Experimental study of the mass transfer behavior of carbon dioxide absorption into ternary phase change solution in a packed tower
2022-04-27YihanYinAoqianQiuHongxiaGaoYanqingNaZhiwuLiang
Yihan Yin,Aoqian Qiu,Hongxia Gao,Yanqing Na,Zhiwu Liang
Joint International Center for CO2 Capture and Storage (iCCS),Provincial Hunan Key Laboratory for Cost-effective Utilization of Fossil Fuel Aimed at Reducing Carbon-dioxide Emissions,College of Chemistry and Chemical Engineering,Hunan University,Changsha 410082,China
Keywords:Monoethanolamine-sulfolane-water Phase change solution Mass transfer Packed tower
ABSTRACT Phase change absorbents for CO2 are of great interest because they are expected to greatly reduce the heat energy consumption during the regeneration process.Compared with other phase change absorbents,monoethanolamine (MEA)-sulfolane-water is inexpensive and has a fast absorption rate.It is one of the most promising solvents for large-scale industrial applications.Therefore,this study investigates the mass transfer performance of this phase change system in the process of CO2 absorption in a packed tower.By comparing the phase change absorbent and the ordinary absorbent,it is concluded that the use of MEA/sulfolane phase change absorbent has significantly improved mass transfer efficiency compared to a single MEA absorbent at the same concentration.In the 4 mol.L-1 MEA/5 mol.L-1 sulfolane system,the CO2 loading of the upper liquid phase after phase separation is almost zero,while the volume of the lower liquid phase sent to the desorption operation is about half of the total volume of the absorbent,which greatly reduces the energy consumption.This study also investigates the influence of operating parameters such as lean CO2 loading,gas and liquid flow rates,CO2 partial pressure,and temperature on the volumetric mass transfer coefficient (KGaV).The research shows that KGaV increases with increasing liquid flow rate and decreases with the increase of lean CO2 loading and CO2 partial pressure,while the inert gas flow rate and temperature have little effect on KGaV.In addition,based on the principle of phase change absorption,a predictive equation for the KGaV of MEA-sulfolane in the packed tower was established.The KGaV obtained from the experiment is consistent with the model prediction,and the absolute average deviation (AAD) is 7.8%.
1.Introduction
With the development of industries and economies,air pollution and the greenhouse effect caused by the use of fossil fuels have seriously threatened the global environment.The International Energy Agency (IEA) predicts that by 2030,the fossil fuels coal,oil,and natural gas will still be dominant [1].Global warming and the greenhouse effect are common challenges in the sustainable development of all countries.Therefore,there is an urgent need to remove CO2from the exhaust gases produced by the combustion of fossil fuels such as in coal-fired power plants.To reduce such a huge amount of CO2emissions,CO2capture and storage(CCS)[2]is the most direct and effective means to achieve CO2emissions reduction.Research and development of CO2capture technology with good industrialization prospects can not only reduce environmental problems caused by large amounts of CO2emissions,but also can effectively use CO2resources for industrial production to provide economic benefits.In a word,it is a work of long-term significance to develop an industrialized technology for more efficient and economical recovery of CO2from flue gas.
As an important gas–liquid separation technology in the current chemical industry,packed towers are widely used in various unit operations such as rectification,absorption,desorption,and ion exchange due to their high mass transfer efficiency,large processing capacity,and good operational stability.When the packed tower is used for gas–liquid reactions in CO2capture,the amine capture agent is sprayed from the top of the tower through the liquid distributor,and the flue gas enters the absorption tower at the bottom.In this arrangement,the gas entering the tower is in countercurrent contact with the amine solvent,and a chemical reaction occurs on the surface of the packing layer,which is the process of CO2absorption.Studying the mass transfer performance of amine solvents to capture CO2in a packed tower is an effective way to evaluate the application prospects of capture agents.
In industrial applications,the development of new low-cost and low-energy CO2absorbents plays a decisive role in reducing energy consumption in the regeneration process,and is also a current research highlight.Research has shown that porous heterogeneous materials can efficiently fix carbon dioxide[3]recently.More generally,in order to reduce the energy consumption of carbon capture,researchers have devoted themselves to studying organic amines and compound amine absorbents of different structures to reduce the heat of absorption,but their contribution to reducing the energy consumption of carbon capture is quite limited.
In 2009,Hu and Adeyiga [4] first proposed the concept of the phase change absorbent,which quickly attracted the attention of researchers and was regarded as an ideal CO2capture agent.The currently developed phase change absorbents are divided into liquid–liquid phase-separated and liquid–solid phase-change absorbents.The phase change absorbent is a homogeneous solution before absorbing CO2,while after reacting with CO2,it is divided into two immiscible phases,namely the lean liquid phase and the rich liquid phase.Only the rich liquid phase needs to be desorbed.Compared with the traditional amine absorbent,it reduces the amount of liquid entering the desorption unit and has a larger CO2cycle load,so it is expected to reduce the energy consumption of desorption [5,6].
Svendsen’s research group[7–11]developed a 2-(diethylamino)ethanol (DEEA)-N,N-Dimethyl and 1,3-propane diamine (MAPA)composite amine phase change absorbent.The composite absorbent can form a phase change absorbent at 5 mol.L-1DEEA+2 m ol.L-1MAPA.In addition,the phase-change absorbent has been tested in the Gloshaugen(NTNU/SINTEF)pilot plant.It proved that the phase-change absorbent can effectively reduce energy consumption in large-scale industrial applications.
Perry et al.[12] developed a liquid–solid phase change absorbent,the main component of which is 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldsiloxane.After the CO2is absorbed,a highviscosity fluid is first generated,and then converted to a solid.This liquid–solid phase change absorbent has a large CO2cycle processing capacity,but it is not conducive to desorption due to its higher thermal stability.After absorbing CO2,the viscosity of this liquid–solid phase change absorbent becomes higher,resulting in unsatisfactory conditions for using traditional absorption towers for absorption.
The sulfolane amine solution was studied and analyzed by Guo et al.[13].It was found that the addition of sulfolane not only promoted the absorption of CO2by the amine solution,but also caused the solution to separate into two phases.The sulfolane amine solution offers good potential for the industrial phase change solvent to absorb CO2due to its large CO2loading capacity and relatively cheap price.
In summary,the focus of the researchers is mainly on the development of phase change solvents and the reduction of thermal energy consumption,while the research on the effect of the solution phase change on the absorption performance has hardly been considered.However,it has become clear that MEA-sulfolane phase change absorbent is one of the most promising solvents for large-scale industrial applications due to its low price,fast absorption rate and low desorption energy consumption [14,15].
In this study,the mass transfer performance of the phase change absorbent MEA-sulfolane-water ternary system in the process of absorbing CO2was studied in a packed tower to realize the industrial process of capturing and recovering CO2with high efficiency and low energy consumption.The total volumetric mass transfer coefficient (KGaV) is used as an index to investigate the influence of liquid feed flow rate,lean CO2loading,inert gas flow rate,and CO2partial pressure on mass transfer performance.In addition,a KGaVequation is established based on the phase change transition absorption process,which provides basic theoretical data for the industrial application of CO2capture by the packed tower and MEA-sulfolane-water ternary phase change absorbent.
2.Theory
At the present stage,the solubility,enhancement factor,and reaction kinetics of the reaction between CO2and organic amines have been intensively studied,but the reaction medium has been limited to aqueous solutions.The newly developed liquid–liquid phase change absorbent with a solvent-based medium that promotes phase separation,adds an opportunity for research on the reaction and mass transfer mechanism.
Some researchers have found[16] that compared with the current two-film theory of mass transfer process as shown in Fig.1,where ZGand ZLare the thickness of gas retention film and effective liquid retention film respectively,the liquid–liquid phase change absorbent has a unique mass transfer model for CO2absorption,which is a‘‘self-concentrated”phase change transition absorption process as shown in Fig.2.From the microscopic analysis,the added solvent to promote the phase separation can be used as the ‘‘carrying and transporting”phase to transport CO2gas to the amine absorption solution as the carrier phase.This process can reduce the mass transfer resistance and increase the gas–liquid mass transfer coefficient,which can significantly increase the absorption rate.Different phase change systems and their concentrations affect the volume ratio of upper and lower liquid phase separation,reaction rate,and CO2loading.
The phase change mechanism of amine sulfolane-based solution for CO2absorption is that the difference in molecular polarity leads to the phenomenon of individual agglomeration,which was proposed by Liu et al.[17].However,The Dortmund group [18]claimed that the presence of carbamate may reduce the lower critical solution temperature of the system causing phase separation.In general,the solution is a homogeneous solution before absorption,while the lower critical solution temperature keeps decreasing with the absorption of CO2and the continuous generation of carbamate.At the same time,the difference between the polarity of the hydrated ammonium group and the sulfolane solvent molecule also increases.Eventually,at a certain critical CO2loading point,the ammonium group and sulfolane begin to agglomerate due to the polar separation,resulting in a sharp decrease in the gas–liquid contact area.In addition,the viscosity of the solution after the phase change increase,resulting in an increase in the diffusion resistance of CO2gas molecules,liquid free amines,and products.During this process,the absorption rate will oscillate and then begin to decrease sharply [19].Because of the high density of sulfolane,the lower layer is mainly sulfolane solvent while the upper layer is rich amine solution with CO2.

Fig.1.Schematic diagram of two-film theory.

Fig.2.Mass transfer diagram of phase change absorbent.
3.Experimental
3.1.Materials
Reagent grade monoethanolamine (MEA) with purity of ≥99%,sulfolane with purity of ≥99%,hydrochloric acid (HCl) with purity of ≥98% and methyl orange were obtained from Aladdin Shanghai Reagent Co.,Ltd.,China.Carbon dioxide (CO2) was supplied by Changsha Rizhen Gas Co.,Ltd.,China with the purity of ≥99.9%.The required concentration of lean solution is configured by MEA and deionized water.
3.2.Experimental set-up and procedure
The flow diagram of the mass transfer experimental device is shown in Fig.3.It is mainly composed of a gas absorption section and other auxiliary devices.The gas absorption section is a packed tower with an inner diameter of 28 mm a height of 1.28 m.The packing type is Sulzer DX structural stainless steel and the tower body is vacuum insulated double-layer glass.Before the CO2absorption experiment,the required concentration of phase change absorbent was heated to the required temperature by using a constant temperature water bath (Beijing Zhongxing Weiye Instrument Co.,Ltd.model DZKW-4).A stirrer (Shanghai Jintan Xinrui Instrument Co.,Ltd.model JJ-1)is used to keep the solution in a uniformly mixed state when using a phase-separated solvent.Then,the preheated phase change absorbent and a certain proportion of gas were fed to the packed tower through a constant flow pump (Baoding Qili Precision Pump Co.,Ltd.,model BT300-01/YZ1515)to control the fluid flow rate.After the reaction was stable,the CO2concentration at different heights was measured by a capnometer (ANALYTICAL SYSTEMS TNC.NIAGARA FALLS.model NY 302WP).
In addition,after the CO2concentration measurement was completed,an appropriate amount of absorption liquid was taken at the bottom of the tower for testing.When the gas–liquid phase error was less than 10%,the experimental results were considered valid.The detailed experimental steps is described in our previous work [20].
3.3.Calculation method of volumetric mass transfer coefficient
The volumetric mass transfer coefficient(KGaV),which has great significance for understanding CO2capture with amines,is the most important basic parameter in the design of a packed tower.At steady state,the mass transfer flux (,kmol.(m2.h)-1) equation is as follows:

where KGis the total mass transfer coefficient of the gas phase(kmol.(m2.h.kPa)-1);P represents the experimental operating pressure(kPa);means the mole fraction of CO2in the gas phase anddenotes the equilibrium mole fraction of CO2in the liquid phase.
According to conservation of materials between the gas and liquid phase,at any micro-element height (dZ),a relationship can be obtained:

where aVshows the effective phase boundary area (m2.m-3);Ggindicates the flow of inert gas (kmol.m-3.h-1) andmeans the mole ratio of CO2.
Combining Eqs.(1)and(2),the calculation equation of KGaVcan be obtained as:
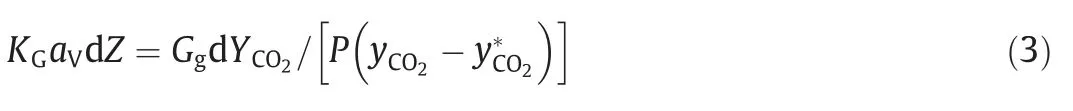
3.4.Conservation of gas and liquid phases
In order to ensure the accuracy of the experiment,material balance calculations are required.This is to ensure that the difference between the CO2reduction in the gas phase and the CO2increase in the liquid phase is less than 10%.The specific calculation formula is as follows:
The reduction of CO2in gas phase (mg):

where G denotes the gas flow rate(L.min-1)and Yinand Youtexpress the mole ratio of import and export CO2respectively.
The increase of CO2in the liquid phase (ml):

where L indicates the liquid flow rate (L.min-1);C represents the amine solution concentration(mol.L-1);αoutand αinsignify the rich liquid and lean liquid load,respectively (mol CO2.(mol amine)-1),and T is the experimental temperature (K).
4.Results and Discussion
4.1.Comparison of initial mass transfer coefficients
It is worth noting that the phase separates when the CO2loading reaches 0.2 at 298 K.It means that under the experimental conditions,the homogeneous solution with the CO2loading of less than 0.2 appears phase separation after entering the absorption tower.In this experiment,the volume ratio of the upper and lower phases after phase separation is approximately 1:1.As a result,the concentration of the upper amine solution is double,which is consistent with Liu’s research results [17].

Fig.3.Flow diagram of mass transfer experimental device.

Fig.4.Comparison of KGav varying with liquid flow rate.
Fig.4 shows the curve of KGavof 5 mol.L-1MEA,4 mol.L-1MEA/5 mol.L-1sulfolane,and 5 mol.L-1MEA/5 mol.L-1sulfolane solutions in the packed tower plotted against liquid flow rate.It can be seen that the concentration of the amine solution has a significant impact on the mass transfer performance.This is because the increase in the amine concentration not only causes a stronger driving force for mass transfer,but also allows for a larger amount of CO2to be absorbed per unit volume of the solution[21–24].However,the higher the amine concentration,the greater the energy that is consumed during regeneration[25].At the same time,it can be seen that the KGavof 5 mol.L-1MEA/5 mol.L-1sulfolane solutions is higher than that of 5 mol.L-1MEA.This is because sulfolane,as an aprotic polar solvent,has a strong affinity for CO2,which can improve the solubility and absorption rate of CO2during chemical absorption without participating in chemical reactions.In this case,the capture of CO2is affected by the interaction of physical absorption and chemical reaction,leading to the higher KGav[25].According to Liu et al.[17],the large addition of sulfolane leads to a decrease in water content,thereby reducing the amount of dissolved bicarbonate,and an increase in viscosity resulting in a slow reaction rate,so the absorption rate of the solution and the upper limit of the absorption load have been reduced.Therefore,a proper concentration ratio should be selected in actual industrial operation.
In Fig.5 it can be seen that the 5 mol.L-1MEA has the lowest KGavat the height of 0.3 m,which is mainly due to the temperature changes during the absorption process [26].However,the KGavof the two MEA/sulfolane solutions showed a sharply decreasing trend from the top of the tower and do not change significantly until the middle of the tower.On the one hand,as the different polarities between sulfolane and amine-CO2-H2O systems after the solution absorbs CO2,the solution drips and then separates into the lactescence,resulting in a reduction in the effective gas–liquid contact area.On the other hand,the enriched amine solution becomes viscous,which is not conducive to the diffusion of amine molecules and formed salts,resulting in increased diffusion resistance.At the same time,it can be seen that there is a slight upward trend in 5 mol.L-1MEA/5 mol.L-1sulfolane and a slight downward trend at the height of 0.4 min 4 mol.L-1MEA/5 mol.L-1sulfolane.This is because the MEA concentration is higher in 5 mol.L-1MEA/5 mol.L-1sulfolane,so more CO2can be absorbed.After a short period of phase separation,the probability of MEA molecules contacting CO2is higher.In 4 mol.L-1MEA/5 mol.L-1sulfolane,when the solution reached the bottom,the absorbed CO2was close to saturation,and even a slight desorption tendency began to appear.
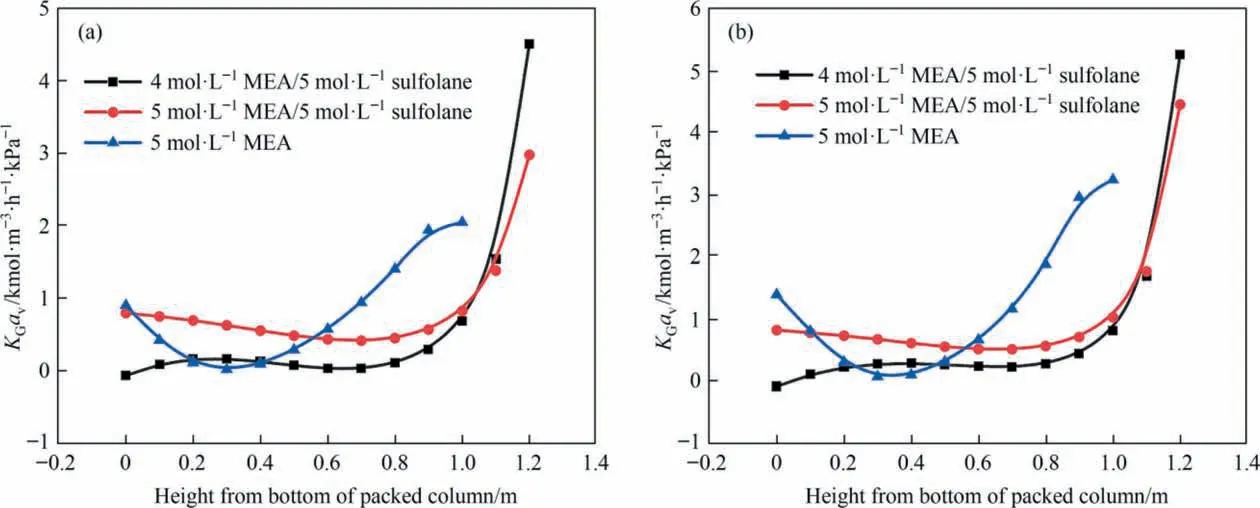
Fig.5.Comparison of KGav varying with tower height at two liquid velocities.(a) Liquid flow rate is 60 ml.min-1.(b) Liquid flow rate is 70 ml.min-1.
The characteristics of 4 mol.L-1MEA/5 mol.L-1sulfolane mentioned in the previous study [17],that are the lowest thermal energy loss and the fastest speed of desorption,along with its high cyclic capacity made it an ideal candidate for this study of its mass transfer performance.
4.2.The effect of liquid flow rate on KGav
The liquid flow rate,as a very important parameter,directly determines the volume and the cost of absorbent.In the absorption process,increasing the flow rate of the absorbent feed liquid increases the wetting rate of the packing surface,so that higher mass transfer efficiency and CO2removal rate can be obtained[27–29].Fig.6 reflects the variation of KGavwith tower height at different flow rates.It can be seen that as the liquid flow rate increases,KGavincreases significantly at the top of the tower.This is mainly because of the increase in the number of free amine molecules in the tower and the more intense fluid turbulence.In this case,the gas–liquid contact is more complete and the interaction is stronger,contributing to an improvement of mass transfer,thereby increasing the mass transfer coefficient of the liquid film[28].In the middle of the tower,the increasing trend of KGavbecomes slows.This is because the phase separation has an influence on the absorption process,resulting in the smaller value of KGavwhich changes little at this time.Therefore,when using a phase-separated solvent for absorption in the industry,it is necessary to consider the entire absorption and desorption process to select an optimal liquid flow rate to make CO2capture economical.
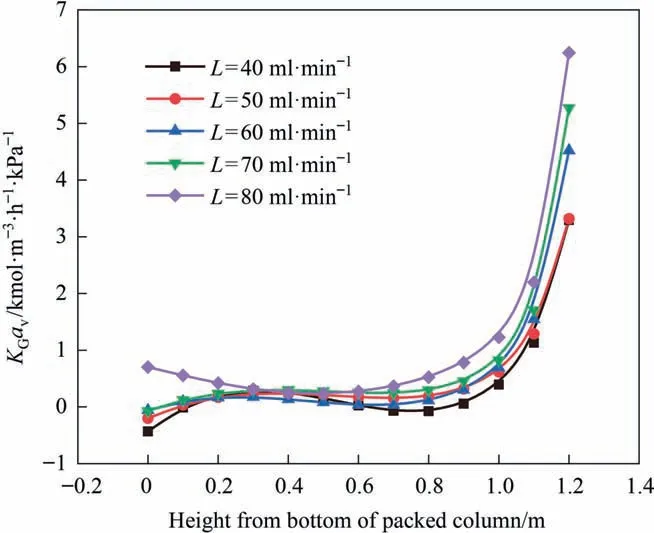
Fig.6.Influence of liquid flow rate on KGav.
4.3.The effect of gas flow rate on KGav
It is found that KGavdecreases with the increase of the inert gas flow rate until the flow rate reaches a certain value,when the growth trend of KGavis gradually slow down as Fig.7.According to the two-film theory,increasing the flow rate of the inert gas can increase the degree of gas turbulence in the tower,which promotes the surface renewal between gas and liquid,and the mass transfer coefficient increases and gradually tends to be constant.However,as the gas flow rate increases,the residence time of the gas components in the tower is shortened,resulting in insufficient contact between the gas and the liquid phase.This also accelerates the renewal of CO2molecules on the surface of the liquid film and consumes more amine molecules,which is not conducive to mass transfer.
4.4.The effect of lean liquid loading on KGav
The lean CO2loading reflects the number of active amine molecules available in the amine solution before absorption of CO2takes place.The more active amine molecules,the faster the absorption rate.It can be seen from Fig.8 that as the lean liquid loading increases,KGavshows a downward trend.A similar phenomenon was obtained by Arashi et al.[27].At too high lean CO2loading,the driving force for mass transfer between gas and liquid becomes smaller.At this point,the reduction of CO2removal efficiency cannot be compensated even if the absorbent flow rate is increased.However,the lean CO2loading cannot be reduced indefinitely because the required energy increases as the lean CO2loading decreases,and the energy demand is not proportional to the degree of loading reduction.Therefore,in the process of CO2capture,the lean CO2loading should be carefully selected to minimize energy consumption and improve removal effectiveness [30,31].

Fig.7.The influence of gas flow rate on KGav.
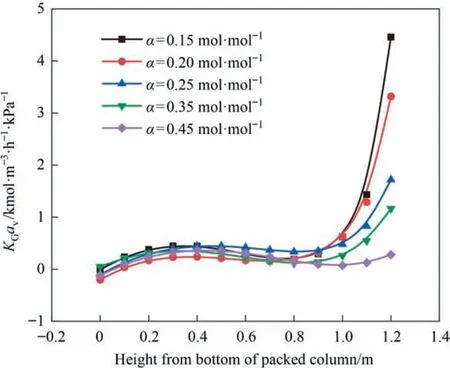
Fig.8.Influence of lean liquid loading on KGav.
4.5.The effect of partial pressure on KGav
The flue gas of traditional power plants has the characteristics of large gas flow,low CO2partial pressure and high inert gas content.Its CO2partial pressure is about 14–16 kPa.Therefore,in order to fit the actual situation,Fig.9 shows the effect of 12–20 kPa CO2partial pressure on KGav.The partial pressure of CO2reflects the proportion of CO2molecules in the gas phase,and it also affects the heat load of the reboiler.It can be seen that KGavgradually decreases with the increase of CO2partial pressure from Fig.9.This phenomenon is mainly because under certain conditions,the increase of CO2partial pressure causes more free amine molecules in the solution to be consumed,while,at the same time,the mass transfer driving force (yCO2-) increases,leading to a decreasing trend in KGav.As for 12 kPa,due to the low CO2partial pressure,it takes longer for the absorbent to reach the critical CO2loading,perhaps in the bottom of the tower,which is reflected in a better mass transfer performance at the top of the tower compared to other partial pressure.

Fig.9.Influence of partial pressure on KGav.
4.6.The effect of liquid temperature on KGav
As shown in Fig.10,when the liquid temperature increases,KGavslightly increases.This is due to the interaction of two contradictory aspects.On the one hand,when the reaction temperature increases,the viscosity gradually decreases,thereby increasing the diffusion coefficient.From the perspective of dynamics,the liquid temperature increase promotes the absorption of CO2.On the other hand,considering that the CO2dissolution process is an exothermic process,when the liquid temperature rises,the dissolution of CO2is restrained,reducing the mass transfer driving force and rate of the liquid film.This is confirmed by Chen et al.[32] that the temperature has a negative effect on the absorption when the gas is absorbed in the liquid.Under the combined effect of the two aspects,as the liquid temperature increases,the total mass transfer does not change much.

Fig.10.Influence of liquid temperature on KGav.
5.Correlation
5.1.Gas-liquid material balance analysis
This experiment uses the gas–liquid phase material balance method to evaluate the accuracy and reliability of the experiment.The scatter plot of the mass balance analysis of the gas phase concentration corresponding to the liquid phase load in the MEA/sulfolane absorption CO2mass transfer experiment is shown in Fig.11.It can be seen from Fig.11 that the CO2loading measured by the amine solution titration method is in good agreement with the data measured by the CO2gas infrared analyzer.The experimental absolute average difference (ADD) is 4.7%,which indicates that the error in the experiment is small and the experimental result is reliable.
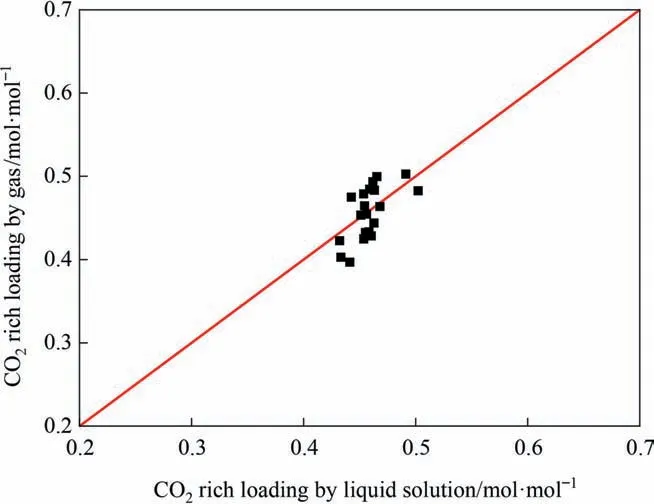
Fig.11.Scatter diagram of the gas–liquid phase loading material balance.
5.2.KGav correlations for MEA-sulfolane-water ternary system
In a packed tower,the calculation of the gas film mass transfer coefficient (kG),liquid film mass transfer coefficient (kL) and effective phase boundary area of the packed tower is very complicated and cannot be accurately obtained.Therefore,it is more practical to use KGavto express the mass transfer performance.Many researchers have tried to correlate the KGavof CO2captured by amine solution in a packed tower.The most widely accepted is the KGavcorrelation formula proposed by Kohl and Riesenfeld [33].

where F indicates the packing factor,μ shows the viscosity of the liquid (cycle per second,cps),αeqdenotes CO2balance load(mol.mol-1),andis the partial pressure of CO2(kPa).
In the actual absorption process,the only variables that can be controlled are operating parameters such as temperature,lean CO2loading,liquid flow rate and CO2partial pressure and these parameters are the key factors affecting the KGavand CO2absorption.Therefore,establishing the quantitative relationship between these operating parameters has practical guiding significance for actual chemical production.According to the literature [31,34–36] combined with our own experiments,the following formula can be preliminarily derived:

The comparison between the predicted value obtained by Eq.7 and the experimental value is shown in Fig.12.The absolute average deviation (AAD) of KGavis 7.8%,indicating that the model can be applied to the MEA-sulfolane phase separation system in the packed tower absorption of CO2.The model also has a good generalization ability,which has important theoretical guiding significance for the selection and optimization of operating parameters,the design of packed tower height and diameter,and the study of the mass transfer mechanism.

Fig.12.Comparison of KGav experimental value and predicted value.
6.Conclusions
The high energy consumption of absorbent regeneration is the main disadvantage of traditional ethanolamine aqueous solutions in the CO2capture process.Other researchers have shown that the CO2desorption rate of the rich amine solution can be significantly increased after the phase change.The amount of water vapor and the energy consumption of heating the solution during desorption process can be greatly reduced although the phase change behavior of the solvent leads to a decrease in the absorption capacity and absorption rate.The research of phase change absorbents is still in the initial stage of laboratory experiment development and performance verification.The focus of such research is mainly on the development of phase change solvents and the reduction of thermal energy consumption.There is little investigation of the effect of the solution phase change on the absorption performance and still a long way to go to achieve production applications.
The mass transfer performance of phase change absorbent MEA/sulfolane in the process of CO2absorption in the packed tower was studied.Research has shown that the KGavdecreases to almost zero at the height of 0.7 m.It can be inferred that phase separation occurs at this time.As the phase separation progresses completely,the MEA layer solution can absorb a small amount of CO2,resulting in an upward trend in KGavat the height of 0.3 m.This provides a basis for selecting the appropriate tower height to improve the mass transfer efficiency in practical applications.At the same time,the KGavincreases with increasing liquid flow rate and decreases with the increase of lean CO2loading and CO2partial pressure,while the inert gas flow rate and temperature have little effect on KGav.In addition,based on the new principle of phase change,the prediction equation of KGavin the packed tower is established.The absolute average deviation between the KGavobtained by the experiment and the model prediction is 7.8%.It shows that the prediction model has a good correlation with the experiment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The National Natural Science Foundation of China (NSFC-Nos.22138002,22078083,and 21978075),the Hunan Key R &D Program Project(2020NK2015),National Key R&D Projects in Changsha (kh2005018),National Key Research &Development Program -Intergovernmental International Science and Technology Innovation Cooperation Project (2021YFE0112800),and the science and technology innovation Program of Hunan Province(2020RC5032)
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
