Progress of vanadium phosphorous oxide catalyst for n-butane selective oxidation
2022-04-27MuhammadFaizanYingweiLiRuiruiZhangXingshengWangPiaoSongRuixiaLiu
Muhammad Faizan,Yingwei Li,Ruirui Zhang,Xingsheng Wang,Piao Song,2,Ruixia Liu,2,*
1 Beijing Key Laboratory of Ionic Liquids Clean Process,State Key Laboratory of Multiphase Complex System,Institute of Process Engineering Chinese Academy of Sciences,Beijing 100190,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
Keywords:N-butane selective oxidation Oxidation of light alkanes Vanadium phosphorus oxide (VPO)Maleic anhydride
ABSTRACT The utilization of lighter alkanes into useful chemical products is essential for modern chemistry and reducing the CO2 emission.Particularly,n-butane has gained special attention across the globe due to the abundant production of maleic anhydride (MA).Vanadium phosphorous oxide (VPO) is the most effective catalyst for selective oxidation of n-butane to MA so far.Interestingly,the VPO complex exists in more or less fifteen different structures,each one having distinct phase composition and exclusive surface morphology and physiochemical properties such as valence state,lattice oxygen,acidity etc.,which relies on precursor preparation method and the activation conditions of catalysts.The catalytic performance of VPO catalyst is improved by adding different promoters or co-catalyst such as various metals dopants,or either introducing template or structural-directing agents.Meanwhile,new preparation strategies such as electrospinning,ball milling,hydrothermal,barothermal,ultrasound,microwave irradiation,calcination,sol–gel method and solvothermal synthesis are also employed for introducing improvement in catalytic performance.Research in above-mentioned different aspects will be ascribed in current review in addition to summarizing overall catalysis activity and final yield.To analyze the performance of the catalytic precursor,the reaction mechanism and reaction kinetics both are discussed in this review to help clarify the key issues such as strong exothermic reaction,phosphorus supplement,water supplement,deactivation,and air/n-butane pretreatment etc.related to the various industrial applications of VPO.
1.Introduction
The oxidation of light alkanes are durable for producing various valuable chemicals and be recruited in many industrial applications.In this scope,n-butane catalytic selective oxidation to maleic anhydride(MA)is the first and so far most effective industrial process.Vanadium phosphorus oxide catalysts (VPO) exhibits the peculiar competency to activate C-H bond and selectively oxidize n-butane to MA [1,2],is commercially well-established industrial catalyst since 1980s,before that merely benzene synthesis was available for MA production.The production capacity of MA around the world in 2023 is expected approximately 3.43 million metric tons;more or less 70 percent MA production obtained from n-butane oxidation,remaining capacity is still achieved from benzene synthesis[3].The trend has shifted from benzene to n-butane oxidation due to the formation of more by products that causes the high budget-value and pollution concerning environmental issues because of its higher toxicity.Further,its moderately lower feed concentrations(<2%(mol))exploited the process stream flammability [4].Because of its industrial significance and scientifically integrating prospects,noteworthy research papers and patents (a few hundreds) have been accounted for selective oxidation of nbutane to MA from VPO catalysts.
The exploration of VPO catalyst is beginning since 1960s,researchers initially used the support of vanadium catalysts for benzene oxidation,was the only option for the manufacturing of MA as an industrial process.After that,the addition of phosphors ligand into vanadium substrate termed as V-P-O complex brought an excellent catalytic performance for producing MA as compared to previously reported methods.The different metal dopants are contributing the unique physiochemical properties of VPO catalysis.For example,doped with 1% Co has positive attitude for phtalic anhydride productivity,meanwhile MA selectivity is mitigated [5].On the other hand,doped with 1% Fe generates least selectivity towards phtalic anhydride and enriched in CO and CO2stream [6].The o-xylene selective oxidation of phthalic anhydride produced the maleic anhydride along with CO and persistent oxidation products.However,available literature inaugurates the intimate aspects affiliated with different molecular-level sagacity,are still a hot issue [7,8].The performance of VPO catalyst is based on active phase,which is crucial for reaction kinetics and mechanism.In such condition,vanadium oxidation state is closes to +4.Meanwhile,the presence of phosphorous at the VPO surface is directly engaged in the catalysis reactivity.The purpose of n-butane activation is to proceed the hydrogen abstraction mechanism and thus considered as rate-determining step.
The VPO catalyst has quite complex structures with the existence of nearly fifteen different phases[9],its surface morphology,phase composition,P/V ratio,and acidity are highly dependent on synthesis,and has a considerable effect on VPO catalysis performance.The main active phase component of VPO catalyst is vanadyl pyrophosphate (VO)2P2O7,which commences from the main precursor i.e.VOHPO4.0.5H2O (HVP),through topotactic transformation under the activation conditions (Fig.1) [10].This catalytic conversion is somewhat convoluted by considering the reaction which consists of 14 electrons transfer and three oxygen insertions that bring about the presence of consecutive reactions as well as parallel reactions [11].Different research groups found that the different phases existed simultaneously and presented the various models of selective and active VPO surface sites and phases for nbutane oxidation[12–14].The VPO catalyst experience many challenges such as small specific surface area(~20 m2.g-1)and low MA selectivity (60%–65% (mol)) [15,16].Consequently,it is remonstrance to attain higher MA selectivity yield with maximum n-butane conversion and engage the tremendous attention [17].

Fig.1.The evolution scheme proposed for the VPO catalyst with respect to time[18].
The evolution of catalyst performance is based on active phase.To enhance the activity of active phase,various preparation methods such as electrospinning,ball-milling,hydrothermal/solvothermal,barothermal,ultrasound,microwave irradiation,calcination,sol–gel method were employed to synthesize the better precursor and improved the physicochemical properties such as surface area and acidity,content of lattice/surface oxygen,crystal phases,P/V ratio,and redox V+4/V+5ratio.In current review,we described the above mentioned factors in the views of overall catalytic performance.In addition to this discussion,we also elaborate the industrial issues such as strong exothermic reaction,phosphorus supplement,water supplement,deactivation,and air/n-butane pretreatment,which are closely related to that catalytic yield.
2.Strategies for the Preparation of VPO
It has far widely recognized that the preparation approach of VPO catalyst can promptly affect the oxidation state,the Lewis acid density,the structure morphology,and the location of the vanadium species.The catalytic behavior of VPO catalyst is observed from the conversion rate and yield of selective product.The method of preparing the VPO precursor is the curial step for the activation of final VPO and then affects its catalytic performance.
2.1.Common methods
As mentioned before the main precursor HVP is the catalytic precursor of active phase (VO)2P2O7.Generally,it involves the reacting substrate of vanadium (V2O5) and phosphorus (H3PO4)with the assistance of reducing agent in the presence of solvents.
2.1.1.Aqueous phase method
Initially,aqueous media used for preparing catalyst precursor,generally utilizing hydrochloric acid as reducing agent and generally alluded as the VPA course (Fig.2):

Fig.2.VPO precursor is prepared from aqueous phase method.
In the beginning,aqueous media is used to prepare the precursor [7].HCl is replaced by different reducing agents such as phosphorous acid,lactic acid and oxalic acid.Extensive efforts have been devoting to explore the alternative sources of vanadium,for example,NH4VO3and VCl3/V2O5introduced as vanadium source instead of vanadium pentoxide for main precursor phase of precursor.The reduction of vanadium pentoxide can be achieved in the presence of cetyltrimethylammonium chloride and phosphoric acid at higher temperature,this strategy has been introducing for the exploration of the ideal precursor.Similarly,in direct reaction of H3PO4and V2O5are followed the same strategy.
2.1.2.Organic phase method
Aqueous medium is replaced with alcohol for achieving large surface area (the VPO route),additionally;alcohol is providing the reducing agent characteristics,such as aliphatic alcohol could be utilized for producing the precursor phase that leads the lamellar morphology,whose basal plan configuration is identical with precursor phase and carbon length of starting alcohol effects the d(001) values.In one-pot alcoholic route,the most common alcohol is isobutanol,further available common synthesis solvents are benzyl alcohol and/or isobutanol for VPO catalyst [2,19–22].Alternatively,either ketones or aldehydes functional groups are applied then reactant product would be VO(H2PO4)2by means of interaction between V2O5and H3PO4(Fig.3) [23,24].

Fig.3.Organic phase method is used to prepare the VPO precursor.
The reduction of VOPO4.2H2O precursor occurred in the presence of both aqueous and alcohol medium named as VPD route.Both long/short chain alcohols are helpful for the preparation the VPO catalyst.HVP is prepared from the VOPO4.2H2O precursor.The whole process is initiated by the reaction of H3PO4and V2O5,and then reduced it with alcohol for better yield (Fig.4).

Fig.4.Two-stage organic phase method is used to prepare the VPO precursor.
Various glycols such as 1,4-butandiol,1,3-propandiol and 1,2-etandiol have the capacity to change the morphology.The organic content of the precursor is related with the ‘aspect ratio’’ of the crystal.Crystallographic stacking is disturbed when large content of organic compounds is retaining.In aqueous and organic phase methods,the role of solvent and reducing agent is very crucial to proceed the reaction.
2.1.3.Role of solvent and reducing agent
Water and hydrochloric acid are abundantly used as solvent and reducing agent in the preparation of VPO precursor.VPO precursor contains the mixture of vanadium ions,which represent the V+3,V+4,and V+5oxidation state.Average oxidation state is an important characteristic of VPO precursor that can be presented with the help of this equation.

It can be assumed that reducing agent played a decisive role in average oxidation state of vanadium while isolate the VPO precursor.Other reported reducing agents are:isobutanol,allyl alcohol,glycerol,crotyl alcohol,allyl alcohol+t-butyl alcohol,HC1+H2C2O4and N2H .2HC1.Large specific area has also been alleged in the presence of benzyl alcohol and isobutanol being a solvent and reducing agent.The average oxidation state of the precursor arbitrated from the strength of the reducing agent.Moreover,reducing agent is also helpful in the determination of reaction temperature at which activated catalyst perform their catalytic actions.
The active catalyst,mainly its preparation method includes the hydrochloric acid digestion of phosphoric acid (H3PO4) and vanadium pentoxide (V2O5) in the presence of non-aqueous solvent or aqueous solvent,such as isobutanol,tetrahydrofuran (THF) or methanol followed by removing the solvents then final catalyst precursor are ready for selective oxidation of n-butane.In ballmilling process,cyclohexane is used as solvent in the absence or presence of organic comb graft copolymers as dispersants for increasing the BET surface areas up to 55 m2.g-1or even more.Different pore modifying agent for instance methylcellulose,glycol,and citric acid,which increases the pore volume from 0.8 to 1 pm are utilized[7].Final VPO catalyst is retained via evaporation of the solvent.In literature reported solvents are:water,ethanol,isobutanol,allyl alcohol,crotyl alcohol and allyl alcohol with tbutyl alcohol.The utilization of organic solvent supports the large surface area,apart from large surface area utility its catalytic performance does not always improve simultaneously.The reaction condition at which final precursor is retained and isolated via filtration and nature of the solvent probably influence the morphology and phase composition of the catalyst.
To date,enormous endeavors have been devoted to acquiring the knowledge about the nature of the active phase of the catalyst.This knowledge is ascribed in physiochemical properties of active phase.Phase properties are predicted from the presence of valance state of vanadium (V+3,V+4and V+5),adsorbed and lattice oxygen,acidity,P/V ratio,redox V+4/V+5ratio,etc.Additionally,surface area and structural configuration such as morphology always have impact on the performance of developed precursor.The effective catalyst needs intensive investigation and proper consideration during synthesis because it will affect the physicochemical properties of VPO precursor and eventually it will have influenced on final yield.
2.1.4.Effect of the starting vanadium species
The VPO precursor has been used for developing the active phase,which have more vanadium species.The precursor is usually utilized the V2O5substrate,however,other vanadium substrate such as NH4VO3could be used during the preparation of precursor[25].The VPO precursor can be prepared in the presence of N2,O2or air(via calcination process)and developed the so many precursors such as NH4HVPO6,(NH4)2[(VO2)2C2O4(HPO4)2∙5H2O]and NH4(VO2)2PO4[26].These precursors especially have following phases i.e.VO(PO3)3,VO(PO3)2or (VO)2P2O7.In addition to that VOSO4,VO[OCH(CH3)2]3and VO(acac)2have been used as vanadium source to develop the mesostructured VPO phases[27],they were totally relying on their activation conditions and conditioning procedure [26].
Researchers found that prepared VPO precursor exist in fifteen different phases such as α1-,α2-,β-,δ-,γ-and X1-VOPO4etc.,which have specific metal oxides.For instance,VOPO4.2H2O precursor is prepared from POCl3and VOCl3in the presence of aqueous and nitrogen stream inside furnace and then powder form of VOPO4-.2H2O was retained [28].Afterwards,it will transform into α-VOPO4and β-VOPO4phases in the presence of nitrogen environment (via calcination process).Finally,active phase of catalyst is produced being initiating the in-situ activation [28].This process is helpful for those reagents,which are more convenient in gasphase reactions.
2.1.5.Modification of VPO via promoters
More efficient and reliable catalysts should be synthesized in selective oxidation of n-butane for producing the higher yield of MA at optimized reaction conditions.Many researchers continuously keep investigating novel preparation methods for the exploration of ideal VPO precursor.These methods include the combination of co-catalyst and mixed metal promoters as a metal dopant.Various structure-directing agents and templates are utilizing to reform the bulk VPO catalyst characteristics.Generally,inorganic metal and organic chemicals are usually introduced as electronic and structural promoters during the synthesis of VPO to improve its catalytic performance.
2.1.5.1.Inorganic metal promoters.Hutchings et al.first time introduced the concept of doping metal promoters,in which they regulates the surface chemistry characteristics [7].In the light of this contention,VPO catalysts doped with a wide range of metal cations have been broadly explored such as Zn,Ag,Mg,Bi,Ni,W,Co,Fe,Zr,Cr,Al,Cu,Mo,Nb,Ce etc.to improve the catalytic performance through metal-oxide(P2O5,Nb2O5,WO3etc.),and nitrate and chloride salt (Table 1) [2,10,17,31,37–40].The selectivity trend of MA has been disclosed that electronegativity properties have been affected due to different type of metal promoter.Guliants et al.have been observed the selectivity trends as Zr Table 1 The characterization results of various metal dopants summarized as a promotional effect based on the performance of VPO precursor for n-butane selective oxidation Table 2 Supposed reaction steps in n-butane oxidation to MA and described the polyfunctional nature of VPO precursor [78] K Y.H.Taufiq-Yap et al.,[38] explored the promoter effect of different metal dopants on active phase.They found that characterization results of temperature programmed reduction (H2-TPR)shown that particular dopants have the capabilities of removing the induced oxygen content through V+4/V+5peaks.Different dopants also have influenced on crystal morphology.The different dopants illustrated the distinguish morphology and surface area. 2.1.5.2.Organic chemical promoters.Organic macromolecules can perform as matrixes,module,cooperative modifiers and affect the nucleate process to assist morphological control.The control of crystal growth mechanism and nucleation development process implicates the special structures,eventually influencing their performance.Structural promoters were normally exploited to extract excess phosphorus and obstruct the development of undesired phases.Various researchers have argued that it is possible to modulate the structure and morphology of the catalyst following through synthesis procedures of the precursor [42].Meantime,synthetic polymer e.g.polyethylene glycol (PEG) has been employed as template agent in the synthesis of VPO catalyst to idealize the crystal formation for achieving high surface area(~45 m2-.g-1)) [43].Moreover,the addition of cationic,anionic or primary alkylamines such as miristyl trimethyl ammonium bromide(MTAB) [44],cetyltrimethyl ammonium bromide (CTAB) [45],cetyltrimethyl ammonium bromide [46],and sodium hexadecane sulphonate [46],and alkyltrimethyl ammonium surfactants (C12-C16) [47] as structure-directing agents or surfactant directs the hexagonal meso-phases or cubic-phases in the synthesis of VPO catalysts to develop the catalytic performances [48].For example,Carreon et al.introduced the hierarchical design of macro-porous vanadium-phosphorus-oxide phases (macro-VPO) as a structuredirecting agent through monodisperse polystyrene sphere arrays,which is experiencing the 3D cluster structure of spherical voids.Surprisingly,the VPO phase exerted the high surface area 75 m2.g-1) and the preferred macro-porous configuration [49].Even more,high surface area of microporous VPO phase(~250 m2.g-1) could be successfully developed while utilizing decyltrimethyl ammonium bromide as a surfactant,etc.[44]. 2.1.5.3.Ionic liquids and deep eutectic solvent.Ionic liquids(ILs) are composed of organic cations and inorganic/organic anions and they exhibit the unique properties,such as excellent dissolving capacity,highly designable by changing the combination of cation and anions have been develop for specific applications [50–52].Our research group first time explored the novel promoters such as Iron-based-ILs as electronic and structure promoter for n-butane selective oxidation.The Fe-ILs i.e.1-octyl-3-methylimidazolium tetrachloroferrate ([OMIM]FeCl4)and 1-butyl-3-methylimidazolium tetrachloroferrate ([BMIM]FeCl4) are full enhanced the development of active phase and played an important for the formation of structure morphology for achieving the higher MA productivity [2].In addition to that various other ILs such as 1-butyl-3-methylimidazolium hexafluorophosphate([BMIM]PF6) [53] and polyoxometalate-ionic liquids (POM-ILs)[54]have already been employed for the investigation of synergistic interaction of cation and anions of VPO catalyst during synthesis(Fig.5).We aforementioned that phosphorus is vital for overall VPO catalytic performance,in order to explore the role of phosphorus into VPO,we utilized the phosphorus based ILs to exploit the promoted and oriented activity of phosphorus [19].In addition to that,ILs have capability to exfoliate the crystalline nanosheets of VOPO4∙2H2O and then can be utilized into various catalytic application and green-energy storage devices[55],which could be helpful for modern world energy requirements [56–58] because ILs shows exclusive compatibility with VPO substrate. Fig.5.Polyoxometalate-ionic liquids (POM-ILs) is synthesized for VPO precursor.Reprinted with permission from Ref [54].Copyright 2016 Elsevier B.V. Deep eutectic solvent(DES)as emerged class of ILs composite as beginner and green solvents has already been utilized in enormous fields [41].We already explored the green and effective promoter DES of choline chloride/oxalic acid (ChCl/OA) for the preparation of VPO catalyst to synthesis the maleic anhydride from selective oxidation of n-butane[20].In detailed investigation we found that DES performs the multifunction such as structural modifier,crystal induced agent,assisting the deposition of single-crystal structure at precursor surface and topological transformation to the singlecrystal active phase under the activation conditions accomplishing the disintegration of DES.We developed the ‘‘rose-like plate”and‘‘stack-like plate”modified structures of precursor.Surprisingly,ChCl/OA DES inhibit the redox characteristic and chemical state;consequently improve its catalyst performance (Fig.6).This green and high-efficiency modifier(DES)are directing the structural promoter and electronic promoter,corroborating boost the selectivity or/and activity of VPO catalyst. Our researcher group further investigation and explored that the biomass-based DES.DES-choline chloride (ChCl) with glucose(GLU)or different metal salts are used for assisting the preparation of VPO precursor[41](Fig.7).Importantly,the developed DES-VPO catalyst is cost-effective and green-precursor as compared to conventional modifier (organic polymers and metal inorganics).The goal of achieving the high crystalline precursors is successfully attained from this procedure.Different chemical properties such as acid-base properties and valance state were significantly changed due to the presence of DES conjugates.Collectively,all these unique features of DES-VPO have been assisting the greater MA yield up to 11%.Additionally,mono-,di-,and tri-metallic salt DES are also investigated for the exploration better catalytic performance [59].Unfortunately,di-and tri-metallic DES catalytic yield are not appropriate as compared to monometallic DES due to their lower Lewis acidity and poor surface area of VPO catalyst. Moreover,the effective and green promoter,DESs-ChCl and polyols(xylitol,1,4-butanediol,ethylene glycol and glycerol)were synthesized to produce VPO precursor for selective oxidation of nbutane [22].The selectivity of MA yield is enhanced extensively because of polyols based DES promoter.ILs and its analogous DESs both have amazing potential for developing an ideal VPO precursor that would bring the revolution in the chemical industry but it needs careful consideration and proper investigation. The performance of VPO catalyst is also depended on how we synthesized the VPO precursor.The preparation method of VPO precursor is deciding the physiochemical properties of VPO catalyst.Therefore,selection of appropriate method is very important.To date,researchers have been explored the different synthesized techniques for obtaining the higher selectivity of n-butane oxidation.In this section,we summarized the all-available strategies in literature in the exploration of effective VPO catalyst. 2.2.1.Hydrothermal/solvothermal synthesis Hydrothermal technique is to produce the crystallizing substances in the presence of high-temperature aqueous solutions.The hydrothermal technique has emerged as a powerful too for the synthesis of vanadates.In a particular synthesis,various factors have an impact on crystal growth and nucleation rate of final products such as precursor,temperature,pH value,starting concentration and reaction time.Zhibin Zhang et al.,focus on solubility factor of initial reactants.They employed the reactant i.e.NH4VO3and confirmed that V+5species were entirely reduced into V+4species.In mixed valance species(V+4and V+5species),catalyst are formed due to the replacement of insoluble reagent(V2O5)with dissoluble(NH4VO3)solvent[60].Further,they develop the two amine-bases VP (vanadium-phosphorus)catalyst such asH2O via hydrothermal process. Fig.6.The VPO catalyst is synthesized from deep-eutectic solvents (DES) for n-butane selective oxidation to MA.Reprinted with permission from Ref [20].Copyright 2019 American Chemical Society. Fig.7.DES prepared from choline chloride (ChCl) and glucose (GLU),have been utilized for the synthesis of VPO precursor then calcined it for the evaluation of VPO catalytic performance.Reprinted with permission from Ref [21].Copyright 2019 American Chemical Society. Ali et al.,first time introduced the novel one-step solvothermal process to synthesis the well-defined crystal of vanadyl hydrogen phosphate hemihydrate (HVP),through the reaction of H3PO4and V2O5in the presence of primary aliphatic alcohol (1-butanol or 1-propanol) at elevated temperature (373–423 K) and highpressure autoclave [61].Further,reaction mixtures straightway provides the precursor main phase (HVP),which is a significant commodity as commercial catalyst precursor for n-butane conversion as selective oxidation for achieving 38% yield of maleic anhydride that is almost double from conventional hydrothermal process.As compared to conventional hydrothermal process,solvothermal process is to prepare the active phase at comparatively lower reaction temperature (up to 50% reduced) and timeon-stream for acquiring equilibrium state.Importantly,reaction temperature in solvothermal condition and carbon chain length in an alcohol medium had a great influenced on physiochemical properties of final catalyst.Interestingly,a liner relationship between n-butane catalytic conversion and surface area of catalyst is obtained. Catalyst precursor HVP was also prepared from solvothermal technique via C2-C4alkanoic alcohols[62].The selective and active phase faces(100)might be controlled for n-butane conversion and selective oxidation for maleic anhydride yield.The linear alcohols(ethanol or n-butanol),and fluctuation with iso-alcohols;intercalate into metastable vanadyl-alkyl-phosphates(100)platelets,thus yield the (VO)2P2O7active phase faces (200) platelets.Iso-alcohol has least potential than linear alcohol for MA selectivity at 440 °C.Ethanol-derived catalyst shows maximum yield (29.5%)and highest n-butane conversion (72%) with the employment of smallest platy crystallites of active phase. The barothermal synthesis is the combination of organothermal and hydrothermal synthesis.This process is complete without the involvement of the solvent,which is different from solvothermal synthesis because it must contain the solvent for the account of synthesis.The VPO precursor is produced form the interaction between vanadium substrate (V2O5) and phosphate substrate (H3PO4) in the presence of oxalic acid under autoclave environment where Teflon is utilized [63].They observed that HVP phases is merely generated under n-butanol medium.It will affect the surface morphology,and surface structure has been change from ‘‘rose”to ‘‘plaits”type morphology.This substantial change shows the selectivity and activity towards n-butane oxidation.Furthermore,they found that‘‘plaits”type morphology is also productive role in oxidative dehydrogenation of ethane[63].However,deep eutectic solvent is follows such pattern of reactivity for n-butane oxidation through which the modification of surface morphology is involved [20–22]. 2.2.2.Sol–gel method Sol-gel process is used to prepared the catalyst by lowering the crystalline size of the precursor and have an impact on the crystal size of active phase[64].The performance of precursor is based on the activation process.Based on this knowledge,ortho-phosphoric acid reacts with triisopropoxide oxide in the presence of aprotic solvent then dried in an autoclave chamber at high pressure.Then produced VOPO4gel has a capability to entrap the incoming molecules then alcohol is introduced for the formation of precursor,which was used in partial oxidation of n-butane [64].In the modification of VPO precursor properties,we could introduce either metal dopants,template or structural directing groups into the precursor. 2.2.3.Electrospinning Vanadium oxide cathode materials can be produced in various shapes such as nanobelts/nanowires or nanotubes/nanoneedles with the help of different techniques such as vapor transport,hydrothermal reaction,and electrospinning.Electrospinning is versatile and convenient method that is capable of preparing the ultra-long hierarchical nanowires (diameter~50 nm,length~100 nm) with quasi-one-dimensional (Q1D) structures.In some research reports,VOxis synthesize from the electrospinning technique.VOxfibers (VFs) are bounded by the solution formulation,indeed for electrospinning.The surface of electrospun composite is crucial for the formation of VOxhierarchical nanowires.As solvent,ethanol is an incredible choice instead of water in electrospinning technique due to high volatility and dielectric constant and advancing the yield of ordinary fibers.In the absence of water,anhydrous crystalline phases are observed.In order to upgrade the spinnability of the ethanolic solution,dimethylformamide (DMF)and poly(vinylpyrrolidone) (PVP) could be introduced.However,developing continuous VOxfibers is still a challenging task for maintaining the variable vanadium oxidation states V+4and/or V+5.The vanadium precursor(ammonium metavanadate,NH4VO3)is used to produce VOxnanofibers consisting of crystallinity structure and variable oxidation states V+4and/or V+5[65].In electrospinning,the nanostructure material of metal oxide is potentially active for selective oxidation.Surprisingly,electrospun process can modulate the morphology of the substrate.The prepared nanomaterial is provides excellent support for VPO catalyst,for instance,bulk mixed VPO oxide (VPO500) and VPO carbon-supported material (VCF200) showed high surface area and presence of fibrous morphology(structure)which undergoes oxidizing conditions (Fig.8) [66]. 2.2.4.Ultrasound In recent decades,chemists have discovered new synthesis technique known as ultrasound synthesis for rapidly preparing new materials and infamous as sonochemistry technique,has application in catalytic reactions,organic synthesis,engineering sciences and environmental engineering.The temptation of ultrasound irradiation is to induce the chemical reactivity and inhibit the surface reaction,the phenomena of sonication is obvious for chemical process by means of utilizing the sound energy.The procedure of newly developed VPO catalyst by sonochemical method is quite similar to conventional method,but difference is that reagent is put inside such water system,which has exposure to the ultrasound energy irradiation [67].After specific time laps,active phase will be appear,and it has major contribution for n-butane selective oxidation [68].Interestingly,catalyst preparation method is short and it will save the time as well.The structurally develop active phase via nano-catalyst is a potential candidate for n-butane partial oxidation.Nanostructure VPO catalyst is structurally tuned from nano-rod of V2O5while subjected with deionized water.The nano-scale particles are sonicated and thus tends to agglomerate the larger particles.Finally,as obtained structurally tuned VPO motif is a potential catalyst for n-butane partial oxidation [42].Bismuth doped VPO catalyst were directly sonically explored via sesquihydrate route,the resulting active phase leads to promote the average oxidation state of vanadium[59].The cavitation phenomena of ultrasound waves are favoring the crystal clusters.Its catalytic activity for alkane oxidation is boost due to Lewis acid sites,provoking the surface defects.The selection of bismuth metal is dependent on their significant contribution for MA productivity.Hence,the available literature of VPO doped catalyst could be helpful for the researchers for the development of most potent selective oxidation catalyst. Fig.8.The VPO catalysis is develop from the phosphorus-functionalized carbon fibers (P-CFs) by electrospinning technique.Reprinted with permission from Ref [66].Copyright 2016 Elsevier B.V. 2.2.5.Microwave irradiation We are familiar that preparation methods played a vital role for the catalyst performance,because of this reason,researchers have always tried to explore the new ways for synthesis the VPO catalyst.Microwave irradiation technique is quite famous in catalysis due to expedite the surface area,selectivity and activity of the catalyst [69].Generically,HVP prepared from the reaction of H3PO4(as phosphorus source) and V2O5(as vanadium source).The microwave-assisted chemistry disclosed the justified performance as compared to conventional heating process[70].The microwave heating is the triggering of this reactivity.In this method,first both reagents and solvents can absorb the microwave energy,and then induce it to heat and delivering the efficient drying and escalating the heating rate.In microwave process,active phase (V+4species)is not merely responsible for selectivity,but VOPO4(V+5)also contributing its role towards n-butane conversion[68].Various-doped VPO catalyst (Co,Mo,Nb,Bi) can be prepared using dehydrate method of microwave for the modification of their physicochemical properties [71].For example,HVP precursor prepared from microwave radiation in the presence of ethylene glycol as reducing agent and water treatment,thus final catalyst activity and performance is significantly improved and other reaction parameters such as n-butane conversion and MA selectivity are 81% and 63%respectively,are enhanced fabulously [72]. 2.2.6.Ball-milling process The ball milling procedure termed as tribomechanical is the mechanical process for the development of main precursor phase.Ball processing results in increased surface area of milled materials,when catalyst precursor is put inside the ball-mill unit,its starts the grinding of the precursor to break it down to smaller scale material and hence increase the surface area and active sites which has direct impact on catalysis activity[73].Main objective of this operation is to reduce the size of HVP crystallite(>5×10-6m to ca.3.5 × 10-8m) [74].Initially,iron balls were utilized,when found that iron balls involved in catalysis inactivity and show adverse effects on catalysis performance,iron balls were replaced with the alumina and stainless steel balls in order to avoid the deleterious of the catalyst.To date,different researchers explain the feature of this methodology.Anyhow,crystal defects normally under considered when high-energy magnitude is applied for grinding in ball mills operations.These imperfections are transmitted to the active phase substrate during precursor transformation,and thus affect its productivity.This will lead to the improvement of selectivity and activity of newly develop catalyst. 2.2.7.Supercritical fluid Alternatively,another approach has been introduced by the researchers for the development of catalysis activity and its performance towards MA yield is via super critical fluids.For example,Hutchings colleagues reported the unique route for exploring the VPO catalyst through an alcohol solution using supercritical carbon dioxide as an anti-solvent[75].Where CO2can be liquid at temperature 293 K and pressure at <42 MPa,or either supercritical fluid condition (P=7.2 MPa,T=304 K).This strategy generated the amorphous micro-spheroidal VPO phases,which have extra potent than crystalline(VO)2P2O7catalyst for n-butane selective oxidation to maleic anhydride.Besides,this novel phase approach did not require a broad actuation period to accomplish full synergist action.Anyhow,crystalline vanadyl (1 V) pyrophosphate is essential for the predominant of catalyst activity and selectivity for nbutane conversion. There are confusing and conflicting observations available in the literature to elaborate the trans-configuration features for the development of active phase starting from the VPO catalyst precursor.However,different patent literatures clarified that preparation of active phase of VPO is the crucial step for the performance of nbutane oxidation products.The available various phases of VPO is depending on different variables and various influencing factors such as time duration,temperature condition,precursor morphology,atmosphere,preparation method,added different dopants,P/V ratio,and structural defects.There are two basic strategies have been defined in order to explore the activation process of the VPO catalyst. 1.In the present case of activation process,it requires the elevated temperature more than 400 °C in presence of an inert oxygen-free atmosphere,following by introducing the mixture of reactant (n-butane present in an air):with this preparation method,a pure and refined crystalline form of vanadyl pyrophosphate is produced after the first step.For the probability of partial oxidation,products are added to the reaction mixture. 2.There was a contradiction observed in such case as compared to previous activation process.It demands the moderately high temperature below 400 °C either in the presence of single or in stage wise calcination,following by introducing the mixture of reactant (n-butane present in an air).It is observed that at specified temperature 280 °C after calcination,catalyst precursor is still retained,although captured alcohol is being removed.Eventually,the overall reaction kinetics interprets the crystal structure and enlarges the surface area. In the temperature range of 380-400°C,the VPO precursor begins to disintegrate in such phase whose characteristics resembles to an amorphous phase including V+5and V+4vanadium oxide.After that adding the reactant mixture(n-butane present in an air),the available amorphous could be dehydrated and converted to vanadyl pyrophosphate precursor or/and other oxidized phase products. Researchers perform a set of different experiments to predict the activity of the oxygen.They found that during VPO pretreatment,oxygen flow is drastically increased when lies in the temperature zone of 410–530 °C [76].They further elaborated that maximum selectivity of MA is dependent on pretreatment condition of the reactants such as oxygen pretreatment temperature is approximately 450 °C.In that pretreated temperature (450 °C)the lattice and adsorbed oxygen interact with organic complex nearly balance the number V+4species in vacant sites[76].Importantly,in pretreated temperature zone,oxygen is adsorbed at this stage,but at elevated temperature zone,adsorbed oxygen species will be desorbed.At slightly higher temperature(~500°C)selectivity of MA is further increased due to the involvement of V+5-phosphate ionic species seems to be accountable when combined with active phase catalyst [76].The presence of strong Lewis acid sites at VPO catalyst surface accommodate the adsorb oxygen and produces the more stable oxygen species and coordinately placed at surface ions of unsaturated vanadyl species [77].At higher temperature,desorbed oxygen species performs the catalytic activity for n-butane oxidation.However,at lower temperature lattice oxygen do the same activity.Hence,same chemical species found at catalyst surface even at anaerobic conditions. The HVP precursor after synthesis is converted into poor crystalline active phase,fresh and impurity phases i.e.over-oxidized V+5orthophosphate,in the presence of oxygen that altered its physicochemical properties and catalytic performance in reactant mixture while on-stream conditioning[78].The term‘‘fresh”catalyst is associated with ‘non-equilibrated’ catalyst in contrast to‘equilibrated’ catalyst that will expose in reaction conditions for substantive duration such as 30 days.A fresh catalyst is less selective but more active in n-butane conversion,specifically at high conversion,because of presence of the V+5orthophosphate impurities and readily oxidize the V+4to V+5species such as in fixed-bed reactor where lower n-butane concentration is more capable to oxidize the reactant mixtures [76]. The objective is to minimize the orthophosphate V+5impurity phases and increase their surface area in the presence of crystallize active phase and n-butane.Different characterization techniques led us providing the information about the bulk VP(V+4) phase such as different surface termination and relatively different interaction with selective planes (200) relying on applied conditioning and synthesis methods.Therefore,the development of VP (V+4) catalyst with high steady-state selectivity associated with the termination of the (200) surface planes owing to progressively disappearing the thin amorphous layer[78]. The VPO crystal structure and surface chemistry properties such as the P/V ratio,content of lattice oxygen,valence state,etc.on the VPO surface influences the C-H bond activation and lattice oxygen transformation[79,80],and their impact on physicochemical properties.Herein,the proportion of phosphorus to vanadium was observed to be significant for the phase composition of the catalyst in vanadium-phosphate composite.The P/V ratio is considerable factor to evaluate the physiochemical properties of VPO catalyst.This variable (P/V) can be helpful to explain the different features of catalyst such as determination of the phase composition of the catalyst,analyzing the redox properties e.g.reducibility of the catalyst via oxidizability and hydrocarbons or hydrogen,and distribution of vanadium species (V+4and/or V+5) in poorly crystalline or amorphous catalysts [75].The gas-phase composition,activation temperature,thermal treatment time,and P/V stoichiometry can all influence the final phase composition of catalyst.Additionally,the temperature and nature of the solvent from which is isolated the precursor via filtration can affect the phase composition of catalyst [81,82].The composition of the ideal catalyst is measured from the excess phosphate by means of HVP chemical equation.A significant phosphate content (P/V=1.5–3.0) at catalyst surface is characterized from XPS techniques.Bulk phase compositions of VOPO4and (VO)2P2O7phases,as characterized by spectroscopy,diffraction,and microscopy [75].The overall catalyst performance(the MA selectivity,the MA yield and n-butane conversion) were affected due to the change phase composition and the impact were eminent while P/V ratio was greater than one [81,82]. The oxidation of hydrocarbons involves the surface chemistry of the metal oxide catalyst with the aid of lattice oxygen atoms.The involvement of lattice oxygen in reactivity is very obvious [83].Lattice oxygen character is controversial such as strongly bonded lattice oxygen is required in selective oxidation,which has a nucleophilic nature but weakly bonded of lattice oxygen is needed for complete oxidation,which has an electrophilic nature [84].However,no one can exempt the role of lattice oxygen in preliminary conversion to more reactive species i.e.O-radical species [85].Subsequently,the surface vacancies are re-oxidized by gaseous O2,the fundamental steps of this process in which electrons are mentioning in order to explore the redox behavior are demonstrated as follows,(Fig.9) [86]. An inferior rate expression is predicting in the view of above mention discussion,considering that vacancies are occupied at fixed position on the surface.Hence,2□and 2□-2deliberated as single unoccupied and occupied surface site,the following form of expression is derived [87]. At VPO surface,three types of lattice species are present with respect to their reactivity.The kinetics of lattice oxygen is hard to generalize in order to illustrate the clear picture of the activation chemistry at oxide surfaces.In gas phase,oxygen species establish an equilibrium with other oxygen species e.g.adsorbed oxygen and lattice oxygen.Substantially attaining the O-2(lattice oxygen)oxidation state by adding electrons while syngeneic interaction with surface ions.For instance,O-2species is formed when V+4ions interact with the precursor O2(adsorbed oxygen),where vanadium ions is placed to the antibonding orbital(Fig.10)[81].The presence of an oxidant either as lattice oxygen ion or as adsorbed oxygen species may be confined by the means of heat and mass transfer restriction.The presence of water content could inhibit the gasphase activation without affecting the utility of lattice oxygen[88].In some cases researchers considered the lattice oxygen for oxidation process in the absence of oxygen feed[89].The adsorbed oxygen species has more significance as compared to lattice oxygen of active phase(Fig.11)[8].We can conclude that throughout the redox mechanism lattice oxygen is required for selective oxidation of n-butane for MA production. Fig.9.Redox oxidation behaviors of hydrocarbons are associated from lattice oxygen. Fig.10.The vanadium occupied the position at antibonding orbital and due to the con-committed administration of V+4 ions with adsorbed oxygen and produces the O-2 species. The presence of oxygen in an environment leads to the oxidation process and exhibits the various phases of V+5-phasphate analogous e.g.(δ-,γ-,β-,αII-,αI-) depending on both reaction condition and intrinsic characteristics of the precursor phase.The(α-VOPO4) phase is enriched in oxygen content and it has influence on specific activity of active phase [7].It has been observed that selective nature of VPO precursor contain those oxygen species which are formed at coordinated surface site of unsaturated vanadyl ions [76]. It would be noted that different variations and additions to the VPO precursor did not alters the binding energy of V(2p3/2-)and P(2p-) electrons,meanwhile binding energy of O (1s-) electrons was declined significantly.The reduction of binding energy of oxygen atom is the evidence of changes the negative charge present at oxygen surface.For instance,the addition of alkaline and alkali earth metal ionic species have an impact on the charge of oxygen species.These metal ions are responsible for the increment of these negative charges of oxygen species and consequently oxidation rate of n-butane increases significantly [90].As negative charge is increasing then its basicity nature of surface also increases.The basicity nature of surface oxygen atoms accelerates the n-butane activation and acidity nature of surface oxygen atom regulating the suitable residence time of intermediates formed during reactions.This phenomenon is prominent during the adsorption of CO2and usually not considered with unprompted catalyst.Certainly,more adsorbed CO2content favors the lower binding energy of O(1s-)electrons[90].The scope of oxygen in the conversion of n-butane to MA elucidated as lattice and absorbed oxygen. Fig.11.The adsorbed oxygen species could be transforming into lattice oxygen due to the redox behavior of oxygen ion species. Oxygen present in gaseous form is readily dissociated and converted into lattice oxygen and exhibit the nucleophilic character rather than producing the adsorbed oxygen species.These ionic species,no matter whether it is strongly or weakly attach with the catalytic surface sites,do not perform its role in selective mechanism for the transformation of hydrocarbons.Similar finding also exploit by another research team and express that lattice oxygen has minor contribution towards the activation of C-H bond[91].Thus,selectivity of n-butane is not dependent on oxidation species.Both oxidant species,lattice oxygen and adsorbed oxygen are limited to their implications due to the limitations of the heat and mass transfer catalogue.Anyhow,at elevated temperature,chemical bond present between the surface sites and organic adsorbed species that might be extensively weaker(due to thermal vibrations of lattice oxide) to ensure the rapid movement of ions. Surprisingly,the impregnation of molybdenum (Mo) onto VPO oxides surface accelerate the oxygen liability of active phase as electronic promoter in the presence of strong Lewis acid sites and indicated that electronic effect is promoting the segregate effect due to the structural changes [40]. The role of phosphorus in VPO heterogeneous and homogeneous catalysis is very dominant for selective oxidation.Phosphorus has the ability to modulate the proportion of V+4/V+5crystal phase,and balance the V+4specie by obviating the over-oxidation of V+4to V+5species [53].The detailed studied on active phase structure will justify the role of phosphorus in VPO catalyst.For instance,well-crystalline structures of VOPO4(αI,αII,β,and δ phases) to active phase,significant structural changes are present at locations of phosphorus atoms,meanwhile no eloquent changes is observed at vanadium atoms[92].One of the research group on the basis of DFT calculations claimed that active center of VPO chemistry found at V+5OPO4surface,where reactive phosphine–oxo [P=O(1)] bonds is present that removes hydrogen atom from n-butane methylene group(-CH3)[93].The bonds[P=O(1)]is very reactive due to the presence of adjacent V+5atom,as vanadium atom has large reduction potentials so it is strongly coupled,hence active the C-H bond of n-butane for selective oxidation[93].Subsequently,similar research finding famous as ‘‘reduction-coupled oxo activation (ROA)”mechanism [92]. Since,the vanadium phosphorus oxide framework is characterized through the presence of different crystalline phases,and then the morphology and structure of the active phase must be deliberated in term of dominant crystal factors such as P/V ratio,oxidation state,and more importantly transformation of crystal phases under reactant conditions.The transformations of these crystal phases are dependent on oxidizing or reducing behavior of the reactants and reaction temperature,and the time-on-stream.The most essential secret encompassing the VPO’s system concerns which phases are liable for the reaction [13]. The (VO)2P2O7phase is such reduced phase of VPO,that endorsed to be active,among five additional VPO oxidized phases–α1-,α2-,β-,δ-,and X1-VOPO4–that has significant role(Fig.12)[13].Among them,three oxidized phase’s α1-,α2-,and X1-VOPO4are sensible candidates,since they are structurally layered and exhibit the stable surfaces.Anyhow,merely X1 phase should not be selective due to various exothermic steps,but α1phase showed a potential towards the selectivity of MA [13].Both oxidized and reduced phases comprising of eight-member rings of alternating phosphorus and vanadium atoms attached with each other by oxygen atoms.Nonetheless,each phase has unique surface characteristics where reactions proceed,such as bridging oxygen (V-O-P)and non-bridging oxygen (P=O,V=O). Fig.12.Phase transformation in VPO system [9].Dotted lines distinguish the reversible redox transformation to(VO)2P2O7(a) from irreversible dehydration (b). The reduced phase active phase includes the motif of oxygen sites bridging (V—OH—P) and V=O groups along with pyrophosphate bonds,which is present in Z direction.Moreover,the reduced phase is exclusive in every vanadium carries as a unique unpaired like spinning that all vanadium atom is an anti-ferromagnetically coupled with an adjacent atom.The threshold energy value that would activate the n-butane is 88 kcal.mol-1(1-kcal=4.186 kJ),due to the lower O-H bond energies.The purpose of active phase is to activate the C-H bond of n-butane for selective oxidation. Formally,vanadium has four-valance state in active phase,although it has been witnessed that V+3ions formed in the course of the catalytic activity,and the couple of V+4/V+5redox is configuring the oxidation gesture.In various research studies presumed that different V+5phases(α11-,β-,ɣ-,δ-,VOPO4)are coexisted during the activity of the catalyst and the performance of V/P oxide catalyst relies upon the synergetic interaction between active phase matrix and surface behavior of V+5phases [94].This summary shows a conflict with industrial V/P oxidative catalyst of n-butane,this issue is raised when studied the changes in reactivity and structural behavior of the catalyst precursor with respect to time-on-stream.At starting,the precursor is poorly crystallized termed as‘‘non-equilibrated”sample,where active phase exists as amorphous too(ɣ-VOPO4and V+4-P-O)but in the final stage‘‘equilibrated”precursor consist of well crystalline active phase.The structural configuration develops when intention to the selectivity of n-butane for MA yield,so concluding that V+5phase also has significance for n-butane selectivity.V+5phases are normally available in catalyst.Some research studies found that V+5species seems to be essential at initial C-H activation for n-butane,hence contribute the catalytic performance [95].The V+4/V+5ratio (one V+4site for four V+5sites) could give the information about the catalysis performance and found that 0.25 value is the ideal ratio for MA reactivity [83].The most common available V+5ions in VPO are anhydrous VOPO4that can readily produces HVP precursor upon hydration[96].Moreover,polyvanadates phases VxOyalso set up for VPO catalysis[97]and has a potential for selective oxidation of alkanes [98]. In generic,the presence of vanadium oxidation state (V+4/V+5)and the ratio of phosphate to vanadium are pivotal parameters of n-butane selective oxidation for MA production [14].Metal oxide supported VPO catalyst (CeO2/P-modified CeO2) indicated that ratio of vanadium oxidation (V+4/V+5) is closely related to the surface area,which is conducting for the development of active phase[99].Similarly,the high and low P/V ratio of VPO catalyst is varying the surface compositions and have influences on reactivity and selectivity of n-butane oxidation products [100].The least MA selectivity associated with the P/V ratio of 1:1,thus an excess amount of phosphorus promotes the selectivity [14].The P/V atomic ratio is increased by adding drop by drop phosphoric acid into the solution for improved selectivity [99].Surprisingly,the P/V ratio could stabilize the vanadium V+4/V+5within the catalyst for a selective and active catalyst. The researchers speculate that changes in VPO phases at variable reaction condition are tentative on subordinate changes in P/V ratio,that might be effect on differing surfaces and observed during ex situ and in situ studies [101].As mentioned in literature the varying P/V ratio changes the intermediate phases from active phase to δ-VOPO4transfiguration.However,intermediate phases seems to be rather unlikely,for instance,VOy+P2O5and α1-VOPO4analogous are observed when P/V ratio is one,but for P/V >1.00,different intermediate analogous such as VOPO4∙2H2O and δ-VOPO4are seen (Fig.13). It is suggested that the phosphorus rich phase is amorphous,esteeming for large surface P/V ratio that is ordinarily noticed in experimentation.A significant large P/V ratio (10:1) is reported too for high surface area [102].The blending of high P/V ratio catalyst and low P/V ratio catalyst could bring the improved catalytic performance e.g.higher yield and better selectivity. The catalytic performance of VPO precursor is readily controlled by the modification of surface basicity/acidity of catalyst and phase constitution through simultaneously functionalization with ordinary mixed metal-oxides [103].While considering both basicity and acidity,higher concentration of weak basic and acidic sites are the prime aspects for improved catalytic activity [103]. Fig.13.The influence of the P/V ratio schematically represent in vanadyl pyrophosphate on both the nature of active layer and catalytic performance [88]. The Lewis and Brönsted acidity present at the VPO surface might be due to V-OH groups and/or surface hydrogen phosphate species (P-OH) [104].Thus,accommodating the fission of C-H bond in spite of C-C bond,and hence improving the selectivity for maleic anhydride [105].For instance,niobium doped VPO catalyst(Nb-VPO) invokes the active phase defects,liable for C-H bond nbutane activation,which have been noticed to be collaborated with low acidity sites of Lewis acid [31]. The VPO precursor is prepared from different approaches with same P/V ratio,found that specific acidity of VPO is independent of synthesis methods [104].But in different P/V ratio found that the content of phosphorus is useful for stabilizing the oxidation species of vanadium (V+4) at particular range.Nevertheless,excess phosphorus content assists the over-oxidation to COx,probably,encouraged the basic sites and alleviates the weak acid site,which elucidate the phenomenon of selective oxidation of n-butane and MA yield [53,106].For instance,one of the research group introduced the self-regulatory system for surface acidity and active phase with the aid of NH4(VO2)HPO4(the VPO precursor) and siliceous mesostructured cellular foams (MCF).MCF performed the dual character;one is influencing on the surface acidity another on surface basicity.They concluded that catalyst surface acidity is dependent on degree of reduction of the V+5,released NH3quantity and surface ratio V+4/V+5as revealed by H2-TPR and XPS investigations [107].The acidity of VPO surface is characterized from the adsorption of ammonia (CO2-NH3)or pyridine [104,106].To characterize the Lewis acid sites present in vanadium-based catalysts,CO is also a useful probe[104].Moreover,the feed of gaseous oxygen is crucial for preceding the reaction;specifically,oxygen content has the capability to tune the oxidation state of vanadium,hence the surface acidity and catalyst performance is also affected. The catalyst performance is dependent on the reactivity of active phase;the so-obtained catalysts structure morphology is modulating by the surface area and crystal structure,hence provide a supportive role for C-H activation.As researchers tapped into the surface area feature of active phase,attention has been diverted towards the development of maximum surface area of HVP precursor.In earlier findings,water has been used and have achieved the surface area up to 10–13 m2.g-1from previous reported 4 m2.g-1[108].Water treatment causes the impurity issues in of the precursor.Recently alcohol has been employed,that has dual advantages such as increasing the surface area and also act as reducing agent [109].Structure directing group such as monodisperse polystyrene has also exhibited the high surface area 75 m2.g-1[110].The required surface area can be obtain by the addition of the surfactant while preparing the HVP precursor,for example decyltrimethyl ammonium bromide is providing high surface area up to 250 m2.g-1[44].Various promoters such as Sm,Co,Nb and Fe are already been employed for the enlargement of the surface area[14].While the addition of different additives during VPO synthesis,some treatments have also been reported for the effective surface area such as mechanical process (i.e.milling process) [73],tribomechanical and solvothermal process,are utilized to produces the high surface area [62].Similarly,microwave irradiation is used to prepare the large surface area precursor as compared to conventional heating method [69,111].Surface area of VPO catalyst is easily evaluated from the characterization technique Brunauer-Emmer-Teller(BET).The catalyst surface area and specific n-butane conversion are in liner relationship with each other[75].Apparently,synthesis process directly effects on crystal morphology. Structure morphology is persuaded by the nature of the precursor and the reagents,and develop VPO catalyst could activate insitu under air/butane atmosphere [112].In-situ observation found that the presence of two V+5phases (α11-,β-VOPO4) support the large surface area [10].The V+5species of HVP precursor existed in β-VOPO4and active phase and large surface area collectively boost the chemical activity for selective oxidation.The (020) plan exhibits the smaller size and could expands the BET surface area[109].The presence of VO(H2PO4)2phase in VPO precursors is casing the impurity for the active phase,removing it through water extraction method resultant the high surface area and better selectivity.In-fact,the removal of impurities claimed the high surface area [109]. In recently explored additives,ILs is such kind of amazing reagent that could be structurally design as per requirement.The modulation of structure morphology is the exclusive feature of ILs.Moreover,the presence of different metal dopant in ILs further assists the structure morphology.For instance,iron-based ILs precursors (i.e.VPO-OMIMFeCl4and VPO-BMIMFeCl4) formed the chrysanthemum-shape clusters,after it was characterization revealed that surface area was significantly increased and the development of surface redox sites truly promotes the active phase reactivity[2].We can conclude that changes in structure morphology can give the breakthrough regarding large surface area,which demands ideal preparation route.Further,organic media as reducing agent might be the best choice for the enhancement of the surface area and controlling the crystallites morphology while synthesizing the effective HVP precursor[61].Obviously,the catalyst activity is quantified from the surface area of the catalyst,and is evaluated through crystal morphology. It is quite interesting that large surface is required for better catalytic yield but it does not mean very large surface area is required.In the case of surfactant [47],researchers were able to achieve very large area,unfortunately,catalytic performance is not improved too much.Porous structures with very large surface area habited the transfer of heat,easily causing hot spot and resulting in the over-oxidation to CO2.Therefore,the optimum surface area is required. Vanadium species V+4and V+5are necessary for the production of MA at higher yield.We are already familiar that the role of V+4in VPO precursor is very crucial but we can’t ignore the importance of V+5species[18].In VPO precursor V+4species associated with oxygen species specifically O-are contributing their role in catalyst activity [32].However,V+5species activity participate in MA production[32].In the presence of different dopants V+4and V+5species are regulated [38].Metal dopants added to VPO precursor as promoter are associated with different redox potential and it has different correlation with V+4/V+5ratio.For instance cobalt (Co+3/Co+2) redox potential is greater than V+4/V+5,in such case when V+4/V+5ratio decrease then conversion of n-butane also decreases[113].On the other hand,iron(Fe+3/Fe+2)has lower redox potential than the V+5/V+4herein,when V+4/V+5ratio decrease then conversion of n-butane is not decreased due to low redox potential of iron stabilizing the n-butane conversion [113]. The VPO catalytic performance can be controlled from the oxidation state of vanadium through the V+4/V+5ratio which are available at catalytic surface [114].From electrical conductivity data found that,most reactive part of VPO precursor is that which are present in oxidized state.Ascribed from p-type semi conductivity[115],it is demonstrated that electric charge present at positive holes h+associated with isolated V+5species at vanadyl position[114]. The electronic vacancies(holes h+)are chemically communicate with O-species conforming from [114]; Such kind of oxidizing species are presumably not only oxydehydrogenate to the n-butane,but also allow insertion into butene C4=substrate and generated the MA.p-Type semi conductivity alludes an excess 1/2ε oxygen anions,as a origin of oxygen atom converted to MA production [114]. The ratio of V+4/V+5is conformed to MA from its intrinsic activity.It has been observed that V+5micro domains are discovered at catalyst surface meanwhile isolated V+5sites are present in the sub layers of VPO structure.The optimal value of V+4/V+5ratio for oxidizing reaction condition (fuel-lean:O2/C4=12) is approximately 0.25 which correlates the four V+4sites from one V+5sites,however,such conditions are not rational in reducing reaction conditions (fuel-rich:O2/C4=0.6) [114].These arguments allows that VPO precursor framework is only suitable for mild oxidation condition of n-butane to MA through the addition of metal dopants into VPO catalyst system.Their localization,optimal concentrations and nature of the metal dopants are still under exploration,anyhow Co and Mo both metal dopants are good compatible for assisting the VPO precursor in fuel-rich conditions [114]. The VPO catalyst and in specific active phase shows the unmatched and unique reactivity in the phenomena of selective oxidation of C4-hydrocarbons for producing the MA.Additionally,the active phase shows a potential behavior in the selectivity of C5-hydrocarbons for producing the MA and phthalic anhydrides.Except VPO,no other catalyst has been explore yet for such kind of comparable catalytic behavior.Surprisingly,the active phase has least potential for the selective oxidation of C2-,C3-,linear-C6-hydrocarbons,but cyclic-C6-hydrocarbon ability to synthesis the benzene as oxidative dehydrogenation.Moreover,in the case of oxidation the alkenes(butadiene and butenes)over VPO catalyst of n-butane at optimal reaction condition to maximize the MA production,as compared to n-butane conversion there is least selectivity for MA production,notwithstanding the common perception of these alkenes are contributing its role as intermediates while n-butane oxidize for MA.The catalysis activity of VPO precursor very much effected from the modality of preparation method,and the crystal structure with more defects seems to be more reliable and preferable,particularly existence of stack defects in-between(100)planes.Anyhow,the researcher found the difference in their behavioral activity of VPO precursor after the concomitant administration with distinguish started alkane reagents i.e.n-pentane or n-butane.Their versatile reactivity is dependent on the modification of local structure of VPO catalyst.Various researcher groups are trying to explore the reactivity characteristics of active phase in the synthesis of MA from n-butane,are based on their nature of planes/sites existed at the characterizing surface of (100 plane) [76]. (1) The correlation exists between Brönsted sites (P—OH) and the Lewis acid sites (coordinated with unsaturated V+4ionic species) for selective oxidation of light alkane specifically nbutane. (2) Generation of peroxide-type species (pseudo-ozonide or ntype) via the adsorption of O2at cations surface of V+4species as coordinately unsaturated and their importance in alkane activation and selective oxidation. (3)The vanadium and phosphorus are bonding each other with the help of bridging the oxygen atom acting a direct role to complete the overall reaction and shows its significance in final oxidation products. (4)Although V+5species has low density in active phase matrix but it shows significant presence with phosphate domains at active phase (100) surface plane. (5) Possibility of one election is present at V+3/V+4and V+4/V+5,and two-electron at V+3/V+5redox reactions. (6) The (100) plane exhibits the labile lattice oxygen,however,restricted to the surface layers. (7) The active and unsaturated oxygen present at specific geometric arrangement adjacent to the adsorption sites. (8) The surface equilibrium present between the V+4+p+(p+=positrons) and V+5species [115],this morphology is observed from the measurements of electric conductivity.An adjacent anion electron (neighboring electron)could occupy the current electron vacancy (O-2+p+↔O-).Hence,formed the oxygen species that urgently required for the activation of alkane bond and selective oxidation mechanism. Generally,few of these outcomes are not practically valid and needs further demonstration.Although some approaches related to reaction mechanism of MA synthesis and exclusive feature of vanadyl pyrophosphate are already explained in literature. Since 1970s,the researchers have devoted their huge efforts in the exploration of ideal catalyst precursor,but the reaction mechanism of n-butane is not clearly explained yet.However,it is obvious that n-butane oxidized for MA production is dependent on oxygen species present at crystal lattice,which is replenish by oxygen uptake due to gas phase.In an earlier work it is hypothesized the viability of cyclic precursor of MA e.g.furan[81,82].In present studies alkoxide route has been described as a non-cyclic mechanism rather than cyclic mechanism as shown in Fig.14 [116]. Fig.14.The oxidation mechanism of n-butane over VPO catalyst is suggested by Xue et al.[116]. In these cyclic and non-cyclic mechanisms,presume that the oxidative dehydrogenation and activation of n-butane to n-butenes(1-butene,2-butene) is the first step and recognized as rate determining step as mentioned in Table 2.In earlier cyclic mechanism the proposed oxidative dehydrogenation step for butadiene,whereas non-cyclic mechanism(an alkoxide route)to put forward the concept of transferring the lattice oxygen to both butenes by the generation of butanal/butanone.In cyclic route,assumed precursors such as butadiene,furan and dihydrofuran are present inside mechanism in the latest research models and are considered to be unstable at the catalyst surface.Consequently,they more readily desorb and are easily predicted in gas phase chamber,while n-butane was oxidized,whereas alkoxic species are still present at catalyst surface and merely detected by FTIR or resembling characterization techniques.Except desorption,the species present in cyclic route might be regressed back to the respective alkoxide species with the help of cleavage in the ring.The final oxidation product of MA concludes the generation of carboxylic species at crystal surface [117]. Numerous research approaches presenting the description of reaction mechanism are reviewed by Dummre research group,arguing intermediates are crucial for the formation of desired product [18].We cannot ignore its role towards the reaction mechanism and its involvement in reaction kinetics;anyhow,many of the research groups have neglected it[1,18].A complete oxidation process is elaborated with reaction rates and reaction mechanism.Therefore,the scheme of reaction network is presented to investigate the detailed reaction mechanism [1].An appropriate reaction network is required for describing the catalyst reactivity with desired reaction of selective oxidation of n-butane to MA in parallel with a non-selective byproducts and total oxidation reaction.On the other hand,after decomposition of MA to oxidation products follow the reaction network.Two different kinetic route have different purposed stoichiometric equations are (Fig.15) [118]: Fig.15.Two different kinetic route of selective oxidation of n-butane to MA supposed to be distinguishing stoichiometric equations. Two different stoichiometric coefficients n and p have a dependency on catalyst activity and reactivity,and kinetic measurements are required to determine these coefficients.Kinetically based designed reactor provides the knowledge about these stoichiometric coefficients.After that it can be easily predict the possible reaction mechanism.However,there are some clues present about the proceeding of the reaction,according to the concept of redox mechanism,each reaction might be further separated into temporally or spatially separated reduction/oxidation of the catalyst (e.g.the concept of DuPont according to circulating-fluidized bed) [119].There is no defined outline as to which kinetic model is ideal for MA synthesis.Some researchers imply the redox mechanism (e.g.Mars and van Krevelen) to describe the kinetics,other used Langmuir-Hinshelwood or Eley-Rideal type rate equations.Pseudo-first-order and power law approaches are also in trending.In spite of considering the feed composition and reaction condition,experiments are carried out for the exploration of oxidation products (H2O,CO,CO2,MA,by-products).Particularly,neglected by-products such as acrylic acid and acetic acid are examined by adding them in the feed section.Additionally,in co-dosing experiments section,reactions are required for the concentrated oxidation products (MA,acrylic acid and acid acid).In this discussion,it will be illustrating that the acrylic acid and acetic acid are readily oxidized to CO2/CO.Nevertheless,each commodity is detected at significant level (each one has a selectivity up to 4%) under industrial operating condition,their oxidation and formation should contribute the additional role in the formation of CO2/CO than previously assumed.Hence,we can conclude that neglecting of both segments is not a good choice for the ideal description of reaction kinetics.It is a rational question whether n-butane directly forms the CO2/CO,except from the abstraction of carbon atoms meanwhile acrylic acid and acetic acid is formed.The reaction network deserve at least nine consecutive reactions,some further reactions are observed during experiments findings (Fig.16) [120]. Fig.16.The reaction network is rationalized in order to cover the all aspects for the generation of COx from the kinetic equations based on consecutive parallel reactions. Upcoming research should focus on dynamic process at VPO surface.Intention requires for the importance of water,different dopants especially phosphorus during the reaction mechanism.Moreover,the reaction mechanism of non-selective by-products,acetic acid,acrylic acid,COx(CO2,CO),and their influence along different species present at phase surface needs more precise and comprehensive investigation. Various experimental and few theoretical investigations on reaction mechanism of catalytic behavior of n-butane for MA formation is still a hot topic.Few researchers proposed that oxidation mechanism of n-butane follows the track of olefinic route and consider that intermediates of reaction are desorbed [121],whereas others suggested that n-butane follows an alkoxide route and predict that n-butane is anchoring at the surface soon-after the activation of C-H bond and is desorbed only when MA has been generated [64].There are always some uncertainties presented in reaction mechanism,a few basic information about which plane/sites at the surface are actively participating in the cleavage of C-H bond of n-butane is still missing.These challenges lead the researchers further investigation in this area,finally they have introduced the numerical concept of reaction mechanism referring to density functional theory (DFT) calculations. More defects present at (100) surface is preferable;in DFT calculations the electronic structure was developed with respect to their different clusters exhibited by variant oxygen species and then simulating the active phase (100) surface according to the cluster model studies [122,123].They found that different structural oxygen species present at (100) surface have distinguished electronic configuration.The reactivity of double and triple coordinated oxygen sites (O2/O3) are evaluating through their electrophilic attack.The strong nucleophilic character of distinct oxygen O3sites (bridging one P atom and two V atoms) and V-3d character of LUMO/HOMO leads the C-H bond splitting and thus activating the organic species.Moreover,they concluded that vacancies of oxygen can be displayed as a source of boosting the selectivity and reactivity of VPO precursor. From literature,it is found that the electronic behavior of active phase (100) surface evaluated form different theoretical techniques such as DFT calculations,periodic model or cluster model,revealed that vanadium species perform the chemisorption domain of hydrocarbons,while simultaneously nucleophilic character of terminal oxygen of P-O (considered the most basic nature of oxygen surface)prosecute the selective oxy-functionalization of the reactant [8,124,125].However,dissociative chemisorption behavior of water showed its importance in eternalization of oxidation cycle.A simulation studies about the active phase (100)and 1-butene discovered that there are huge difference was existing while comparing the active phase (100) with n-butane,possibly not being an intermediator during n-butane selective oxidation [8]. In contrast to the above discussion about the active center of active phase present at either chemisorbed O2or V-O bonds.Active center found at [P=O(1)] V+5OPO4surface because VOPO4surface is exhibiting the more reactivity of phosphine-oxo bond[P=O(1)],which can extract hydrogen from n-butane methylene groups (-CH3) and possess the barrier energy of just 13.5 kcal∙mol-1(1 kcal=4.186 kJ),in coherent with DFT calculation data [93].Finally,they concluded that the capacity of [P=O(1)]specie is to break the C-H bond because of their strong basic nature and communicate with adjacent V+5ions (Fig.17).In the support of previous conclusion about the active center,it also involves the [P=O(1)] bond at [VOPO4] surface,which is responsible to initiate the VPO chemistry.In this morphology,hydrogen bond is also extracted from n-butane reagent.They employed the quantum mechanical (QM) studies of DFT and observed that juxtaposition of P=O bond adjacent to strong reducible V+5ionic species but surprisingly makes an oxygen bridge to enhance the reactivity of P=O for the abstraction of proton from an n-alkane,meanwhile electron are transferred from V+4to V species [92]. The role of active surface and each catalyst constitute role during reaction mechanism that still needs to be more justified.The dynamic behavior of the active surface and sophistication of precursor phase under operating conditions integrate serious hindrances for the comprehension of these interpretations. When VPO is explored as catalyst in n-butane oxidation then it has substituted the selective oxidation of benzene to produce MA due to the greenhouse gas emissions and higher productivity.As industrial trend is inclined towards the selective oxidation of n-butane form VPO precursor,then following issues are arising in chemical plant. The strong exothermic or endothermic reactions of VPO elucidate the overall performance of the chemical reactor.These analogy cases due to temperature excursions (either cold-spot or hotspot),thus evaluate the efficiency of the catalyst.Particularly,in the course of higher conversion of partial oxidation,the limitations of radial heat transfer bring a significant parametric sensitivity that dropped the both catalyst activity and product selectivity.In endothermic reactions such as steam reforming,an insufficient heat is transferred between heat carrier and catalyst that has adverse effect on catalyst efficiency. Fig.17.Pyrophosphate oxidation has been illustrated through valence-bond and found that active center present at V+5OPO4 surface which is responsible for C-H bond activation of alkane [93]. The sensitivity of temperature for MA synthesis is not as high as anticipated,the utility of isothermal mechanism is approximately inadequate,thus yield trend inclined towards MA and bounded from 4% to 56%–58% [126] and release large amount of heat as follows: The detailed simulation studies can acknowledge these findings.The kinetic model investigations with Langmuir-Hinshelwood,Eley-Rideal,and Redox-mechanism displayed nonsubstantial diversity among all these kinetic models.Anyhow,the feed concentration and temperature dependency are easily predicted from Eley-Rideal kinetic model. The n-butane partial oxidation to MA with VPO is considered as a strong exothermal reaction (Fig.18).When oxidation reaction exhibits the strong exothermic behavior,it means that nonisothermal activity is dominant and encourage the large-scale production from fixed-bed reactor [117].In each strong exothermic partial oxidations,a hot-spot is generated in the first quarter of catalytically activated zone [126].This hot spot leads to selectivity losses.In order to avoid the hot-spot,various techniques are available in literature.For example,in an analyzer dilution technique various cooling circuits are utilized for optimizing the temperature of the reactor in order to improve the heat transport properties.The strong exothermic phenomena can be effectively controlled by introducing the wall reactor concept in a conventional fixed bed reactor by designing a parallel plate inside the reactor to avoid the radial heat transfer.Importantly,this wall-reactor shows the isothermal operation [126].The phenomenal activity of nonisothermal operation must be evaluated and be quantified.This behavioral quantification of non-isothermal operation is granted by redox coupling kinetics and dynamic evolution of the reactants in the supervision of reaction thermodynamics and finally applying the suitable mathematical model for reactor designing and simulation.In fixed bed reactor,more than one cooling zones might be good selection for overall reactor performance.The extra heat energy of strong exothermic oxidation reaction is removed with great priority. It is indeed for the designing of new reactor with the concept of strong exothermic reactions that offers the behavior of isothermal reactor as well as easily simulated with cooled fixed bed reactors. Fig.18.Various combustion products and heat are generated during n-butane oxidation. In order to improve the performance and prolong the catalyst lifetime of VPO precursor,the addition of phosphorus compounds as supplement would play a curial role.It will affect the reaction exotherm ‘‘hot spot”beyond shifting into the catalyst bed.The main purpose of the exotherm shifting towards catalyst bed is to assist the process for the manufacturing of MA [127].Patent 4701433 reports a series of phosphorus substances,which have been widely used for phosphorus supplement [128].The valance state requirement of phosphorus is lower than +5,for instance,phosphorus oxide,hypophosphite and di-,tri-,tetra-alkyl phosphate,or mixture of these.Phosphorus supplement is represented in following structure: The ester of orthophosphoric acid (RO)3P=O (R=H,or C1-C4alkyl)has been elected for phosphorus compound due to its better reactivity.Patent US 4810803A[129]and US 4515899A[130]confirmed that trimethyl phosphate and trimethyl phosphate have an excellent performance as phosphorus supplement after screening various phosphorus reagents. There is presumption that water content and phosphorus compounds both have a capacity to boost the selectivity of the product[127].It could be experienced with/without continuous flow of oxygen or/and hydrocarbon.The preferred technique for the enhancement of catalyst performance is the intermittent or continuous addition of the water and the volatile organophosphorus compounds to the gaseous stream of the oxygen-containing gases and hydrocarbons both simultaneously entering into the reactor.The catalyst exotherm can be moved down towards the catalyst bed through the addition of 0.005–5 grams per kg per day of phosphorus compounds and the addition of water content in this stream is about 1–40 g per kg of total gas feed stream[127].From this strategy,the activity and reactivity of the catalyst precursor is sustain through stabilization or improvement.The main advantage of this method is the production of MA does not have to be fluctuated. In earlier stage for the development of VPO precursors,water is used for enhancing the surface area,later on trend has been shifted towards the organics solvent because of its dual significance;one is providing the large surface area as compared to water and acting as reducing agent too.Initially water is considered as surface area prospective.Later studies described that water based VPO precursor has a significant impact on their partial/overall catalytic performance.The presence of water in VPO catalyzed system have an positive impact on partial oxidation of acrylic acid [131].The nature of conventionally prepared VPO precursors such as generation of new acid sites and reduces the V+5species,are changed dramatically in water treatment.In aforementioned statements,the oxidation of light alkanes has a negative-effect for the presence of V+5species in VPO catalytic management [131].Same observations seen for maleic anhydride oxidation,such as water vapor displays higher crystallinity in PEG-derived VPO precursors as compared to non-aqueous feed/reflux [43].Water present in either direct feed or in reflux,both have same opportunities.Therefore,An optimum aqueous content is essential for the improvement of catalysis performance [43]. The intrinsic inhibition effect of water is deciding the performance of the catalyst.The higher concentration of water feed reduces the reaction rates,then conversion decline and MA selectivity rises.Anyhow,catalyst activity is boosted when water feed is rising in the presence of trimethyl phosphate (TMP) [132].One of the imperative function of water feed is to disperse the phosphorus species along the entire catalyst bed [133].The concentration of phosphorus at catalyst surface is changed because of water presence and then measure by its adsorption equilibrium.Hence,the induced phosphorus course act as rate determining step in order to evaluate surface dynamics [132].Water is involved in both catalyst synthesis and activation.The water–vapor treatment and water-reflux both are collectively prevailed the surface hydroxylation and thus remodeling the surface structure and acidity.Moreover,the role of water as vapor stream is very interesting during reaction,such as it transfers the heat and mass energy to the catalyst bed of the reactor and enhances the product selectivity. With the assessment of global warming,world paved their attention to carbon neutrality.The development of industrial sector,unfortunately,leads the global warming issues because of carbon emission form various chemical process such as petroleum oil refinery that produces the lighter alkane i.e.n-butane as byproduct,which can be converted into useful industrial reagents such as maleic anhydride with the aid of vanadium-phosphorus-oxide catalyst. The exploration and application of VPO catalysts for selective oxidation of low-hydrocarbons has continual at an eminent rank throughout chiefly foregoing in past four decades.Particularly,recent years have affirmed to grab the attention in research on vanadium phosphate substrate and improvement for the utilization in selective n-butane oxidation.The production of maleic anhydride is achieved via n-butane oxidation.The interest for maleic anhydride comes chiefly from the production of agricultural chemicals,food additives,value-added products,fumaric acid,unsaturated polyester resins,lubricating oil additives,pharmaceuticals and numerous other fine chemicals.Because of its great importance,researchers are seeking to explore the novel synthesis of MA from VPO.In VPO catalysis,develop the active phase(VO)2-P2O7is the curial step for overall catalyst performance and MA productivity,but this active phase intrinsically based on synthesis methods such as hydrothermal,sol–gel method,electrospinning,ultrasound,microwave irradiation,ball milling.And all these will affect its physicochemical properties such as surface area,lattice oxygen,crystal phase,valance state,acidity,redox ratio of V+4/V+5and P/V ratio.At surface V+4and V+5species are present,among them V+5species assist the higher selectivity while V+4endorse the conversation rate.Collectively,V+4/V+5redox ratio have an influenced on catalytic performance.The selectivity and reactivity of VPO precursor controlled from the vanadium species.C-H bond activation is one of the prime aspect of n-butane oxidation;in current scenario,vanadium and phosphorus both are equally entertain the oxidation mechanism.The removal of hydrogen bond from n-butane and produces methylene group (-CH3) is possible due to the presence of reactive phosphine–oxo [P=O(1)] bonds and it will be more reactive because of adjacent V+5species [93].Based on the knowledge we could extend the VPO catalyst for producing different oxidation products and employed in several other reactions such as dehydrogenation,ammoxidation,dehydration etc. Presently,it is satisfying to articulate that preparation techniques are being exploited and consolidated to contain the utility of seeds [134],structure-directing agents and unique supported materials.These advanced precursors could be more reliable and appropriate for the development of modern catalyst,which could be more compatible with reactor technologies,such as membrane reactors and fluidized-bed.As prepared conventional catalysts are mostly use in the technology of fixed-bed reactors whereas the advantages such as modest activity and on-stream activation within the restraints of low n-butane concentrations have been inestimable.The synthetic and green approaches might be the key to improve the selectivity of advanced VPO catalyst.Recently,explored ionic liquids and its analogues deep eutectic solvents were develop the VPO precursor is recognize itself as green and cost effective promoter.It could works as a structure and electronic promoter to regulate the physiochemical properties according to the catalytic performance.If reaction condition is optimal and temperature is low,consequently,selectivity is high and more carbon content is available for reactivity and produces the lower acidity(COx).For the industrial application of VPO catalyst in chemical plant,there is possibility to address the many industrial issues such as strong exothermic,phosphorus supplement and water supplement.In order to reduce the greenhouse gas emissions and develop the more sustainable growth,renewable resources are used as chemical industry feedstock such as oxidehydration of bio-1-butanol could be used for producing MA from vanadyl pyrophosphate catalyst in spite of n-butane and benzene reagents. In addition to the selective oxidation of n-butane,VPO have also been employed for the oxidation of other lighter alkanes including ethane to acetic acid[98],isobutane to methacrylic acid[135],propane and propylene to acrylic acid [136].Moreover,VPO catalysis explores the ammoxidation of picoline [137],methylaromatics,toluene [138],propane [139],and methylpyrazine [140].Surprisingly,the VPO catalyst exploits the oxidative dehydrogenation of ethane and propane[79,97].Therefore,VPO would be a good catalyst to effectively utilize the low hydrocarbons into useful industrial chemicals and benefit for achieving the carbon neutrality. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements This work was supported by the National Key Research and Development Program of China(2017YFA0206803),the innovation Academy for Green Manufacture of Chinese Academy of Science(IAGM2020C17),the Key Programs of the Chinese Academy of Sciences (KFZD-SW-413),the National Nature Science Foundation of China(21808223),the Key Programs of Fujian Institute of Innovation,CAS(FJCXY18020203),Chinese Academy of Sciences,the One Hundred Talent Program of CAS.


2.2.Novel methods



2.3.Activation through calcination
3.Physicochemical Properties of VPO
3.1.Role of lattice and surface oxygen




3.2.Role of phosphorus
3.3.Crystal phase

3.4.Valence state
3.5.P/V ratio
3.6.Acidity

3.7.Surface area
3.8.Redox V+4/V+5 ratio

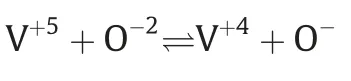

4.Reaction Mechanism



5.Industrial Application of VPO
5.1.Strong exothermic reaction



5.2.Phosphorus supplement

5.3.Water supplement
6.Conclusions and Future Prospects
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
