CdS nanoparticles decorated hexagonal Fe2O3 nanosheets with a Z-scheme photogenerated electron transfer path for improved visible-light photocatalytic hydrogen production
2022-04-27FengGuoHaoranSunYuxingShiFengyuZhouWeilongShi
Feng Guo,Haoran Sun,Yuxing Shi,Fengyu Zhou,Weilong Shi
1 School of Energy and Power,Jiangsu University of Science and Technology,Zhenjiang 212003,China
2 School of Material Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang 212003,China
Keywords:Photocatalysis Hydrogen production 0D/2D CdS α-Fe2O3 Z-scheme
ABSTRACT Photocatalytic water splitting for hydrogen production (H2) is one of the main potential applications of photocatalytic technology,which can use solar energy as the energy required for chemical reactions to alleviate the energy crisis.In this work,zero-dimensional/two-dimensional (0D/2D) contact surface CdS/α-Fe2O3 (CF) heterojunction photocatalyst was synthesized via a simple solvothermal method.Photocatalytic hydrogen production experiments revealed that the CF-15 sample shows the optimal photocatalytic H2 rate (1806 μmol∙h-1∙g-1) and apparent quantum efficiency (AQE=13.7% at λ=420 nm).The enhancement of photocatalytic performance is mainly attributed to the contact of 0D/2D interface and the synergistic effect of Z-scheme electron transfer mechanism.This work provides an effective way for modified composite semiconductor photocatalyst by constructing special interface heterojunction to achieve highly efficiently catalysis.
1.Introduction
Heterogeneous photocatalytic technology for hydrogen (H2)production using solar and semiconductor photocatalysts has been widely recognized as one of the most environmentally friendly and sustainable strategies.Since Fujishima and Honda discovered that TiO2photoelectrodes can photocatalytic water splitting to produce hydrogen,various semiconductor photocatalysts have been developed,such as metal oxides [1,2],nitrides [3–6],sulfides [7–9],etc.From the point of view of solar light utilization efficiency and illumination time,the energy of visible light (wavelength 400–780 nm)accounts for 43%of solar energy,which is more beneficial to practical application.Thus,the development of visible light responsive photocatalyst is highly desired.
Cadmium sulfide(CdS)with a suitable conduction band(2.4 eV)and excellent visible-light-response capacity has been extensively studied as a promising photocatalyst for water splitting[10].Nevertheless,main defects of CdS including fast recombination of electron-hole pairs and few catalytic sites due to nano-sized structure agglomeration,which dramatically limited its further application in photocatalysis field [11].To address above two drawbacks,the construction of zero-dimensional (0D)/two-dimensional (2D)heterojunction has been recognized as an excellent method.This is mainly due to the following aspects:(i) 0D nanomaterials could offer more active sites for further accelerating the separation of photogenerated carriers of the 2D substrate materials [12–14];(ii) 2D nanosheet materials represented will afford more contact areas in heterojunction,ensuring a well-defined support to prevent agglomeration of 0D nanomaterials [15–17].For instance,Zhang et al.reported that 0D/2D Ag/CDots/BiOCl heterojunction synthetized via a solvothermal method possesses excellent photocatalytic activity and the agglomeration is obviously ameliorated[18].Ye et al.prepared 0D vanadium quantum dots/2D g-C3N4composites,showing ultrathin and highly dispersed nanostructures with strong synergistic effects leading to excellent photocatalytic performance [19].Hence,the preparation 0D/2D CdS-based heterojunctions is a feasible method to further improve the photocatalytic water splitting performance.
Recently,hexagonal (hematite) α-Fe2O3nanosheets have been successfully fabricated and exhibit several attractive properties,such as low-cost,nontoxic,high stability,suitable energy band structure and high utilization of solar light,is favorable to the photocatalytic reaction and practical application [20,21].Inspired by the above discussion,it is assumed that the dispersion of 0D CdS nanoparticles on 2D hexagonal α-Fe2O3nanosheets to form 0D/2D heterostructure is an effective method with great potential for photocatalytic H2production.In addition,CdS/α-Fe2O3heterojunction composite photocatalysts have been reported in other previous researches.For example,Zhang et al.synthetized 3D hierarchical CdS/α-Fe2O3type II heterojunction via a simple solution method [22].The CdS nanoparticles were generated on the surfaces of α-Fe2O3microflowers,which resulted in the broaded visible-light response and reduced recombination of electronhole pairs,thus improved the photocatalytic activity for reduction of Cr(VI).Wu et al.reported that 0D/1D α-Fe2O3/CdS nanostructure was prepared by solvothermal deposition method [23].The α-Fe2O3nanoparticles were distributed on the surfaces of CdS nanowires to form composite nanostructure.The absorption capacity of visible light and transfer efficiency of photogenerated carriers were improved,leading to the enhanced photodegradation performance of methylene blue.Nanorod-shaped CdS/α-Fe2O3Z-scheme heterojunction was prepared by Heo et al.[24].The particles of both CdS and Fe2O3were connected via van der Waals forces,which hindered recombination between electrons and holes and extended the lifetime of electrons,so that increased the photocatalytic activity for CO2reduction.Regrettably,although excellent results have been made in the field of CdS/α-Fe2O3heterojunction photocatalyst,there are few works centering on the form of 0D/2D structure between CdS nanoparticles and α-Fe2O3nanosheets to avoid agglomeration phenomenon and promote photocatalytic H2generation.
In this work,0D/2D CdS/α-Fe2O3Z-Scheme heterojunction photocatalyst was synthesized via a simple solvothermal method.The photocatalytic capacity of novel photocatalyst was tested by photocatalytic H2evolution under visible light irradiation(λ >420 nm).Some relevant characterization tests to confirm the microstructure and photochemical properties of as-prepared heterojunctions.The corresponding photocatalytic mechanism was detailly investigated based on the electronic spin resonance(ESR) spectra and band structures.
2.Experimental
2.1.Synthesis of 2D hexagonal α-Fe2O3 nanosheets
1.092 g FeCl3.6H2O was dissolved in a mixed solution of H2O(2.8 ml) and CH3CH2OH (40 ml) under stirring for 30 min.Then,3.2 g CH3COONa was added into the above solution to stir for another 60 min.The prepared solution was transferred to 100 mL autoclave and reacted 12 h at 180°C.After cooling to room temperature,the red precipitate was collected through centrifugation and washing for several times.
2.2.Synthesis of 0D/2D CdS/α-Fe2O3 heterojunction
The 0D/2D CdS/α-Fe2O3heterojunctions were synthetized via a general solvothermal method,and the corresponding flowchart is shown in Scheme 1.Firstly,the different amounts of Cd(CH3COO)2-.2H2O (0.018 g,0.025 g,0.037 g,0.055 g and 0.074 g) were dissolved in 80 ml DMSO under magnetic stirring for 60 min.Then,0.2 g hexagonal Fe2O3nanosheets were added into the above solution.Moreover,the mixed solution was poured into the reactor and heated at 180 °C for 12 h.When cooled to room temperature,the obtained precipitates were washed with alcohol for several times and dried at 70 °C.The products were abbreviated as CF-x (x=5,7,10,15,20),where x represents the mass ratio of CdS in CdS/α-Fe2O3composite products.CdS was prepared via the same method without adding Fe2O3.

Scheme 1.Schematic illustration of the solvothermal route to prepare 0D/2D CdS/α-Fe2O3 heterojunctions.
2.3.Photocatalytic hydrogen production
The photocatalytic hydrogen production performance was investigated in an on-line photocatalytic reaction system (CELPAEM-D8,Beijing China Education Au Light Co.,Ltd,China).Briefly,50 mg photocatalyst was added into the 100 ml deionized water of containing 0.25 mol∙L-1Na2S and 0.25 mol∙L-1Na2SO3as sacrificial reagents and H2PtCl6(sufficient to achieve a 3%(mass)loading)as co-catalysts.A xenon lamp with a cut-off filter was utilized as the visible-light source (λ >420 nm).The temperature of solution inside the photoreactor is maintained at 6 °C by the cooling water circulation system.Before the light,system needs to be exhausted for 20 min to drain the air completely.Then,the produced gas was collected at 60 min intervals for 4 h and the yield of the produced gas was measured by gas chromatograph.The apparent quantum efficiency (AQE) over as-prepared photocatalyst was calculated through equation as follows:

The specific characterizations are listed in the Supporting Information.

Fig.1.XRD patterns of pristine CdS,α-Fe2O3 and CF-15 heterojunction.
3.Results and Discussion
The X-ray diffraction (XRD) patterns were employed to reveal the crystal structures of as-fabricated CdS,α-Fe2O3and 15% CdS/α-Fe2O3(CF-15) and displayed in Fig.1.From the curve of CdS(light blue),it can be seen that three diffraction peaks located at 26.5°,43.8° and 51.7° are corresponded to (1 1 1),(2 2 0) and(3 0 0) crystal planes,respectively,which are in accordance with the previous reports [25].The peaks at 24.1°,33.0°,35.5°,40.8°,49.4°,54.1°,57.6°,62.4° and 63.9° are attributed to (0 1 2),(1 0 4),(1 1 0),(1 1 3),(0 2 4),(1 1 6),(0 1 8),(2 1 4),(3 0 0),(1 0 1 0) and (2 0 0) crystal planes of α-Fe2O3respectively [21].All the diffraction peaks belonging to CdS and α-Fe2O3can be observed in the XRD patterns of CF-15 composite,indicating the successful synthesis of CdS/α-Fe2O3heterojunctions through the facile solvothermal method.
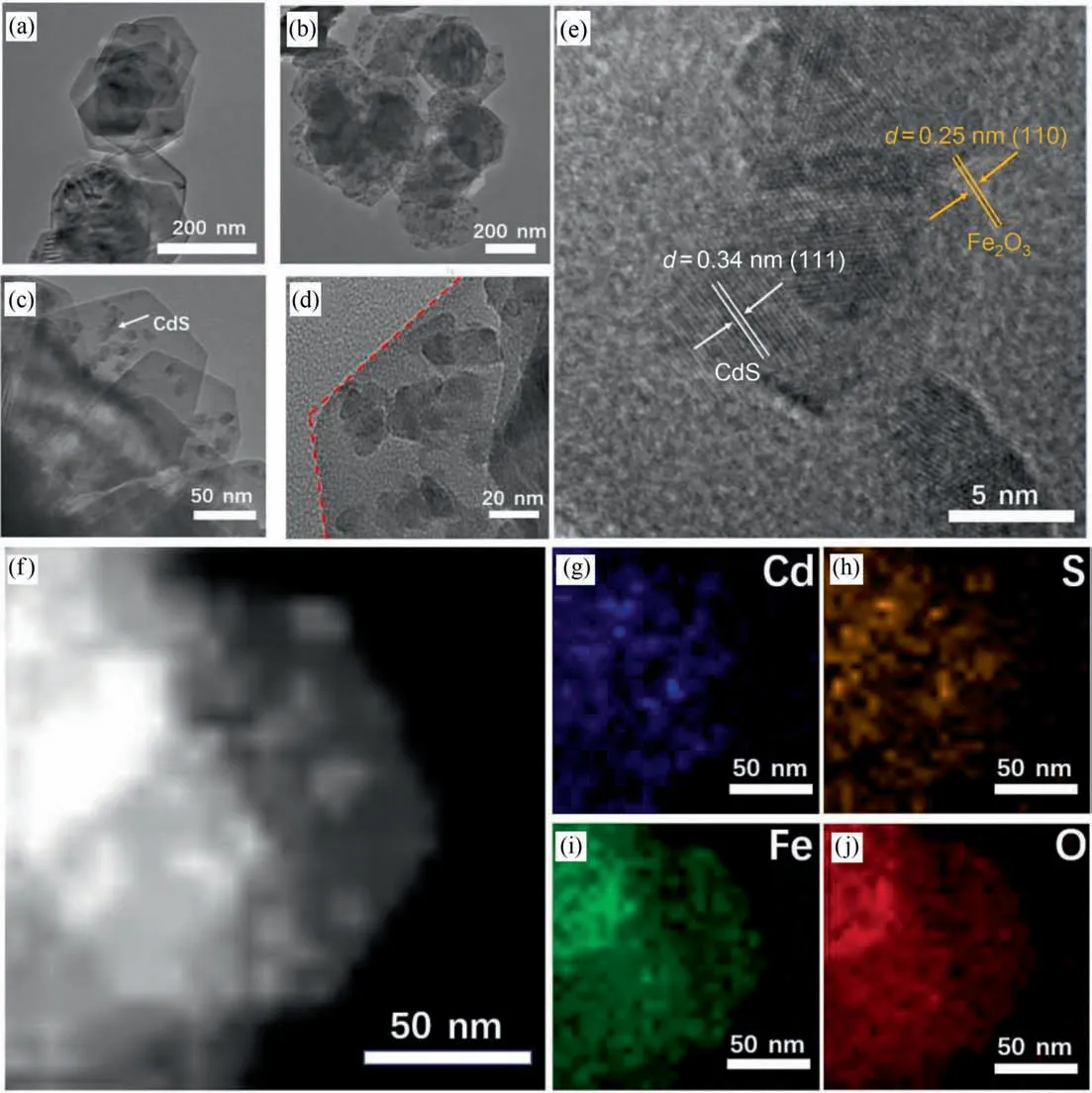
Fig.2.(a)TEM image of hexagonal α-Fe2O3,(b–d)TEM images and(e)HRTEM image of CF-15.(f)HAADF-STEM image of CF-15,(g–j)elemental mapping images of Cd,S,Fe and O from (f).
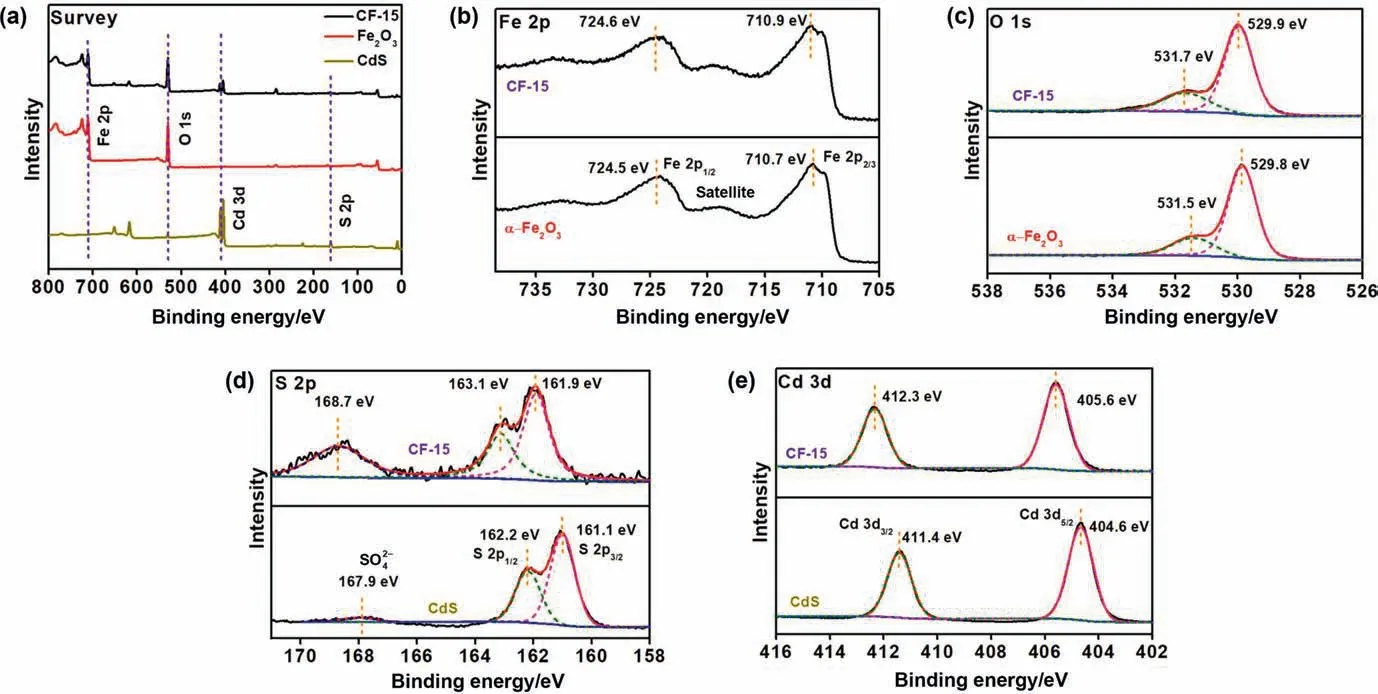
Fig.3.(a) XPS survey spectra of CdS,α-Fe2O3 and CF-15.High-resolution XPS spectra of (b) Fe 2p,(c) O 1s,(d) Cd 3d and (e) S 2p for CdS,α-Fe2O3 and CF-15.
In order to determine the microstructures and morphologies of hexagonal α-Fe2O3and CF-15,scanning electron microscope(SEM)and transmission electron microscopy (TEM) were performed and the results were shown in Fig.2.From the Fig.2(a),the pure α-Fe2O3possessed uniform hexagonal nanosheet structure with average radius size of around 100 nm.Moreover,after solvothermal reaction,it can be seen that CdS nanoparticles were anchored on hexagonal α-Fe2O3nanosheets closely to form 0D/2D heterostructure (Fig.2(b)–(d)),further implying that the comprehensive scheme we designed is reasonable and feasible.The corresponding high-resolution TEM(HRTEM)image(Fig.2(e))exhibited the lattice fringes with two different orientations.The lattice fringes with width of 0.31 nm and 0.25 nm are correspond to CdS crystal plane(1 1 1)and α-Fe2O3crystal plane(1 1 0),respectively [25,26].In addition,the high-angle annular dark field scanning transmission microscope (HAADF-STEM) image of CF-15(Fig.2(f)) and corresponding elemental mapping images (Fig.2(g)–(j)) showed that all elementals belonging to α-Fe2O3and CdS can be seen in CF-15 heterojunction.
The surface chemical elements compositions of CdS,α-Fe2O3and CF-15 were determined by the X-ray photoelectron spectroscopy (XPS) and the corresponding spectra are shown in Fig.3.Four main elements of Fe,O,Cd and S can be seen in XPS survey of CF-15 (Fig.3(a)) and there is no obvious impurity element.The detailed element states of as-prepared photocatalysts were revealed in Fig.3(b)–(e).The spectrum of Fe 2p of α-Fe2O3can be divided into Fe 2p1/2and Fe 2p3/2that located at 724.5 eV and 710.7 eV (Fig.3(b)),and satellite peaks are located at about 8 eV higher than the main peak (Fe 2p3/2) for α-Fe2O3[27,28].For O 1s of α-Fe2O3XPS spectrum (Fig.3(c)),the surface lattice oxygen and adsorbed oxygen are approximately located at 529.8 eV and 531.5 eV [29].As shown in Fig.3(d),the Cd 3d of CdS XPS spectrum can be decomposed into two peaks of Cd 3d3/2and Cd 3d5/2that located at 406.6 eV and 411.4 eV,corresponding to Cd2+in CdS [30].Three peaks for S 2p3/2,S 2p1/2andcan be seen in S 2p of CdS XPS spectrum (Fig.3(e)),which located at 161.1 eV,162.2 eV and 167.9 eV,attributing to the S2-in the metal sulfide and S2-in sulfate,respectively [31,32].The significant change of peak (S2-in sulfate) intensity between CdS and CF-15 was due to more S2-in sulfate arising from the release or exposure of S ions in CF-15 [33].Moreover,compared with CdS and α-Fe2O3,the positions of peaks for CF-15 were shifted to higher angles,which may due to the construction of heterojunctions leading to the changes in the physical and chemical environment.
The UV–vis diffuse reflectance spectra of CdS,α-Fe2O3and CF-15 are displayed in Fig.4(a) to investigate the optical absorption performance.It can be clearly seen that the CdS and α-Fe2O3possess strong visible light absorption with absorption edge at around 550 nm and 650 nm,respectively.The optical absorption spectra of the CF-15 are similar to those of the α-Fe2O3after forming the heterojunction,which is significantly higher than that of the pure CdS,which means that the heterojunction maintains excellent absorption performance.Based on the Kubelka–Munk function,the energy gaps of CdS and α-Fe2O3can be calculated to be 2.42 eV and 2.0 eV (Fig.4(b)).In addition,the positions of valence band (VB) for CdS and α-Fe2O3were measured by VB-XPS (Fig.4(c)),which estimated to be 1.85 eV and 2.35 eV,respectively.Thus,according to the above information about energy gaps,the values of conduction band (CB) for CdS and α-Fe2O3can be calculated to be-0.57 eV and 0.35 eV,and the corresponding energy band structure is displayed in Fig.4(d).
The visible light photocatalytic H2production efficiency of asprepared samples was tested to evaluate the photocatalytic activity.Before the photocatalytic reaction,0.25 mol.L-1Na2S and 0.25 mol.L-1Na2SO3as the hole sacrificial reagents and 3% (mass)Pt regarded as co-catalysts were introduced into the photocatalytic system.As exhibited in Fig.5(a),the H2production of pure α-Fe2O3was almost negligible,while the pristine CdS possesses certain hydrogen evolution activity.Surprisingly,all of the compounds present higher photocatalytic H2production rates than pure CdS,which suggests that the construction of 0D/2D CdS/α-Fe2O3heterojunction can effectively improve the production performance of photocatalytic H2.Moreover,with the increase of CdS loading,the H2production efficiency was improved first and then decreased,CF-15 showed the highest photocatalytic H2production rate (1806 μmol.h-1.g-1),which was 3.6 times higher than that of pure CdS(Fig.5(b)).The initial increase was due to the formation of heterostructure resulting in the decreased of photogenerated carriers recombination rate for enhancing photocatalytic activity.Nevertheless,the excessive loading of CdS may hinder the light absorption efficiency of α-Fe2O3and lead to the decreased of photocatalytic activity [34].For investigation of practical application effects,cycle stability test was employed and displayed in Fig.5(c).After four photocatalytic cycles(4 h per cycle),the H2production efficiency remained above 90%of the first cycle,meaning that CF-15 possesses the excellent cyclical stability.Moreover,the XRD patterns of CF-15 composite before and after four cycles of photocatalytic reactions were investigated and shown in Fig.S1.Obviously,no apparent crystalline structure changes could be observed in the XRD patterns of CF-15 after photocatalytic reactions,further confirming that CF-15 composite photocatalyst in the Na2S and Na2SO3sacrificial solution is quite stable.Furthermore,the apparent quantum efficiencies (AQEs) of CF-15 at 420 nm,535 nm and 630 nm were calculated to be 13.7%,4.3%and 1.4%,respectively (Fig.5(d)),which were consistent with the UV/vis light absorption spectrum of CF-15.

Fig.4.(a) UV–vis diffuse reflectance spectroscopy of CdS,α-Fe2O3 and CF-15.(b) Band gap energies,(c) VB XPS spectra and (d) band structures of CdS and α-Fe2O3.
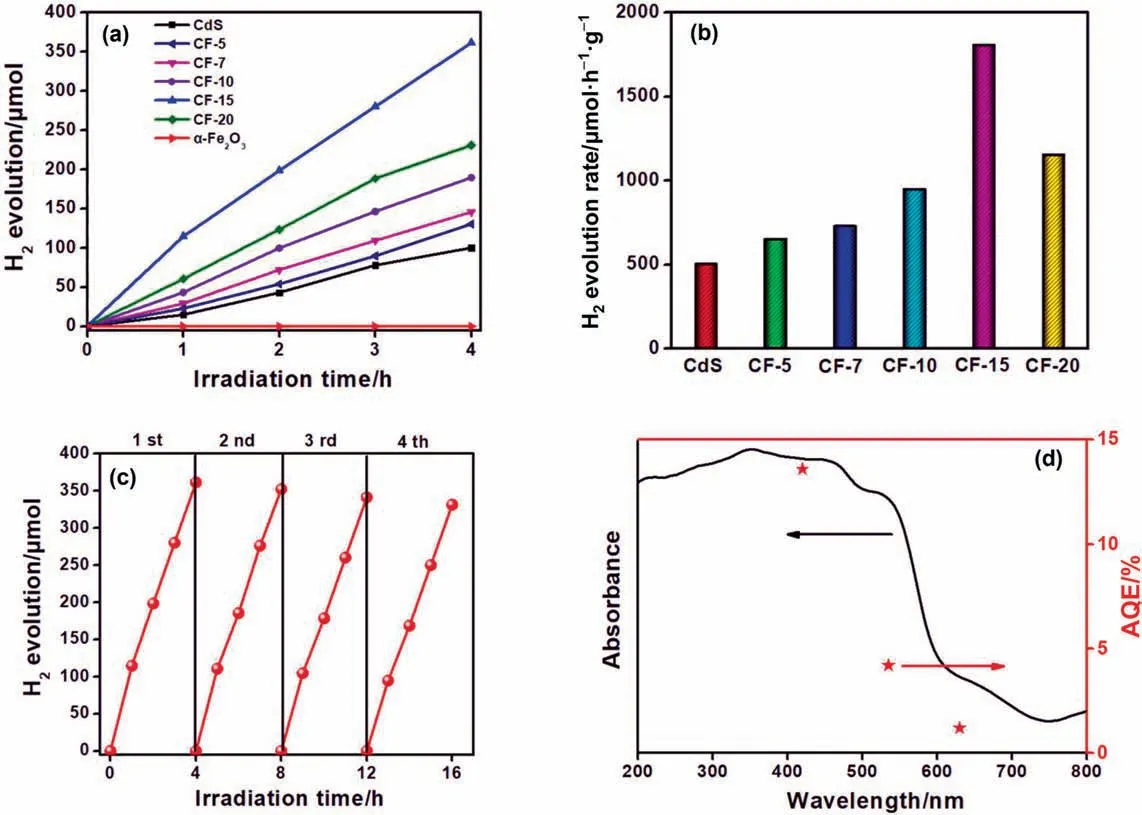
Fig.5.(a)Photocatalytic H2 production and(b)comparison of photocatalytic H2 evolution rates over as-prepared samples.(c)Cycling stability tests for CF-15 photocatalyst.(d) Wavelength dependent AQE of H2 evolution over CF-15 (right axis),UV/vis light absorption spectrum of CF-15 (left axis).
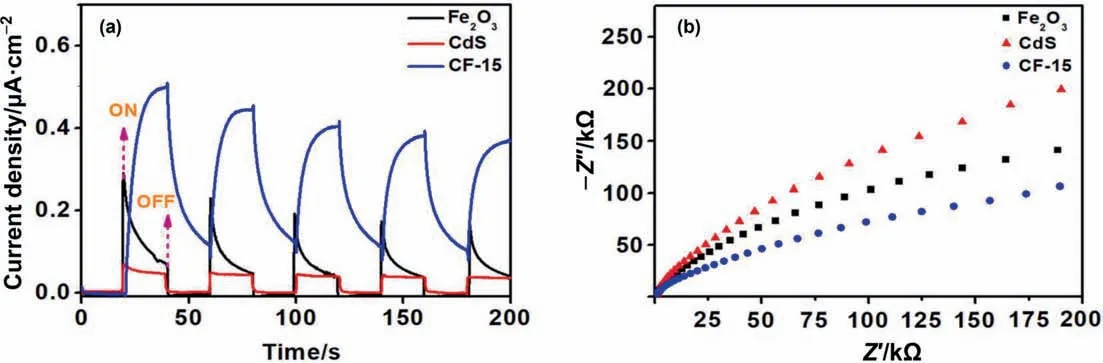
Fig.6.(a) Transient photocurrent curves and (b) EIS spectra of CdS,α-Fe2O3 and CF-15.
The photochemical characteristics of as-prepared samples were checked out and corresponding transient photocurrent curves and EIS spectra were given.From the Fig.6(a),the photocurrent intensity tests for CF-15,α-Fe2O3and CdS from high to low is consistent with the results of previous report[22],which indicates that CF-15 possesses better photogenerated charge transport rate compared with the single component photocatalytic material.Fig.6(b)shows the electrochemical impedance spectroscopy (EIS) spectra of the samples and CF-15 has the smallest arc radius,demonstrating the lowest charge transfer resistance in comparison of other samples [35–38].In addition,photoluminescence (PL) spectra can reflect the recombination and separation of photogenerated charges[39–41].Generally,the obtained PL emission peak demonstrates that electrons and holes recombine in a radiant manner[42–46].As can be seen from Fig.S2,a PL emission peak of pristine CdS at around 500 nm was observed.After coating CdS nanoparticles on the α-Fe2O3hexagonal nanosheets,the intensity of PL emission peak has been decreased,suggesting the photogenerated charges in composite materials has excellent separation and transfer efficiency.

Fig.7.Nitrogen adsorption-desorption isotherms of α-Fe2O3 and CF-15 samples.
The specific surface area of photocatalyst is one of the key factors affecting its photocatalytic activity.The specific surface area of Brunauer-Emmett-Teller (BET) can be obtained from the nitrogen adsorption-desorption isotherms.The α-Fe2O3and CF-15 composite display type-IV isotherms with SBETof 15.96 and 16.68 m2.g-1,respectively (see Fig.7).Due to the successful formation of 0D/2D contact surface,compared with α-Fe2O3,the specific surface area of CF-15 was improved,which can provide more active sites for improving photocatalytic activity.
In order to determine the transfer path of photogenerated electrons in CdS/α-Fe2O3heterojunction,the electronic spin resonance(ESR) spectra of α-Fe2O3and CF-15 were carried out and the corresponding signals were represented in Fig.8(a) and (b).It can be seen that no obvious signals of dimethyl pyridine N-oxide superoxide radicalswere found in α-Fe2O3sample when visible light was turned on and irradiated for 10 min,which due to the valance band(VB)of α-Fe2O3(0.35 eV vs.NHE)was more positive than that of the reduction potential of(-0.33 eV vs.NHE)[47].Somewhat differently,the strong signals of DMPO-can be clearly observed in CF-15 sample under visible light irradiation,indicating the photogenerated electron transfer path in CdS/α-Fe2O3heterojunction was not Type II heterojunction mechanism(Fig.8(c)).Moreover,as the supplement,the ESR spectra of pure CdS are displayed in Fig.S3.The CB and VB positions from CdS are -0.57 eV and 1.85 eV,respectively,which spans the following two energy level potentials:(i)(-0.33 eV vs.NHE) and (ii)H+/H2(0 eV vs.NHE).However,the position of VB for CdS is 1.85 eV,which is more negative than that of the potential of OH-/·OH(2.4 eV vs.NHE).Thus,only signals ofcan be seen in CdS.If the electrons electron transfer path attribute to type II rather than Z-scheme,the electrons located on the CB of CdS will be transferred to the CB of α-Fe2O3,leading to no generation ofand H2in FC-15.As a result,the co-existence ofand H2in CdS/α-Fe2O3photocatalytic system can further prove the Zscheme approach (Fig.8(d)).Based on the above experimental and test results,a possible photocatalytic mechanism of H2production for CdS/α-Fe2O3heterojunction was proposed and exhibited in Fig.8(e).Specifically,α-Fe2O3/CdS heterojunction were exhibited to produce photo-generated electrons and holes through the visible light irradiation.Moreover,according to the Z-Scheme,the electrons on the CB of α-Fe2O3can combine with the holes in VB of CdS;while the electrons on the CB of CdS can react with H+to produce H2,and the holes in VB of α-Fe2O3will react with NaS and Na2SO3,which makes the electron-hole pairs effective separation and transfer.

Fig.8.ESR spectra of (a) α-Fe2O3,and (b) CF-15 under visible light irradiation for detecting DMPO-·O2- in methanol dispersion.(c,d) Schematic illustration of possible mechanisms for charge transfer over CdS/α-Fe2O3 heterojunction.(e) Photocatalytic mechanism of H2 production for CdS/α-Fe2O3 heterojunction.
4.Conclusions
In summary,the CdS/α-Fe2O3heterojunction photocatalyst was synthetized by a general solvothermal method.Under the synergistic effect of 0D/2D contact surface and Z-scheme photoinduced electron transfer mechanism,the hydrogen production rate of 15% (mass) CdS/α-Fe2O3was enhanced dramatically and reached to 1806 μmol.h-1.g-1,which was 3.6 times higher than that of pure CdS.Meanwhile,after four cycles,the production of H2remains above 90% of first cycle,implied that the favorable cyclical stability of CdS/α-Fe2O3.The improved photocatalytic H2production can be attributed to the larger specific surface area,the more active sites,the higher redox potential and the reduced recombination of electron-hole pairs in CdS/α-Fe2O3.This work provides a direction for the modified semiconductor photocatalysts through contact synergistic action of surface contact and electron transfer path.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the founding support from the National Natural Science Foundation of China(21906072 and 22006057),the Natural Science Foundation of Jiangsu Province(BK20190982),‘‘Doctor of Mass entrepreneurship and innovation”Project in Jiangsu Province,and Doctoral Scientific Research Foundation of Jiangsu University of Science and Technology (China) (1062931806 and 1142931803).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.03.055.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
