Therefore, the selective removal of NCCs from crude gasoline has become a global research hotspot.
Metal organic frameworks (MOFs) are classes of organic coordination polymer materials, which are regarded as an important class of materials due to their controllable structure, pore size and high specific surface area[7−9]. Because of its unique structural characteristics, MOFs show excellent performance in various adsorption based applications, such as gas sensing materials[10−12], gas storage materials[13−15],catalytic materials[16−18], etc. For example, MIL-101(Cr)has strong adsorption performance[19], but the efficiency of catalytic fuel denitrification is relatively low. TiO2, a class of photocatalyst in semiconductor material, has excellent photoelectric and photocatalytic properties.However, it only responds to UV and it can't efficiently utilize solar light[20,21]. While MOFs often have responded in visible light. Therefore, the combination of TiO2and MOFs to form composite materials may enhance the response to visible light and improve its catalytic performance[22]. It provides a promising method for selectively removing NCCs from crude gasoline.
In this work, TiO2@MIL-101(Cr) was successfully synthesized by a simple method. The structure and properties were characterized by XRD,SEM, TEM, FT-IR, UV-vis, DRS and BET. The performance of photocatalytic fuel denitrification was tested by simulating fuel (pyridine/n-octane).
1 Experimental
1.1 Materials
Chromium(Ⅲ) nitrate nonahydrate (Cr(NO3)·9H2O), terephthalic acid, hydrofluoric acid (HF),N,Ndimethylformamide (DMF) and tetrabutyl titanate were supplied by Aladdin Reagent Co., Ltd. All chemicals are of analytical grade and used as received. Deionized water was used in all experiments.
1.2 Fabrication of MIL-101(Cr)
In a typical experiment, 1.2 g of Cr(NO3)·9H2O,0.5 g of terephthalic acid were dissolved in 15 mL deionized water to obtain a homogeneous solution. The mixture was transferred into a Teflon-lined stainlesssteel autoclave and heated at 220 °C for 8 h. The precipitate A was collected by centrifugation, then washed with ethanol for 3 times. The precipitate washed with hot DMF and hot ethanol several times,respectively. The MIL-101(Cr) was collected by a centrifugation and washed three times with ethanol,and then dried at 65 °C for 8 h.
1.3 Fabrication of TiO2
Specifically, 1 mL tetrabutyl titanate was added into 40 mL of ethanol under stirring for 30 min, then the resulting mixture was transferred into a Teflonlined stainless-steel autoclave and heated at 220 °C for 3 h. The TiO2was obtained by centrifugation, after which it was washed several times with ethanol and deionized water. The obtained white powder was dried at 65 °C for 8 h.
1.4 Fabrication of TiO2@MIL-101(Cr)
For preparation of 5%TiO2@MIL-101(Cr),54.3 mg tetrabutyl titanate and 250 mg MIL-101(Cr)was added into 40 mL of ethanol under stirring for 30 min, then the resulting mixture was transferred into a Teflon-lined stainless-steel autoclave and heated at 220 °C for 3 h. The 5%TiO2@MIL-101(Cr) material was collected by a centrifugation and washed three times with ethanol, then dried at 65 °C for 8 h.x%TiO2@MIL-101(Cr) with other TiO2ratios (xis the mass ratio of TiO2) were synthesized by similar methods.
1.5 Characterizations
X-ray diffraction (XRD) patterns were obtained using a Bruker D8 Advance X-ray diffractometer.Fourier-transform infrared reflectance (FT-IR) spectra were measured using a Shimadzu IRPRESTIGE-21 spectrophotometer. Transmission electron microscopy(TEM) and high-resolution TEM (HRTEM) images were obtained using a FEI Talos F200X instrument.Ultraviolet-visible diffuse reflectance spectra (UV-vis DRS) were obtained using a Shimadzu UV-2700 UVvis spectrophotometer. The Brunauer-Emmett-Teller(BET) surface areas of the samples were measured using an ASAP 2460 apparatus. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo Scientific ESCA Lab 250 spectrometer.
1.6 Photocatalytic performance
First, 70 mg of pyridine was dissolved in 1.0 L octane to prepare 100 μg/g simulated NCCs-containing gasoline fuel. Second, 50 mg photocatalyst and 50 mL pyridine/octane solution (100 μg/g) were put into a quartz reactor with magnetic stirring, and then the suspension was stirred in the dark for 4 h to ensure the adsorption-desorption equilibrium was reached. Third,the suspensions were irradiated using a 300 W Xe lamp(PLS-SXE 300), which equipped with a UV-cut filter to cut off light of wavelength shorter than 420 nm.Last, 1.5 mL of the sample was centrifuged at intervals.At selected time intervals, aliquots of the suspension were removed and centrifuged. The residual concentration of pyridine in the supernatant was monitored using a Varian Cary 60 spectrometer.
2 Results and discussion
2.1 Characteristics of the prepared catalysts
As illustrated in Figure 1, X-ray diffraction pattern of TiO2, MIL-101(Cr),x%TiO2@MIL-101(Cr)composites were investigated in the scanning range 5° < 2θ< 80°. It can be observed that the typical peaks of MIL-101(Cr) corresponded to the simulated MIL-101(Cr), suggesting the successful preparation of the MIL-101(Cr). After incorporation of TiO2, the position and relative intensity of main diffraction peaks can be indexed to the MIL-101(Cr), which indicate that the crystalline structure of MIL-101(Cr) is retained. What’s more, the typical peaks at 25.3°, 37.8°, 48°, 53.9°,55.1°, 62.7° and 68.8° correspond to the (101), (004),(200), (105), (211), (204) and (116) planes of anatase TiO2(JCPDS no.21-1272), respectively. The results indicate that the successful preparation of TiO2@MIL-101(Cr) composites. Interestingly, the characterization peaks of TiO2in 5%TiO2@MIL-101(Cr) composite is not obvious, which suggest some TiO2grow in cages while a small amount of TiO2cover on the surface of MIL-101(Cr) when the mass ratio is small. In addition,with the mass ratio of TiO2increase, the relative intensity of TiO2are enhanced on the surface of MIL-101(Cr).
The SEM images in Figure 2(a)−2(e) reveal the morphologies of MIL-101(Cr) andx%TiO2@MIL-101(Cr) (x=5, 10, 20, 50). As shown in Figure 2(a), the synthesized MIL-101(Cr) has a uniform octahedral morphology. As shown in Figure 2(b)-2(e), the number and density of the particles increase on the surface with increasingx%. It can be seen that the particles are well dispersed. In addition, the change of the amount of TiO2added to the composite has no obvious effect on the morphology of the composite.
The TEM image (Figure 3 (a)) clearly identify some of the black particles, which indicate TiO2nanoparticles are tightly wrapped around the surface of MIL-101(Cr). 20%TiO2@MIL-101(Cr) still shows octahedral morphology, indicating that the introduction of TiO2has no significant effect on the morphology of MIL-101(Cr). As shown in Figure 3(b), the lattice fringe with a lattice spacing of 0.33 nm can correspond to the (101) plane of anatase TiO2. According to elemental analysis in Figure 3(d)−3(f), C, Cr, Ti are uniformed dispersed in octahedron, indicating TiO2is uniformly dispersed on the surface and inside of the MIL-101(Cr).
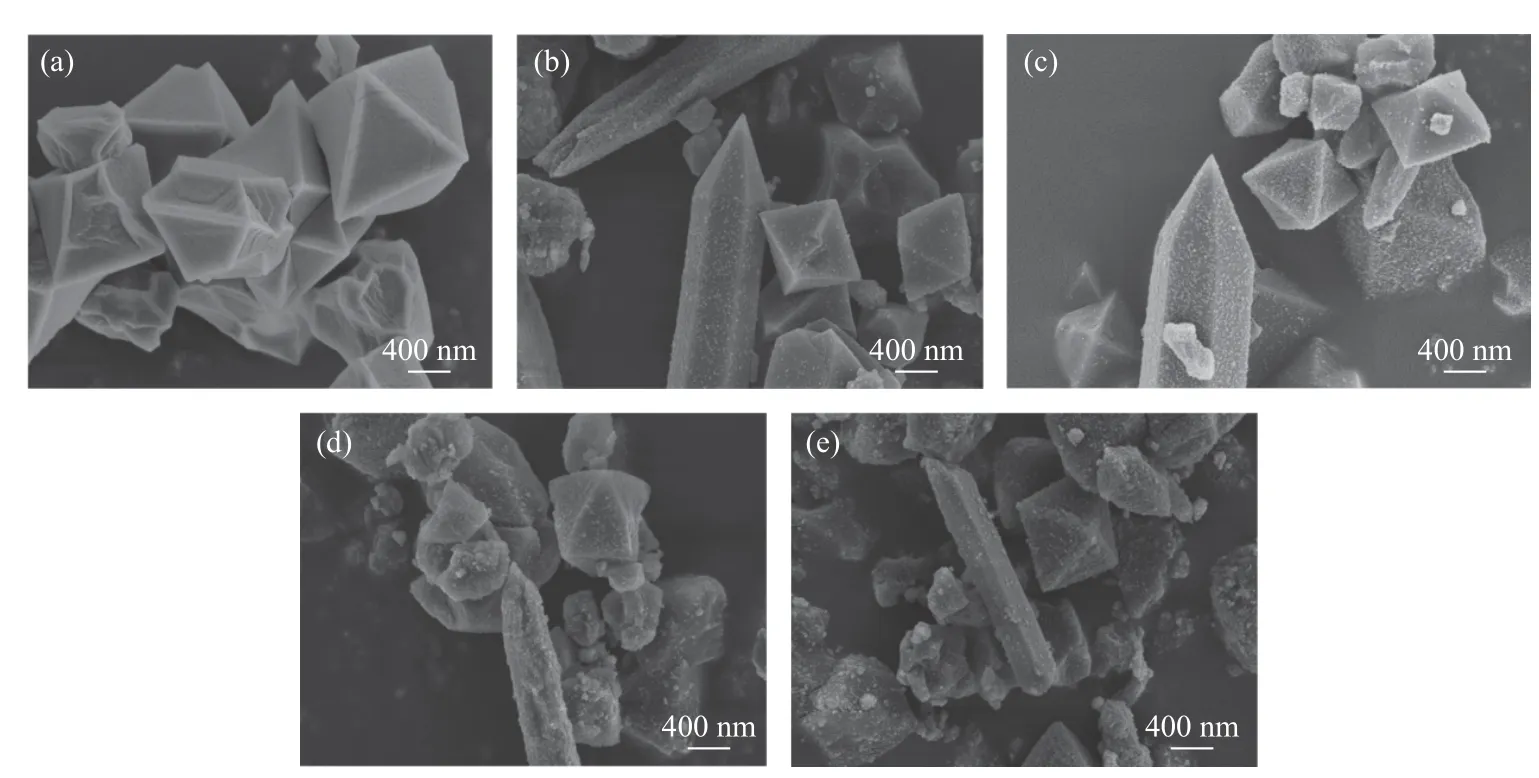
Figure 2 SEM images of (a) MIL-101(Cr), (b) 5%MIL-101(Cr), (c) 10%MIL-101(Cr)(d) 20%MIL-101(Cr) and (e) 50%MIL-101(Cr)

Figure 3 (a) TEM of 20%TiO2@MIL-101(Cr), ((b), (c)) HRTEM of 20%TiO2@MIL-101(Cr), (d) C, (e) Cr, (f) Ti
Figure 4(a) shows the FT-IR spectroscopy images for MIL-101(Cr) and 20%TiO2@MIL-101(Cr). The absorption bands at approximately 1619 and 1396 cm−1in the spectrum of H2BDC could be attributed to the O−C−O asymmetrical stretching vibration and symmetrical stretching vibration,respectively. It shows that the material contains dicarboxylate group. The band at 1015 and 748 cm−1are attributed to the C−H of benzene ring. And the band at 663 cm−1is attributed to Cr−O vibration mode.
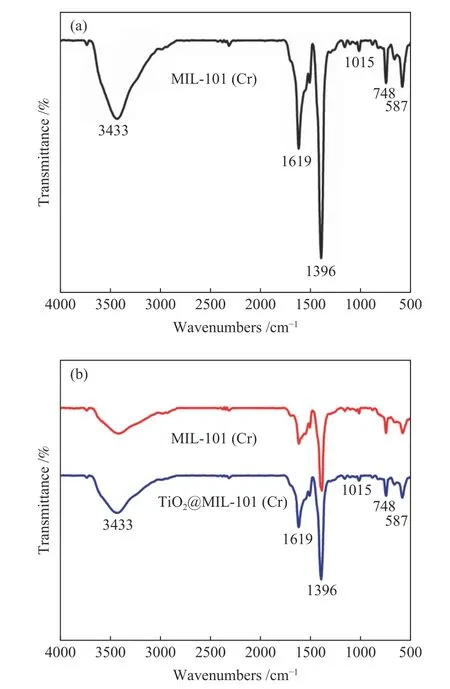
Figure 4 (a) FT-IR spectra of MIL-101(Cr) and (b) comparisonFT-IR spectra of MIL-101(Cr) and 20%TiO2@MIL-101(Cr)
In order to determine whether the structure of MIL-101(Cr) was destroyed after incorporation of TiO2, FT-IR spectra of MIL-101(Cr) and 20%TiO2@MIL-101(Cr) are shown in Figure 4(b). In the 20%TiO2@MIL-101(Cr), the typical absorption peaks of MIL-101(Cr) still exist, which indicate that the structure of MIL-101(Cr) is not damaged after incorporation of TiO2.
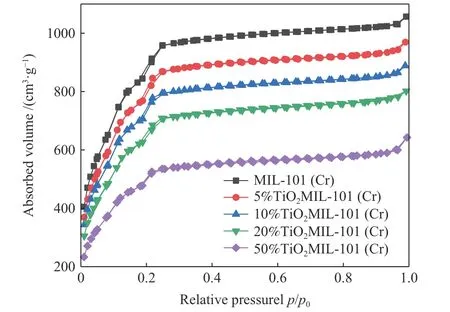
Figure 5 N2 adsorption/desorption isotherms curves of MIL-101(Cr) and x%TiO2@MIL-101(Cr) (x=5, 10, 20, 50)
The N2adsorption/desorption isotherms curves of MIL-101(Cr) andx%TiO2@MIL-101(Cr) (x=5, 10, 20,50) are shown in Figure 5. As shown in Table 1, the BET surface area and pore volume of MIL-101(Cr) are determined to be approximately 3341.1767 m2/g and 1.63 cm3/g, respectively. As the mass ratio of TiO2increase, the BET surface areas and pore volumes ofx%TiO2@MIL-101(Cr) (x=5, 10, 20, 50) decrease significantly. This is because the crystalline TiO2grow in the channel or the surface. Although the addition of TiO2contributes to the improvement of catalytic activity, the low specific surface area may limit the catalytic efficiency. Therefore, it is necessary to find the optimal TiO2addition amount.
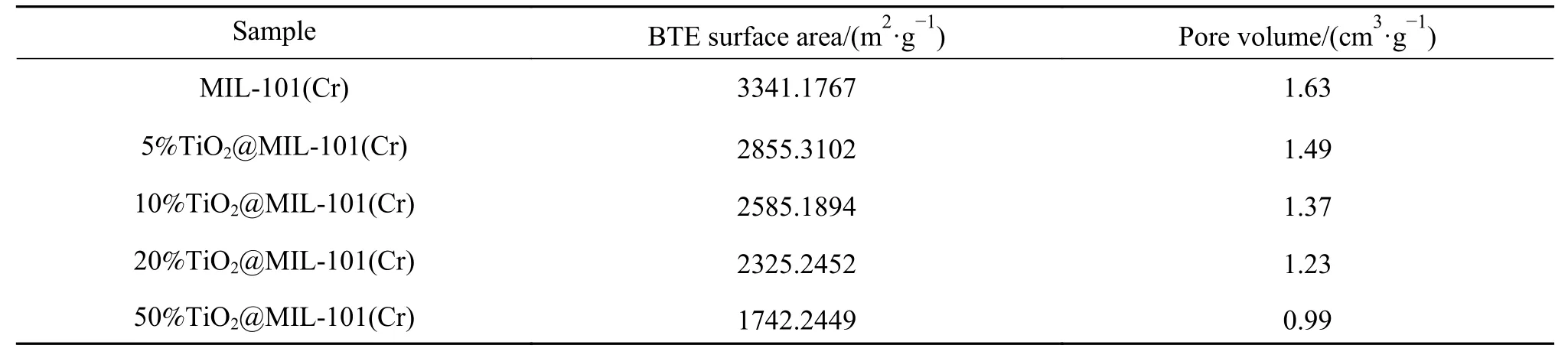
Table 1 BET surface Area, pore volume of TiO2@MIL-101(Cr) composites
Figure 6(a) shows the UV-vis spectra ofx%TiO2@MIL-101(Cr), MIL-101(Cr) and TiO2. TiO2shows an absorption edge about 400 nm. Based on the Kubelka-Munk function, the band gaps can be calculated. Obviously, the band gap of MIL-101(Cr) is about 2.42 eV. As showed in Figure 6(b), the band gaps of 20%TiO2@MIL-101(Cr) and 50%TiO2@MIL-101(Cr) are 2.37 and 2.40 eV, respectively. The band gaps of the MIL-101(Cr) composite materials decrease after incorporation of TiO2, which may promote the separation of photoinduced electron-hole pairs.
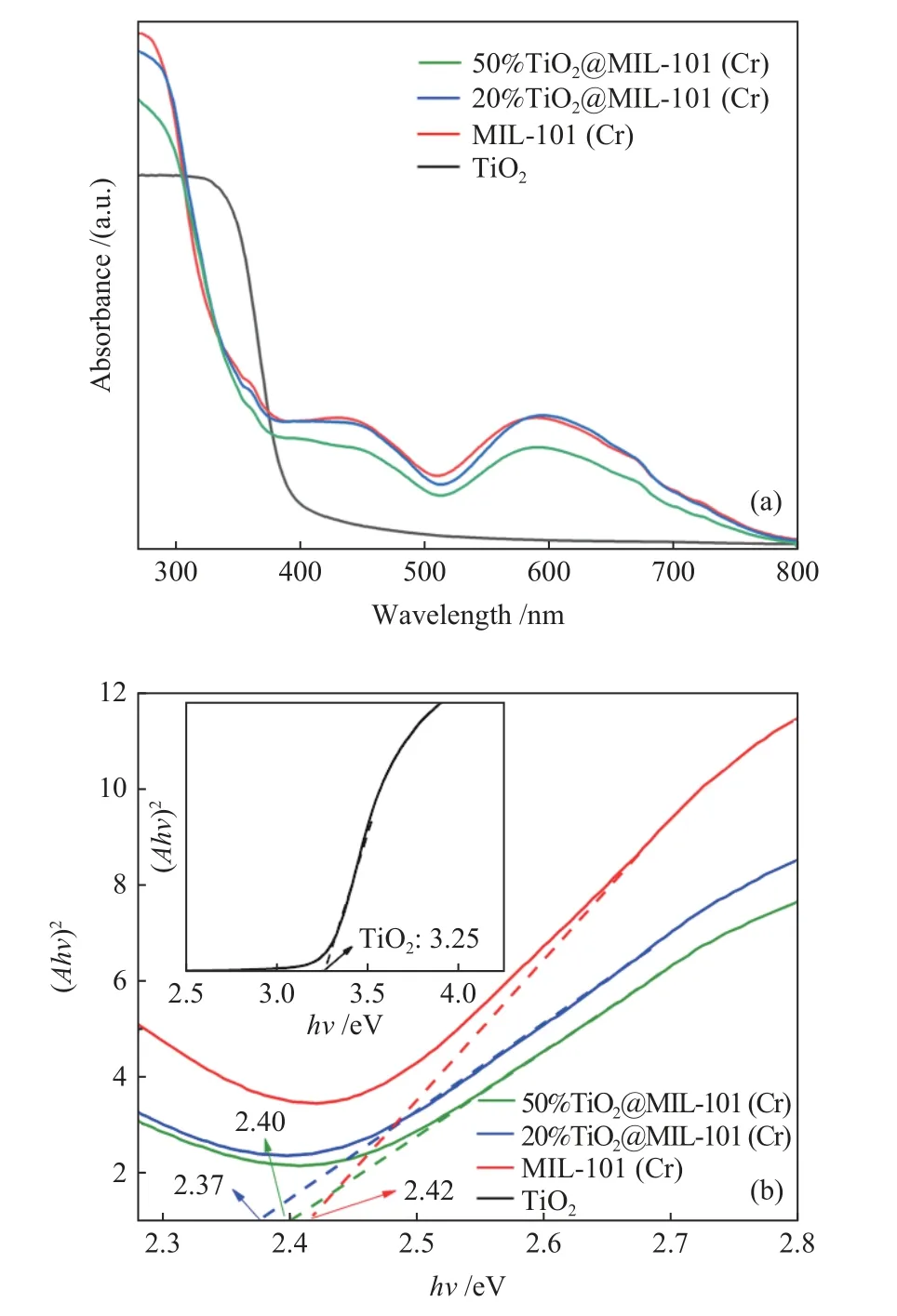
Figure 6 (a) UV-vis DRS spectra of TiO2 and x%TiO2@MIL-101(Cr) composites and (b) (Ahν)2 vs hν of (a )MIL-101 (Cr), (b)20%TiO2@MIL-101 (Cr) and (c) 50%TiO2@MIL-101 (Cr)
2.2 Photocatalytic performance
The photocatalytic activities of MIL-101(Cr) andx%MIL-101(Cr) for the denitrogenation of NCCs have been evaluated using visible light (λ≥ 420 nm). As illustrated in Figure 7(a), 20%TiO2@MIL-101(Cr) has high active of catalysis (70%) within 4 h, while MIL-101(Cr) and TiO2show no activity for the denitrogenation. To further understand the effect of TiO2for the photocatalytic activity of TiO2@MIL-101(Cr) composites,x%TiO2@MIL-101(Cr) (x=5, 10,20, 30, 40, 50) were tested by photocatalytic denitrogenation. The results show that 20%TiO2@MIL-101(Cr) has optimal active of catalysis. The reason for this phenomenon is considered that suitable proportion of TiO2and MIL-101(Cr) promote photocarriers (holes and electrons) generation. The catalytically active sites of composites are enclosed with the increasing of TiO2,which will lead to reduction in denitrogenation.
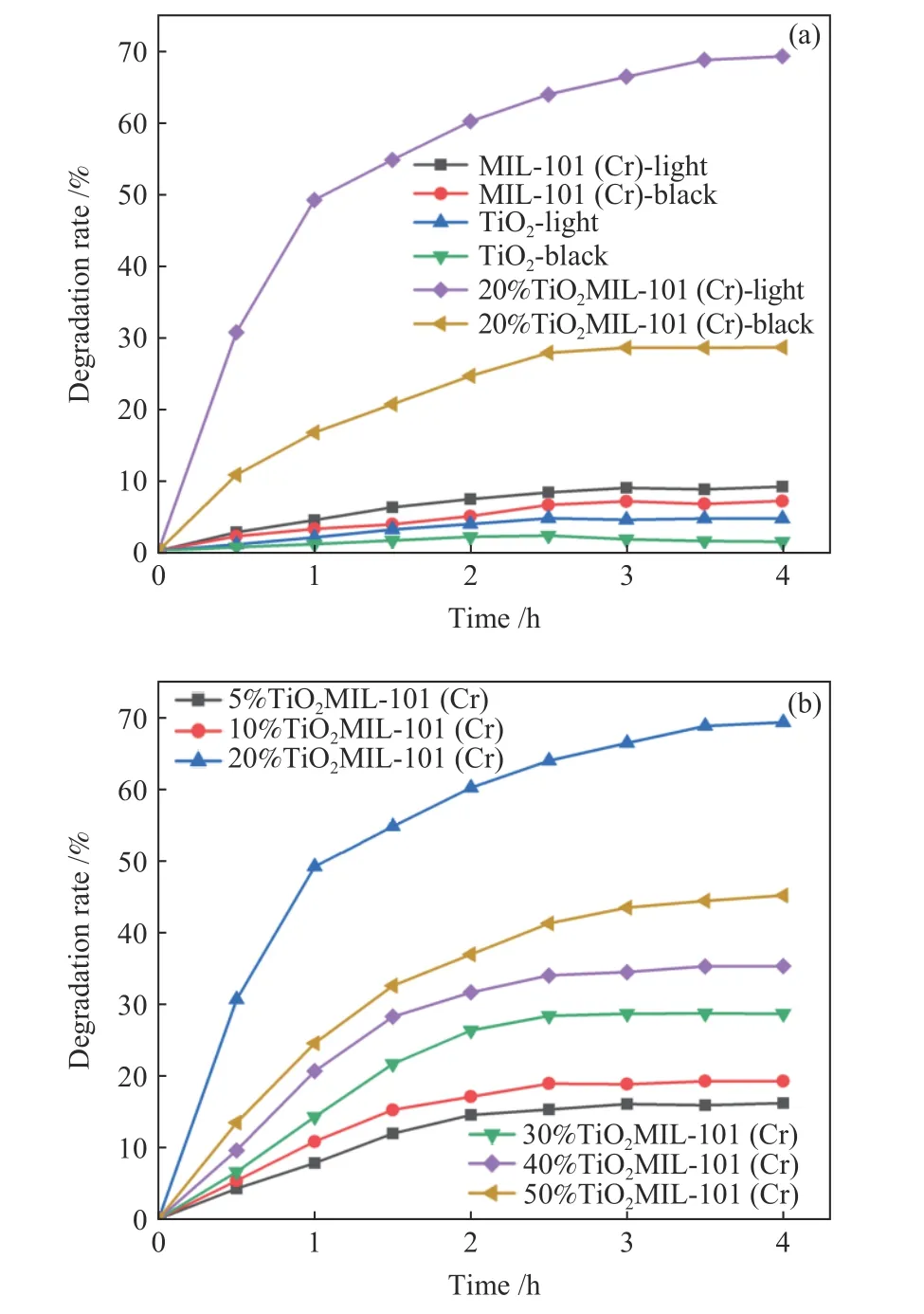
Figure 7 (a) Photocatalytic denitrogenation of pyridine over MIL-101(Cr), TiO2 and 20%TiO2@MIL-101(Cr) under visible light and black, (b) photocatalytic denitrogenation of pyridine at different content of TiO2
2.3 Photocatalytic mechanism
The generation and migration of photogenerated carriers under light irradiation was studied by photoelectric chemistry experiment. As shown in Figure 8(a), the photocurrent intensity of 20%TiO2@MIL-101(Cr) is higher than MIL-101(Cr), indicating that the lifetime of photogenerated electron-hole pairs of 20%TiO2@MIL-101(Cr) is higher than MIL-101(Cr). Moreover, to better understand the excellent charge carrier transmission performance of 20%TiO2@MIL-101(Cr), EIS Nyquist plots was obtained.Compared with MIL-101(Cr) and 20%TiO2@MIL-101(Cr), the semicircle of 20%TiO2@MIL-101(Cr) is smaller, which indicate the significantly increased charge-carrier transfer of 20%TiO2@MIL-101(Cr)compared with MIL-101(Cr) (Figure 8(b)). This result is in good agreement with the photocurrent response,indicating that the addition of TiO2can effectively improve the separation of charge and carrier.
The HPLC-MS spectrometry results are displayed in Figure 9. After 4 h irradiation, the peak intensity of pyridine at approximatelym/z= 79.04 is greatly decreased, which implying that the denitrogenation of pyridine is successful. In addition, two new peaks at 46.03, 85.04 gradually appears, indicating that pyridine has been transformed into C4H4O2and CH3NH2, which are the protonated intermediate products. The most reliable and direct method for investigating reactive species is ESR. In the presence of 20% TiO2@MIL-101(Cr), it is difficult to detect the signal of DMPO-·O2even after 5 min of irradiation, implying that ·O2is not the main active species during this reaction (Figure 10(a)). The characteristic quartet peaks of the DMPO-·OH adduct can be easily detected after visible light irradiation for 5 min (Figure 10(b)), indicating that·OH radicals have been generated. As illustrated in Figure 10(b), the ESR signal of TEMPO decreased,confirming the production of photogenerated holes.Furthermore, as shown in Figure 11, the possible denitrogenation pathway of pyridine are consistent with the information reported in one of our previously published papers[23−25].
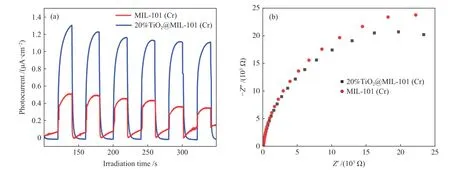
Figure 8 (a) Transient photocurrent responses of MIL-101(Cr) and 20%TiO2@MIL-101(Cr), (b) Nyquist impedance plots of MIL-101(Cr) and 20%TiO2@MIL-101(Cr)
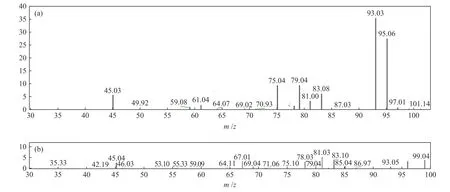
Figure 9 High-performance liquid chromatography profiles of pyridine after different irradiation times: (a) 0 h and (b) 4 h
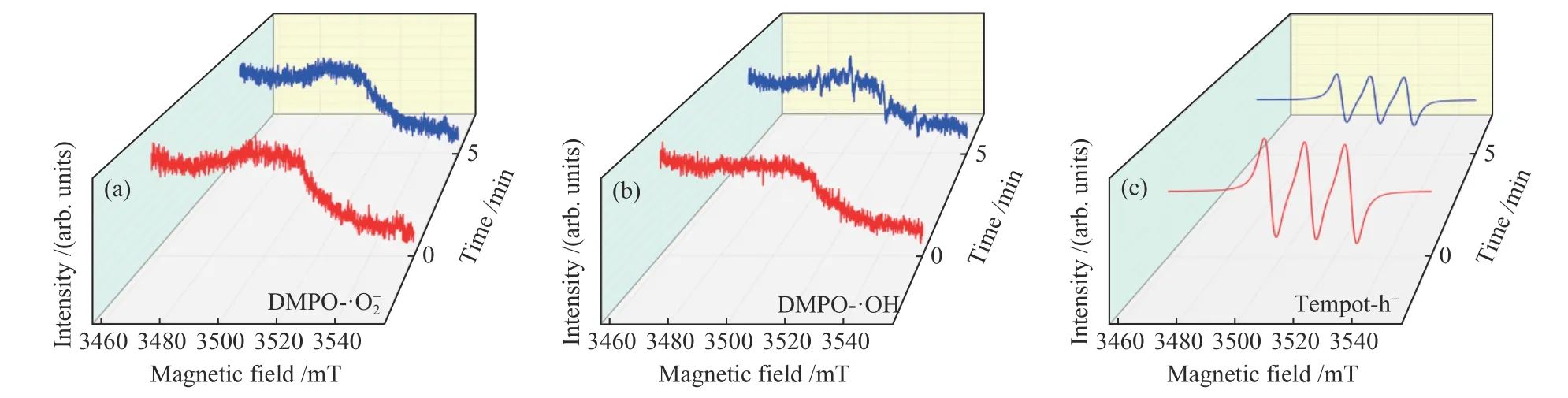
Figure 10 Electron spin response spectra of various radical adducts
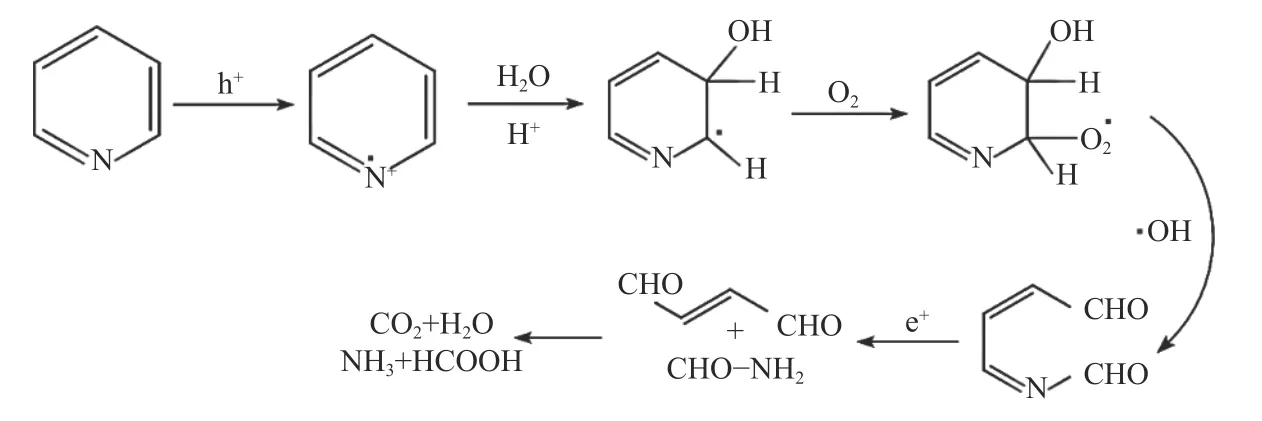
Figure 11 Possible denitrogenation pathway of pyridine
3 Conclusions
Solvothermal synthesis of the MIL-101(Cr) has high surface area (3341.1767 m2/g) and pore volume(1.63 cm3/g). The results show that the photodenitrogenation performance of MIL-101(Cr)increase greatly owning to composite with TiO2.20%TiO2@MIL-101(Cr) has the highest catalytic activity, and the denitrogenation ratio can reach 70%within 4 h under visible light. The excellent photocatalytic performance can be attributed to the introduction of inorganic semiconductor TiO2into MIL-101 (Cr), which increases the generation and mobility of photocarriers. This work provides a new perspective for the realization of efficient photocatalytic performance.
