Auto-thermal reforming of acetic acid for hydrogen production by ZnxNiyCrOm±δ catalysts:Effect of Cr promoted Ni-Zn intermetallic compound
2022-04-27XuanyiJiaXiaominHuQiaoWangBaiquanChenXingyueXieLihongHuang
Xuanyi Jia,Xiaomin Hu,Qiao Wang,Baiquan Chen,Xingyue Xie,Lihong Huang,2,*
1 Department of Chemical and Pharmaceutical Engineering,Chengdu University of Technology,Chengdu 610059,China
2 Richard G.Lugar Center for Renewable Energy,Indiana University-Purdue University,Indianapolis,IN 46202,United States
Keywords:Ni-Zn intermetallic compound Zn-Cr layered double hydroxide Hydrogen production Auto-thermal reforming Biomass Catalysis
ABSTRACT A series of ZnxNiyCrOm±δ catalysts were synthesized via a typical co-precipitation method,in which Zn-Cr layered double hydroxides(LDHs)were found and Ni-Zn intermetallic compound(IMC)was formed after reduction in hydrogen.During auto-thermal reforming (ATR) of acetic acid (HAc),the Ni-Zn IMC was transformed into Ni/(amorphous-ZnO)-ZnCr2O4 species with uniformed distribution and appropriate interaction within these Ni-Zn-Cr-O species;besides,the adsorbed oxygen promoted the activation and transfer of oxygen species;therefore,deactivation by oxidation,sintering and coking was inhibited.And the optimized Zn2.37Ni0.63CrO4.5±δ catalyst presented high activity and stability in a 45-h ATR test with HAc conversion near 100%and hydrogen yield at 2.7 mol-H2/mol-HAc,showing potential for hydrogen production via ATR of HAc.
1.Introduction
Hydrogen can be produced via acetic acid(HAc)which obtained from fast-pyrolysis of biomass.Generally,several catalytic processes,including steam reforming (SR),partial oxidation (POX)and auto-thermal reforming (ATR),have been employed to produce hydrogen from HAc [1].Among these ways,ATR presents the superiority for its self-heat sustainability and diverte HAc transformation route for hydrogen production via Eq.(1) [1]:
Ni-based catalysts can effectively promote the conversion of HAc for its excellence in the cleavage of C–C and C–H bonds in reforming of hydrocarbons and oxygenates [2].However,coke can be found over Ni-based catalysts because of deposition of carbonaceous species over Ni metal[3];besides,the oxygen is mainly consumed at the leading edge of the fixed-bed reactor,which leads to the high temperature up to 1000°C in the up-stream of the catalyst bed under the oxidative atmosphere of ATR [4].As a result,oxidation and sintering of Ni metal can be found during ATR [5].Meanwhile,hydrogenation of CO/CO2by Ni metal brought about high CH4selectivity,which compromised hydrogen generation[6].
To address these concerns,additives may need to improve reactivity via modification of structures and textures within Ni-based catalysts,while alloys and/or intermetallic compounds can be a solution to tailor active sites with Ni metal [7].Zn turns out as a promoter for Ni-based catalysts,in which Ni-Zn intermetallic compounds (IMC) can be fabricated and shows influence on the electronic and geometric aspects within Ni-Zn alloys or IMCs [8].Furthermore,the Ni-Zn IMCs have also been capable to suppress methanation in the SR of methanol by inhibiting cleavage of C-O bonds [9].
Considering variable compositions of layered double hydroxides (LDHs) with uniformly dispersed divalent and trivalent metal ions[10],LDHs precursor with Ni-Zn can be a promising candidate to fabricate well dispersed Ni-Zn IMC species.To form a LDH precursor with divalent ions of Zn/Ni,Cr2O3species stands out for its thermal stability with high melting point and variable valence,and was also found effective to improve adsorption/activation of reactants like CH4/H2O,as reported in catalysts of Pt/Cr2O3and Ni/Cr2O3[11,12].Meanwhile,Cr species also promoted oxidation of formaldehyde,acetone,toluene and ethyl acetate,which may be formed as by-products during ATR of HAc [13].
Inspired by the above reports,ZnxNiyCrOm±δcatalysts were prepared by co-precipitation and then Ni-Zn IMC species were obtained after reduction.The catalysts were screened for hydrogen production in ATR of HAc,and characterizations were conducted to explore role of IMC in ATR.
2.Experimental
2.1.Catalyst preparation
Precursors of ZnxNiyCrOm±δcatalysts were prepared by coprecipitation method.Solution of nitrate salts including Zn,Ni and Cr was prepared,and drop wisely added in a baker with mixed solution of sodium carbonate and sodium hydroxide at CO32–/OH–=1/16 and (total of metal cation electron charge)/(OH–)=1/8,while the co-precipitation conditions were maintained at pH=10 ± 0.5 and 78 °C under vigorous stirring.The resulting precipitates were aged at 78 °C for 12 h,filtered and washed with deionized water for three times,dried at 105 °C for 12 h,and calcined at 700 °C for 4 h.These obtained catalysts with 15 wt% NiO for different molar ratios at (Zn+Ni)/Cr=1/1,3/1 and 6/1 were denoted as ZNC1-1,ZNC3-1 and ZNC6-1,respectively,as listed in Table 1.

Table 1 List of ZnxNiyCrOm±δ catalysts as prepared
2.2.Characterizations
X-ray diffraction (XRD) patterns were recorded by an X-ray diffractometer (DX-2700,Haoyuan Instrument) with Cu Kα radiation.H2temperature-programmed reduction(TPR)was conducted in a fixed-bed reactor at 10 °C.min-1in a 5.0% H2/N2flow.X-ray photoelectron spectroscopy (XPS) was screened by a Kratos Axis-Ultra DLD spectrometer (Kratos,U.K.) using Al Kα radiation(1486.6 eV).The binding energies were calibrated relative to the C1speak from the carbon contamination at 284.8 eV.The reduced catalysts for characterizations were reduced in H2at 700°C,cooled down to room temperature in high purity nitrogen,and stored in vials with high purity nitrogen before testing.TG-DTA analysis was carried on an DTG-60 apparatus (SHIMADZU,Japan) in air from 50°C to 800°C at 10°C∙min-1.The morphology of spent catalysts was recorded by Scanning electron microscopy (Quanta FEC250).
2.3.Catalytic performance test
ATR of HAc was carried out in a fixed-bed reactor.Typically,the catalysts were reduced in hydrogen at 700 °C for 1 h before reaction.The mixture of water and acetic acid was fed by a liquid pump(P230II,Elite Instruments) and vaporized at 240 °C,mixed with oxygen and nitrogen at a molar ratio of HAc/H2O/O2/N2=1.0/4.0/0.28/3.0,and introduced into the reactor.The products were analyzed online using a gas chromatography (SC-3000B,Chuanyi Instrument).The HAc conversion (XHAc),selectivity to carboncontaining products (Si) and hydrogen yield (YH2) were calculated by Eqs.(2)–(4),respectively.The carbon balance over all catalysts was recorded within a ± 5% error range.


Fig.1.Catalytic performance of ZnxNiyCrOm±δ at 650 °C and 24840 ml.(g cat)-1.h-1 for ATR of HAc.

In the above equations,Fi,inoroutis the molar flow of i species at the inlet or at the outlet of the reactor,and niis the molar ratio between the carbon-containing products and acetic acid.
3.Results and Discussion
3.1.Catalytic performance of the ZNC series catalysts
The ZNC series catalysts were tested in ATR of HAc for 45 hours.Over the ZNC1-1 catalyst,as shown in Fig.1,the HAc conversion and the hydrogen yield reached 99% and 2.6 mol H2∙(mol HAc)-1in the first 20 hours,but decreased to 70% and to 1.6 mmol H2∙(mol HAc)-1,respectively,in the end.Meanwhile,the CH4selectivity increased to about 3%,and trace of acetone was found (0.3%).
The ZNC3-1 catalyst with t-NiZn IMC presented a better activity:The HAc conversion was near 100% with a stable H2yield at 2.7 mol H2∙(mol HAc)-1during the 45 h test,while a hydrogen yield rate at 332.3 mmol H2∙(g cat)-1∙h-1was recorded.In addition,methanation were suppressed with only trace of CH4detected in the product.At the maximum conversion level,the outlet concentrations of products after water trap were recorded as followed:N2(the balance gas as calibration standard for GC analysis),38.9%;H2,35.1%;CO2,16.9%;CO,9.4%;by-products of acetone and methane were almost near zero.
For the ZNC6-1 with low Cr content in the 45 h test,the hydrogen yield was continuously decreased from 2.6 mol H2∙(mol HAc)-1to 1.1 mmol H2∙(mol HAc)-1,and the acetic acid conversion decreased from 100% to 60%,while the CH4selectivity increased to 4.2%.

Fig.2.Catalytic test of ZNC series catalysts on effect of temperatures:(a) ZNC1-1;(b) ZNC3-1;(c) ZNC6-1.

Fig.3.Catalytic test of ZNC3-1 catalyst on (a) ratios of O2/HAc and (b) effect of GHSV at 650 °C.
In addition,to further evaluate reactivity of ZNC catalysts,effects of temperatures,O2/HAc and gas hourly space velocity(GHSV) were tested over these catalysts,as shown in Fig.2 and Fig.3.Over the three catalysts,the hydrogen yield recached maximum at 650 °C.When decrease the temperature,both hydrogen yield and HAc conversion decreased,accompanying with the higher selectivity to the methane and acetone.In contrast,the hydrogen yield also slightly decrease when the temperature is above 650 °C,which originates from the occurrence of reverse water gas shift reaction.The results indicate that 650 °C can be an optimal temperature for ATR of HAc.
Besides,as shown in Fig.3(a),a set ratio of O2/HAc were tested in ATR of HAc.When the ratio was zero,despite a higher hydrogen yield at 2.9 mol H2∙(mol HAc)-1was recorded,the process was exactly the SR of HAc that needs external heat supply.And the hydrogen yield decreased with further addition of oxygen.Therefore,the ratio of O2/HAc at 0.28 can be accepted for ATR with consideration of both heat balance and the hydrogen yield.In addition,the effect of GHSV was evaluated as shown in Fig.3(b).Though a wide range of space velocity from 24840 ml.g-1.h-1to 49680 ml.g-1.h-1was applied,the ZNC3-1 catalyst was capable to transform acetic acid and achieve high hydrogen production,indicating its potential for practical application.As a comparison,a blank test without catalyst was conducted at 650°C,and the conversion of HAc was less than 40% with a low hydrogen yield at 0.02 mol H2∙(mol HAc)-1,suggesting the ZNC3-1 catalyst is crucial for acetic acid conversion and especially for hydrogen production.
3.2.Characterization of catalysts
3.2.1.Precursors of catalysts
Precursors of ZNC catalysts were scanned by XRD,as shown in Fig.4(a).No obvious peaks were detected in ZNC1-1 catalyst with less Zn content,indicating that there were mainly amorphous species.With increasing Zn content in ZNC3-1,there were peaks of Zn-Cr hydrotalcite structure in a form ofas layered double hydroxides (LDHs) [14].For ZNC6-1 with more Zn,strong peaks of ZnO(PDF#:36–1451) were found,and the ZnO species can be derived from LDHs:as reported by Chen [15],excess Zn species brought about precipitate of Zn(OH)2in the beginning of precipitation and transformed into ZnO after aging.
3.2.2.Oxides of catalysts.
XRD patterns of calcined catalysts were presented in Fig.4(b).After calcination,precursors of ZNC1-1 were converted from amorphous state to spinel phases as NiCr2O4(PDF#:54–0961) and ZnCr2O4(PDF#:22–1107)),while weak peaks of NiO (PDF#:44–1159) were found as well.Over the calcined ZNC3-1 with more Zn,the Zn-Cr hydrotalcite phase disappeared and transformed into spinel phases,and phases of NiO and ZnO were also formed.With excess Zn species and less Cr species in the ZNC6-1 catalyst,the ZnO species turned out as the main phase.In order to investigate whether it is possible to form a solid solution,the standard patterns of ZnO,NiO,Cr2O3and CrO2were analyzed with the patterns of the LDHs-derived oxides,as shown in Fig.S1 (Supplementary Material).It can be found that there were none of the three catalysts presenting diffraction peaks of Cr2O3and CrO3,and the positions of the diffraction peaks of ZnO and NiO are basically consistent with their standard spectra without significant shift.At the meantime,spinel of ZnCr2O4and NiCr2O4present similar crystal parameters and species of (Ni,Zn)Cr2O4can be formed as a mixed solid solution.
Texture was also checked by N2adsorption–desorption isotherms,as shown in Table S1 and Fig.S2.According to the classification of IUPAC,the isotherms in Fig.S2(A)as type II with H3-type hysteresis loop were recorded.Fig.S2(B)shows that the pore diameters of the ZNC1-1,ZNC3-1 and ZNC6-1 catalysts are centered near 2.4 nm,2.6 nm and 2.7 nm,respectively,suggesting that they were mesoporous structures.
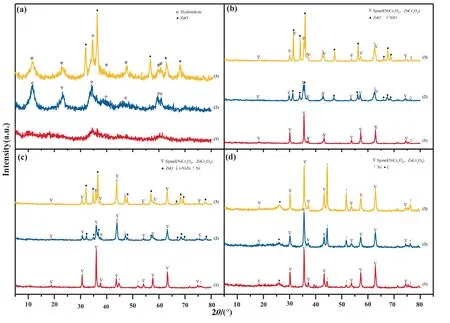
Fig.4.XRD patterns of (a) precursors,(b) calcinated catalysts,(c) reduced catalysts and (d) spent catalysts:(1) ZNC1-1;(2) ZNC3-1;(3) ZNC6-1.

Fig.5.TPR profiles of the calcined ZnxNiyCrOm±δ catalysts.
3.2.3.Reduced catalysts
Catalysts were reduced at 700 °C and screened with XRD,as shown in Fig.4(c).For the ZNC1-1 catalyst,spinel phases still exist,while the NiO phase disappeared with emergence of metallic Ni(PDF#:04–0850).With increasing Zn content in ZNC3-1 and ZNC6-1,peaks of spinel and ZnO became weaker,while a new phase of t-NiZn (PDF#06–0672) was detected as an intermetallic compound (IMC) with a tetragonal L10structure [16].Formation of the t-NiZn IMC can be accounted to the Ni0species:Ni0promoted activation of H2and produced H*species,which then spillover to adjacent Zn2+species within (Zn,Ni)Cr2O4spinel and ZnO,promoting Zn2+reduced to Zn and forming t-NiZn alloy.
Fig.5 illustrates the H2-TPR profiles of the ZNC series catalysts,while the deconvolution of the H2-TPR profiles is listed in Table S2.For ZNC1-1,the peak at 449°C can be ascribed to the reduction of surface nickel oxide species,while a main peak near 750°C can be attributed to the reduction of nickel in the NiCr2O4spinel structure[11,17],which was lower than that of NiAl2O4spinel [11].
With increasing Zn content in ZNC3-1 and ZNC6-1,a peak near 290 °C can be attributed to the reduction of amorphous NiO.The deconvolution results of H2-TPR show that the integrated area of the peaks at 650–750 °C of ZNC3-1 and ZNC6-1 is much higher than that of ZNC1-1.Studies by Wang et al.[18],Pan et al.[19]and Anjaneyulu et al.[20] demonstrated that ZnO can be partially reduced to Zn0species with promotion of Ni.Furthermore,the XRD patterns of reduced catalysts indicate that t-NiZn IMC species were formed within ZNC3-1 and the ZNC6-1 after reduction,suggesting that NiO,ZnO and NiCr2O4could be reduced in the mean time.
In addition,the results of quantitative calculation show that the integrated area of the two peaks in ZNC6-1 catalyst is smaller than that of ZNC3-1 catalyst.According to Wang et al.[18],Vagia et al.[21] and Toebes et al.[22],this is because there is a strong metal-support interaction (SMSI) between Ni and ZnO species,and more surface Zn oxides species may hinder the adsorption of hydrogen on surface Ni atoms and restrain reduction of Ni-Zn species.
X-ray photoelectron spectroscopy(XPS)experiments were conducted over the reduced catalysts to find the state of Ni species,as shown in Fig.S3.Within the Ni2p3/2region,the peak near 852.6 eV and 855.6 eV can be attributed to Ni0and Ni2+,respectively,while the shake-up peak of Ni2+was detected near 861.1 eV [23].The results of Fig.S3 indicate that Ni species over all catalysts were partly reduced;for the reduced ZNC3-1 catalyst,the peak of Ni0was strong with a ratio of Ni0/(Ni0+Ni2+)=38.8%,according to quantitative analysis.Meanwhile,the reduced ZNC6-1 catalyst was recorded with a ratio of Ni0/(Ni0+Ni2+) at 23.9%.
3.2.4.Spent catalysts
To find possible variation during ATR,the spent ZNC catalysts were screened by XRD and SEM,as presented in Fig.4(d) and Fig.6.For ZNC1-1,a broad peak emerged at 25.3° in XRD,which can be ascribed to graphitic carbon (PDF#:41–1487).Meanwhile,large amount of carbon can be observed in the SEM image (Fig.6(a)),which was consistent with the peak of carbon in XRD.Besides,in TG/DTA profile(Fig.S4(a)),a mass gain of 0.49%near 250°C was recorded and can be attributed to oxidation of Ni metal[24],while a major mass loss of 27.2% near 500 °C can be attributed to combustion of carbon deposition [25].Within the ZNC1-1 catalyst,there were spinel phase of NiCr2O4and NiO after calcination,and Ni0particles were formed after reduction as the active surface species,while no t-NiZn IMC species was found.Overtime,the ZNC1-1 catalyst surface was gradually covered by carbon species and there was no enough Ni species available for conversion of HAc,thus a poor catalytic performance was observed.
For the ZNC3-1 catalyst derived from the LDH precursors,the t-NiZn IMC formed from species of (Ni,Zn)Cr2O4/NiO/ZnO could modify the interaction and promote the reduction of nickel species,as suggested by the shift of peak and corresponding integral area in H2-TPR profile (Fig.5) [26].However,peaks of t-NiZn IMC species disappeared in Fig.4(d),while ZnO was not found as well.To find whereabouts of the t-NiZn IMC and ZnO phases in ZNC3-1,the ATR test was stopped at 1 h,5 h,10 h and 45 h,respectively,and the working ZNC3-1 catalyst was then scanned with XRD;As shown in Fig.S5,it was found that t-NiZn IMC disappeared as early as in the first hour,and was converted into Ni metal and amorphous ZnO (a-ZnO) species.The results suggest that under the oxidative atmosphere (O2and H2O) within ATR of HAc,the Zn located near the surface of t-ZnNi IMC was partly oxidized,which led to the formation of amorphous ZnO[27];then,t-ZnNi IMC decomposed and metallic nickel was formed over amorphous ZnO.As a result,over a stable ZnCr2O4skeleton in ZNC3-1,mixed oxides of Ni/(a-ZnO)-ZnCr2O4was formed with dispersed species of Ni0and a-ZnO,as shown in Fig.4(d) and Fig.S5.Meanwhile,as indicated by the SEM image (Fig.6(b)) and TG-DTA profile (Fig.S4(b)),a low mass loss at 4.9% by coke gasification was recorded.In addition,the spent catalyst was cooled down in nitrogen,transferred and stored into vials with nitrogen,and then charged for XPS scanning;the XPS results in Fig.S3(b) indicate that during the ATR process,Ni metal as an active component remained relatively stable with Ni0/(Ni0+Ni2+)=27.7%,suggesting the species of Ni/(a-ZnO)-ZnCr2O4with active sites of Ni0derived from t-NiZn IMC was stable and active to convert HAc [28].Therefore,a high catalytic performance with the hydrogen yield stabled at 2.7 mol H2∙(mol HAc)-1was obtained in the 45 h ATR test,while coke deposition was relieved at the same time.

Fig.6.SEM images of the spent catalysts:(a) ZNC1-1;(b) ZNC3-1;(c) ZNC6-1.
For the ZNC6-1 catalyst with more Zn and less Cr,the interaction was stronger than the other two catalysts and a lower reducibility of nickel was obtained,which provides fewer active sites for the conversion of HAc.Additionally,the XPS of Ni2p (Fig.S3(c) and(d)) showed that the ratios of Ni0/(Ni0+Ni2+) of ZNC6-1 catalyst decreased from 23.9%to 16.6%,indicating that the ZNC6-1 catalyst was partially oxidized during ATR.Meanwhile,there is a high mass loss of 25.1%of coking gasification(Fig.S4(c)and Fig.6(c)).Therefore,except for the strong interaction inhibited the reduction of nickel,partial oxidation of Ni metal species and coking can be the major factors for deactivation of the ZNC6-1 catalyst in ATR.
4.Conclusions
The ZNC series catalysts were prepared by co-precipitation method.Precursors of LDHs were formed and then transformed into spinel of (Ni,Zn)Cr2O4species,which were further converted into t-NiZn IMC species that modify the interaction of nickel and support.During the ATR,the t-NiZn IMC was transformed into Ni/(amorphous-ZnO)-ZnCr2O4species with uniformed distribution and appropriate interaction within these Ni-Zn-Cr-O species as active sites.Besides,only trace coke was observed over the active sites.Therefore,deactivation by oxidation,sintering and coking was inhibited.As a result,over the Zn2.37Ni0.63CrO4.5±δcatalyst,the HAc conversion maintained near 100% and the hydrogen yield reached 2.7 mol H2∙(mol HAc)-1,showing potential in application for hydrogen production via ATR of HAc.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by International Cooperation Program from Sichuan Science and Technology Program (Nos.2019YFH0181,2015HH0013) and the National Natural Science Foundation of China (No.21276031).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.04.017.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
