Hydrate formation from liquid CO2 in a glass beads bed
2022-04-27NanLiJingYuKanChangYuSunGuangJinChen
Nan Li * ,Jing-Yu Kan# ,Chang-Yu Sun ,Guang-Jin Chen
1 State Key Laboratory of Heavy Oil Processing,China University of Petroleum-Beijing at Karamay,Karamay 834000,China
2 State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Beijing 102249,China
Keywords:Liquid CO2 Hydrate formation Sequestration Kinetic Water conversion
ABSTRACT CO2 sequestration in marine sediments as solid hydrates is a potential way to capture and store anthropogenic CO2.In this study,hydrate formation from liquid CO2 in marine sediments was simulated in a glass beads bed,and the factors affecting the kinetics of hydrate formation were investigated.The results indicated that the rapid initial hydrate formation with a high driving force always increases the mass transfer resistance,which slows down hydrate growth.The final ratio of water conversion is higher under conditions of low temperature and higher pressure.A smaller particle size is conductive to initial CO2 hydrate growth,but the water conversion ratio in a bed with larger particles is slightly higher.Compared with other factors,the change in water saturation has an obvious effect on the final water conversion.To inhibit the initial hydrate formation during the injection process,in this paper,a kinetic inhibitor is proposed for pre-injection into marine sediments.This work shows that at a low pressure,a lowconcentration inhibitor has an obvious inhibition effect on hydrate growth.However,at a high pressure,it is necessary to increase the concentration of inhibitor to produce an obvious inhibition effect.
1.Introduction
Carbon dioxide(CO2)is the main contributor to global warming.Excessive CO2emission from human activities has led to serious environmental risks.CO2sequestration has been proposed to significantly capture and store anthropogenic CO2.Sequestrating CO2in deep-sea sediments as hydrates is a potential means for long-term storage of CO2due to the thermodynamic and mechanical stability of CO2hydrate[1,2].When pressurized CO2is injected into low-temperature marine sediment,the part combined with pore water will be transformed into solid hydrates,which are stable and can prevent the leakage of injected CO2[3].Thermodynamic and kinetic studies on the CO2hydrate formation and dissociation are of energy and environment double significance,which have attracted many researchers’attention.The influences of thermodynamic and kinetic factors on CO2hydrate formation in the bulk phase have been extensively studied [4,5].However,for the perspective of geological CO2sequestration,the understanding of CO2hydrate formation in porous media is still limited,which has been concerned in recent works.
After CO2injection in cold marine sediments at high pressure,the storage capacity,migration range of CO2and physical properties of CO2hydrate bearing sediments all depend on the rate of CO2hydrate growth and amount of formed CO2hydrates.The key factors affecting the growth of CO2hydrates are the existence of additives,lithology of the sediment,the seawater’s salinity,and the temperature and pressure conditions of the injection point.
Kang et al.studied the kinetic properties of CO2hydrates in porous media and the effect of a kinetic promoter,sodium dodecyl sulphate(SDS),on the formation process[6].The results showed that when the driving force is greater than 1 MPa,the initial rate of hydrate is significantly affected by the driving force;additionally,when SDS is added,the initial rate of hydrate increases significantly.Yang et al.studied the effect of SDS/tetra-hydrofuran(THF) on the formation and decomposition of CO2hydrates [7].Their experimental results show that when the SDS concentration is 1000 mg.L-1,the induction time of hydrate formation is the shortest,and the hydrate saturation is the highest.Yang et al.studied the effect of salt concentration in pore water on the growth process of CO2hydrates [8].The presence of salt doesn’t significantly inhibit the growth of CO2hydrates,but the conversion of the CO2hydrates is slightly reduced.The gas consumption at the initial stage of hydrate formation in brine is even higher than that in pure water.Dicharry et al.studied the growth kinetics of CO2hydrates in porous media that had different pore sizes and the influence of surfactants[9].They found that although the presence of porous media and surfactants promoted the growth of hydrates,the promotion was weaker than the increase in hydrate growth impetus.Mekala et al.studied the effects of sand size and pore water salinity on the growth kinetics of CO2hydrates [10].They found that when the average grain size of sand is 0.46 mm,it is most conducive to hydrate formation.This result also provided a basis for the selection of a submarine sandstone reservoir for CO2storage.Bhattacharjee et al.also studied the growth kinetics of CO2hydrates in four kinds of sediments with different particle sizes [11].The experimental results showed that the growth process of hydrate dynamics increases with a decrease in particle size.
Besides the experimental studies,several models have been developed to explain the kinetic behaviours of CO2formation.Takahashi et al.established a prediction model of CO2hydrate growth based on the kinetics experiments[12].The model divides the growth of CO2hydrates into two parts:(1) gas phase diffuses through a CO2hydrate membrane and contacts with water phase,making the hydrate membrane grow and thicken gradually.(2) A new interface of gas and water appears during the process of hydrate film thickening and spreading and becomes a new growth point for CO2hydrates.Yu et al.proposed a kinetic model for the formation of CO2hydrates in the process of CO2migration in sediment pores [13].The model divides the CO2hydrates formation into three parts:(1)Hydrate formation in the CO2front,(2)hydrate formation between interface between CO2and interstitial water behind the CO2front,and (3) hydrate formation on the surface of sands behind the CO2front.And the second one was verified as the main part making a great contribution to CO2hydrate saturation.
These research results have greatly improved the understanding of the growth kinetics of CO2hydrates.However,the reported kinetic studies on CO2hydrate formation in sediments have mostly focused on gaseous CO2,in which the driving force of CO2hydrate formation is small[14–18].During the process of seafloor injection,CO2exists in liquid form [19],but reported studies have been less involved the growth process of hydrates in the system of liquid CO2–water–sediment.The hydrate formation from liquid CO2has a higher driving force,which makes the transfer of mass and heat during hydrate formation significantly different from that at low pressure.During the process of injection,if a CO2hydrate is formed at the beginning of CO2migration,the injection channel will be blocked [20].To avoid injection plugging,generally,the injection temperature of CO2should be increased so that its temperature gradually cools to the temperature required for hydrate formation after a certain migration distance.To further limit the formation of CO2hydrates in the process of injection and make CO2migrate longer,a certain amount of hydrate kinetic inhibitors can be preinjected into the sediments.Thus,hydrate formation can be inhibited in front of the injected CO2before reaching the target zone.Additionally,the inhibition effect can be gradually removed by inhibitor dilution in the migration front which consistently contacts the original water in sediments.However,the irregular particle surface in the sediment is conducive to hydrate nucleation.Whether kinetic inhibition plays a corresponding role requires comparative study.Reported studies always focus on hydrate formation in the presence of promoters;however,very few studies have reported hydrate formation in a CO2–water–inhibitor–sedi ment system [17,21].Inhibex 501 (a copolymer of vinyl caprolactam and vinyl pyrrolidone in 2-butoxyethanol) is a commercial hydrate inhibitor,which has been shown to be an effective hydration kinetic inhibitor.Chen et al.carried out the assessment of hydrate kinetic inhibitors with visual observation,they found that Inhibex 501 had the moderate inhibiting performance [22].Two kinetic hydrate inhibitors (Inhibex 501 and 713) were tested their performance during methane hydrate formation by Mark et al.,both of which obviously increased the induction time and decreased the hydrate growth rates[23].
In this study,the kinetic behaviours of hydrate formation from liquid CO2in sediment were studied,and the effects of temperature,pressure,water content,particle size,and kinetic inhibitor on the growth of CO2hydrates were investigated.
2.Experimental
2.1.Apparatus
As shown in Fig.1,the device used in this study mainly consisted of five parts:a hydrate formation reactor,CO2storage vessel,constant temperature water bath,gas intake/exhaust system,and data acquisition system.The inner diameter of the hydrate formation reactor was 30 mm,the outer diameter was 53 mm,the effective volume was 63 ml,and the maximum pressure it could bear was 40 MPa.The effective volume of the CO2storage vessel was 500 ml with a built-in piston.The vessel’s bottom port connects with a high-pressure manual pump,which was applied to pressurise the pure water below the piston to inject CO2into the hydrate formation reactor.There were a set of temperature and pressure sensors in the CO2storage vessel and hydrate formation reactor.The temperature sensor was a Pt100-type thermocouple with an accuracy of ±0.1 K.The precision of the pressure sensor was ±10 kPa,the temperature control accuracy of the constant temperature water bath was±0.1 K,and the temperature and pressure data were collected by Monitor and Control Generated System(MCGS) software.
2.2.Materials
In this study,glass beads of different particle sizes(from As-One Company,BZ-01,0.105–0.125 mm;BZ-02,0.177–0.250 mm;BZ-04,0.350–0.500 mm) were used to represent seafloor sediment particles and had a reference density of 2.6 g ml-1.The glass beads beds were packed loosely,and the porosity of BZ-01,BZ-02 and BZ-04 were 42.04%,41.82%,and 40.30%,respectively.A 3.5%(mass)NaCl aqueous solution was used to represent natural seawater.CO2gas was supplied by Beijing AP BAIF Gas Industry Co.,Ltd.and had a purity of 99.99%.Inhibex 501 was acquired by American International Specialty Products (ISP).
2.3.Experimental procedure
First,glass beads were mixed with a NaCl solution to achieve the respective water saturations (volume fraction occupied by water in pore space,20%,40%,and 60%).Premixed glass beads and water were then filled and pressed into the hydrate formation reactor,and the reactor was evacuated.The water bath temperature was adjusted to 282.15 K,and when the water bath temperature was stable,liquid CO2was injected from the CO2storage vessel into the hydrate formation reactor.The amount and pressure of the injected CO2was controlled by adjusting a manual water pump.When pressure in the hydrate formation reactor reached 6 MPa,we stopped injecting CO2and allowed it to stabilize for 30 min to saturate the pore water.The water bath temperature was then adjusted to the target temperature,and hydrates gradually formed in the pores of the glass bed during the cooling process.The same experimental procedure was conducted on low-pressure gaseous CO2for comparison.In contrast,the gaseous CO2was injected at an initial pressure of 3.3 MPa,and the water bath temperature was maintained at 277.15 K.

Fig.1.Apparatus for the kinetic study of CO2 hydrate formation.
To investigate the effect of inhibition on the growth process of CO2hydrates,the water-soluble kinetic inhibitor,Inhibex501,was used to prepare salt solutions (3.5%(mass) of NaCl) with concentrations of 0.1%(mass) and 0.5%(mass) in several runs.The experimental conditions for each group are listed in Table 1.
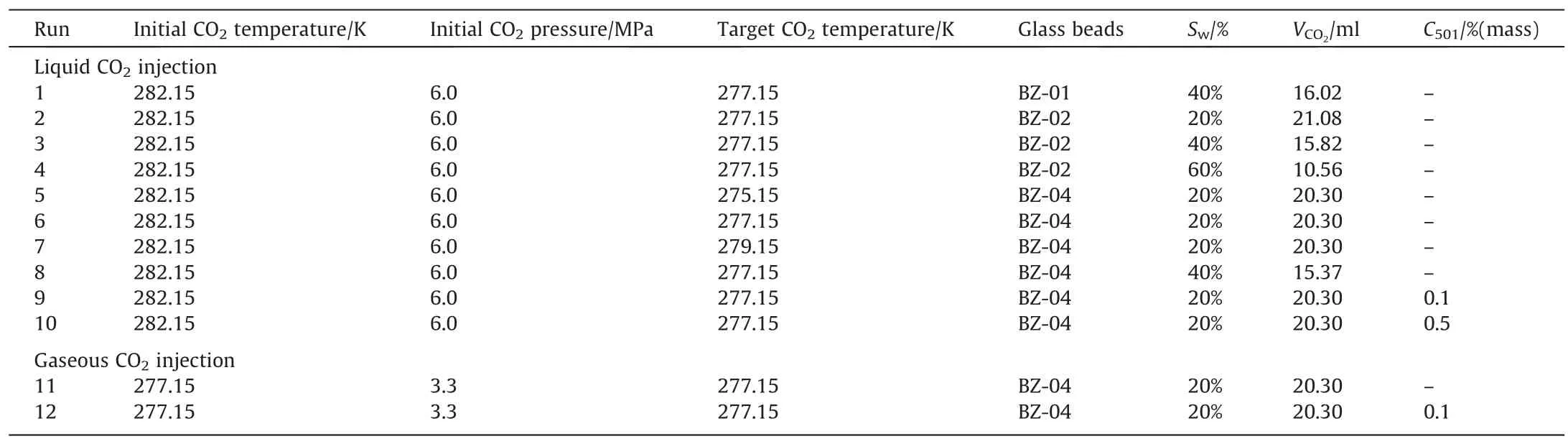
Table 1 Experimental conditions for CO2 hydrate formation in the glass beads bed
2.4.Data processing
The consumption of CO2,Δntat time t during the hydrate formation was calculated by:

where,P0and Ptare the pressure in the reactor before hydrate formation and at time t,respectively;T0and Ttare the reactor temperature before hydrate formation and at time t,respectively;z0and ztare the compressibility factor of CO2in the reactor before hydrate formation and at time t,which are calculated by the Benedict–We bb–Rubin–Starling equation of state(BWRS EoS) and Duan’s Model for gaseous CO2and liquid CO2,respectively [24,25].Vporeis the pore volume of the glass bead bed in the reactor.Vwis the volume of free water in the pores of the glass bead bed before hydrate formation.Vpore-Vwis the initial volume of injected CO2,which can also be measured by the manual pump.VHis the volume of hydrate in the pores at time t.Vw,tis the volume of free water remaining in the pores at time t during hydrate formation,which equalswhere 1.25 is the ratio of the molar volume of the empty hydrate lattice to that of water.VHcan also be calculated by:

where 5.75 is the hydration number of CO2hydrate,and Mwis the mole mass of water and ρwis the density of water.Vpore-Vw,t-VHis the residual volume of CO2at time t,which equals Vpore-Vw-0.2VH.Thus,Eq.(1)in the manuscript can be simplified as:

Eq.(3) was used to calculate the data of all the tables and figures in the manuscript.It also has been used in our previous work for the calculation of formed CH4hydrates [26].
In addition,the solubility of CO2was not considered in this study for two reasons.Firstly,since the CO2solubility in water is very low,the amount of dissolved CO2can be ignored.Secondly,in this study,liquid CO2was kept stable under a constant high pressure for a period of time to saturate the water before cooling,thus the pore water was pre-saturated in this stage.Therefore,the consumption of CO2can be regarded as the amount of CO2converted into hydrate phase during the cooling process.
The conversion ratios of water to hydrates was calculated by:

where nw0represents the initial water content and 5.75 is the hydration number of CO2hydrates [27].
3.Results and Discussion
3.1.Changes in temperature and pressure during hydrate formation
Fig.2 shows the P–T history curves,and changes in pressure and temperature with time during CO2hydrate formation from gaseous CO2and from liquid CO2,respectively.Fig.2 (a) and (b) show the experimental results from run 11,where the injected CO2was initially in a gaseous state[3,25],the glass beads were of type BZ-04,the water content was 20%,and the target temperature of the water bath was 277.15 K.
In run 11,an induction period of approximately 50 min was experienced before hydrate formation.As the CO2hydrate was gradually formed,after approximately 10 h,the pressure in the hydrate formation reactor gradually approached the phase equilibrium pressure[28].Fig.2(c)and(d)show the experimental results of run 7,where the injected CO2was initially in a liquid state [3],the glass beads were of type BZ-04,the water content was 20%,and the target temperature was 279.15 K.P–T curves were used here to determine the time points when the phase equilibrium temperature of the CO2hydrate formation zone is reached as shown in Fig.2c,and the time points in Fig.2d,can be used as the zero time when calculating the water conversion rate.The figures show that after the injected warm liquid CO2was rapidly cooled to the hydrate formation zone,the pressure decreased,and there was no obvious induction period and no significant exothermic reaction.After 40 min,the reactor pressure became stable,and the final reactor pressure was significantly higher than the equilibrium pressure of CO2hydrates and even higher than the liquefaction pressure of CO2at 279.15 K [3,25,28].By comparing these two CO2hydrate formation processes,we found that the rate of hydrate formation from liquid CO2was significantly greater than that from gaseous CO2.
3.2.Effect of temperature and pressure on the formation of CO2 hydrates
Runs 5–7 investigated the growth of CO2hydrates at different temperatures (275.15 K,277.15 K,and 279.15 K) in the glass bead bed (BZ-04) with a water saturation of 20%.The changes in the water conversion ratios with time during the hydrate formation for runs 5–7 are shown in Fig.3a.
For runs 5–7,the water bath cooling processes were the same,during which the driving forces for CO2hydrate growth were very similar(temperatures are almost the same).When the reactor temperatures reached the respective pre-set temperatures for each run,the CO2hydrate growth driving forces were different;thus,the hydrate formation rates were different.Therefore,as displayed in Fig.3a,the initial rates of hydrate formation at different temperatures were very close.Not as expected,further hydrate formation at 273.15 K with the highest driven force didn’t have the highest growth rates in the three runs until 10 min.As reported in the literature [29],initial hydrates were in the form of thin hydrate film on the interface between CO2and water.The hydrate film was denser under high driving force,which increased the resistance of mass transfer and led to sluggish hydrate formation [30,31].Fig.3a also revealed that,at higher temperatures (runs 6 and 7),hydrate formation rates decreased much rapidly after the rapid growth process and hydrate formation was stopped before 20 min.This is due to two reasons:(1)As mentioned above,formed hydrate acted as ‘‘barrier”of mass transfer between the interface;(2) ‘‘Salt-removing effect”of hydrate formation caused increase in pore water’s salinity,which increased the equilibrium temperature of CO2hydrate and decreased the driving force for hydrate formation.The ratio of water conversion in run 5 undertook the runs 6 and 7 at 10 min and became stable at 65% much higher than that obtained in the other two runs.This indicated that lower temperature was in favour of CO2storage,which provided higher driven force for hydrate formation to overcome the resistance of mass transfer and negative salinity enrichment.

Fig.2.Changes in the pressure and temperature during runs 7 and 11:(a)P–T curve during CO2 hydrate formation from gaseous CO2,(b)changes in pressure and temperature with time during hydrate formation from gaseous CO2,(c)P–T curve during CO2 hydrate formation from liquid CO2,and(d)changes in pressure and temperature with time during hydrate formation from liquid CO2.

Fig.3.Ratios of water-hydrate conversion during CO2 hydrate formation at different temperatures (a) and pressures (b).
Fig.3b shows the changes in the water conversion ratios during the process of CO2hydrate formation under different pressure conditions.As shown in the figure,the rate of hydrate formation from low-pressure gaseous CO2was far less than that under highpressure liquid CO2,and the water conversion ratio under highpressure conditions was higher.Under high pressure,the ability of liquid CO2to invade the pore channel is stronger,and the water is dispersed into small water droplets,which is conducive to the deep conversion of water.Moreover,in,due to the sufficient CO2,the process of hydrate formation from liquid CO2always maintains a high driving force.
3.3.Effect of water saturation and particle size on CO2 hydrate formation
The growth process for CO2hydrates in the glass bead bed(BZ-02)at different water saturations(20%,40%,and 60%)was investigated in runs 2,3,and 4,respectively.The change rule of the water conversion ratios with time during the hydrate formation process is shown in Fig.4.Obviously,the initial rate of CO2hydrate formation decreased with an increase in the water saturation,and the final conversion ratio was higher.This is because the lower the water saturation,the more uniform the water dispersion,resulting in a larger gas–liquid interface.Under the condition of low water saturation,the pores in the sand bed have good connectivity,and CO2is distributed in the pores as a continuous phase.When hydrates are formed,the pore channels are not easy to block,which is conducive to mass transfer and heat transfer.

Fig.4.Ratios of water–hydrate conversion during CO2 hydrate formation at different initial water saturations.
Runs 1,3,and 8 were used to investigate the growth process of the CO2hydrate in three glass beads with of different sizes(BZ-01,BZ-02,and BZ-04).The change rule of water conversion ratios with time during the hydrate formation process is shown in Fig.5.As shown in the figure,the smaller the particle size,the faster the initial growth rate of the CO2hydrate.This is because the smaller the particle size,the larger the specific surface area of pores in the bed.As also shown in the figure,the water conversion ratio was very close in the bed with different particle sizes but slightly higher in the bed with large particles.This was due to the rapid growth rate of the CO2hydrate in the small particle bed,which led to an increase in the mass transfer resistance with the increase in CO2hydrates,making the final conversion ratio of water slightly smaller.

Fig.5.Ratios of water–hydrate conversion during CO2 hydrate formation in glass beads of different sizes.
3.4.Effects of the kinetic inhibitor
Runs 9–12 were performed to determine the effects of inhibitors with different concentrations (0%(mass),0.1%(mass),and 0.5%(mass)) on hydrate formation from gaseous and liquid CO2.The experimental results are shown in Fig.6.As shown in Fig.6a,the growth of the CO2hydrate at low pressure was significantly inhibited when the 0.1%(mass) inhibitor was added.The growth time for the CO2hydrate increased from 20 to 90 h.However,during the initial stage of hydrate formation,the inhibition was not obvious.In the first hour,the hydrate conversion with inhibition was close to that without the inhibitor.However,after hydrate formation,with continuous consumption of water,the inhibitor concentration in the solution gradually increased,and the inhibition effect became more obvious;thus,the hydrate formation speed gradually slowed down.At low pressure,the addition of inhibitor had a significant inhibitory effect on the growth of the hydrate,but it did not have a significant impact on the final water conversion.As shown in Fig.6a,the final water conversion ratio was close to that obtained by adding or not adding inhibitors.In contrast to low pressure,as shown in Fig.6b,the addition of the 0.1%(mass)inhibitor did not significantly inhibit the hydrate growth.When the water conversion ratio reached 42%,the formation rate for the CO2hydrate slowed down gradually;however,the final conversion ratio of water slightly increased.When the inhibitor concentration was increased to 0.5%(mass),the initial hydrate formation rate slowed down,and the final conversion ratios of water was lower than that when the inhibitor concentration was 0.1%(mass).

Fig.6.Ratios of water–hydrate conversion during CO2 hydrate formation at different concentrations of hydrate inhibitor:(a) hydrate formation from gaseous CO2 and (b)hydrate formation from liquid CO2.
3.5.Comparison of different factors
Fig.7 compares the effects of different factors on the final water conversion during hydrate formation from liquid CO2under high pressure.As shown in the figure,the change in water saturation had an obvious effect on the final water conversion,followed by the temperature,whereas the particle size and inhibitor concentration had little effect on the final water conversion.The water saturation determines the distribution of CO2in the pores.At low water saturation,CO2is in a continuous phase in sediment;at high water saturation,CO2presents as a dispersed phase(bubbles or droplets)in sediment.This determines the initial hydrate growth interface size and pore connectivity;thus,the change in the water content will significantly change the final water conversion ratio.At a high driving force(high pressure and low temperature),the hydrate formation rate and final conversion ratio of water to hydrate were both high;thus,the change in temperature (the driving force of hydrate formation) had little effect on the change in hydrate conversion ratio.

Fig.7.Comparison of the effect of different parameters on the final water– hydrate conversion during hydrate formation from liquid CO2.
The results improved the understanding of CO2hydrate growth in a water–inhibitor–sediment system,which can provide reference for CO2sequestration in marine sediments as hydrates.However,there are some limitations of our study.Firstly,the influence of different ions in seawater on the kinetic behavior of hydrate formation was not considered,and the glass beads used here were different from the natural seafloor sediments.Secondly,in this study,the hydrate formation pressure and the injection pressure were relatively low (Less than 6 MPa).Since sequestrating CO2in deep-sea sediments is a potential means for long-term storage of CO2,it’s necessary to investigate behaviors of CO2hydrate growth in sediments under higher formation pressure and injection pressure (more than 10 MPa).In addition,the mechanism of using kinetic inhibitors to regulate the growth of carbon dioxide hydrate during injection needs to be further studied.
4.Conclusions
In this study,the effects of temperature,pressure,water content,particle size,and kinetic inhibitors on the growth of CO2hydrates in a sediment system were studied.The conclusions are as follows:
The initial rate of hydrate formation increased with a decrease in temperature;however,at high undercooling,the growth rate slowed down after the initial hydrate formation,which increased the mass transfer resistance.The results showed that the lower the temperature,the higher the hydrate conversion.The hydrate formation rate from gaseous CO2was much lower than that from liquid CO2,and the final water conversion ratio was higher at high pressure.The initial growth rate of CO2hydrates increased with decreases in the water saturation and the final conversion ratio.A smaller particle size was conductive to initial CO2hydrate growth,but the water conversion ratio in the bed with large particles was slightly higher.Compared with other factors,the change in the water saturation had an obvious effect on the final water conversion.At low pressure,the low-concentration inhibitor had an obvious inhibition effect on the hydrates growth.However,at high pressure,it was necessary to increase the concentration of inhibitor to produce an obvious inhibition effect.
CRediT Authorship Contribution Statement
Nan Li:Conceptualization,Methodology,Investigation,Writing– original draft.Jing-Yu Kan:Methodology,Investigation,Visualization,Writing– review &editing.Chang-Yu Sun:Supervision,Validation.Guang-Jin Chen:Supervision,Validation,Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No.22008258),Tianshan Youth Program in Xinjiang Uygur Autonomous Region (2019Q089) and the Scientific Research Program of Universities in Xinjiang Uygur Autonomous Region (XJEDU2019Y069).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
