Influence of zinc state on the catalyst properties of Zn/HZSM-5 zeolite in 1-hexene aromatization and cyclohexane dehydrogenation
2022-04-27DiGaoYiboZhiLiyuanCaoLiangZhaoJinsenGaoChunmingXuMingzhiMaPengfeiHao
Di Gao ,Yibo Zhi ,Liyuan Cao ,Liang Zhao,*,Jinsen Gao ,Chunming Xu ,Mingzhi Ma,Pengfei Hao
1 State Key Laboratory of Heavy Oil Processing,China University of Petroleum (Beijing),Beijing 102249,China
2 Hebei Jingzhi Technology Company Limited,Cangzhou 061000,China
Keywords:Zinc state Catalyst Catalysis Hydrogen transfer Dehydrogenation Moleclar sieves
ABSTRACT Rational design of Zn-containing HZSM-5 zeolite (Zn/HZSM-5) with high reactivity and excellent aromatization performance for olefin aromatization is crucially desired.We develop a new and uncomplicated method to synthesize Zn/HZSM-5 (IMX/Z5) with superior aromatization performance in the paper.Compared to incipient wetness impregnation (IMP/Z5) and mechanical mixing (MIX/Z5),the asprepared IMX/Z5 presents a higher amount of surface ZnOH+species(2.87%)while keeping identical bulk zinc content.As a result,more surface ZnOH+favor both the aromatization of 1-hexene and cyclohexane dehydrogenation.For the two olefin aromatization pathways(hydrogen transfer and dehydrogenation),it is the first time found both the hydrogen transfer ability and the dehydrogenation ability increase linearly with the amount of surface ZnOH+species while keeping identical bulk zinc content.We believe that the linear relationships are essential to design next generation olefin aromatization catalysts.
1.Introduction
Catalytic transformation of olefin in FCC gasoline into aromatics with high octane number has been taken as an effective method to adjust the olefin content while maintaining the octane number of FCC gasoline.HZSM-5 zeolites are widely used as the catalysts for the olefin aromatization (OTA) process due to the unique shape-selective structure for aromatic molecules and the tunable acidic property [1–5].The OTA reaction over HZSM-5 can be schematically represented as a two-stage process:(i) olefin interconversion and (ii) olefin aromatization.It has been well known that olefin interconversion simply involves olefin isomerization,cracking,and oligomerization [6].However,olefin aromatization is a multi-step consecutive reaction that includes isomerization,oligomerization,hydrogen transfer,and cyclization that occur at the Brønsted acid sites of the zeolite[7–9].Following the proposed olefin aromatization mechanism that occurs on HZSM-5,the first step is the hydrogen transfer between olefins to form olefinic carbenium ions,which are the dienes adsorbed on the Brønsted acid sites [10].In the hydrogen transfer reaction between olefins,olefins act as both hydrogen acceptors and donors for forming hydrogen-rich byproducts (alkanes) and target products (dienes)[11].Comparing the rate constants of a series of reaction steps in the kinetic model for light olefins aromatization over HZSM-5,the formation of diene by hydrogen transfer reaction between olefins is the slowest reaction step[11].So promoting the formation of dienes is essential to enhance olefin aromatization.
Modifying HZSM-5 with zinc has been demonstrated to promote aromatics through strengthen the conversion from olefins to dienes [12–15].Due to the presence of zinc species,there is a new reaction pathway in the olefin aromatization.Olefins can form allylic species by directly abstracting a hydrogen ‘atom’ (or ion)[11],and aromatics could be directly polymerized by two allylic species without the formation of alkanes.The molecular hydrogen replaces the alkane becoming the new by-product [16].So the more carbon atoms would turn from the reactants to aromatics.And zinc act as the new active site in catalysts promoting the olefin aromatization process.However,there are different zinc states in Zn/HZSM-5 and the effect of zinc states for olefin aromatization is still rather elusive.
Zn/HZSM-5 can be synthesized in several manners:hydrothermal synthesis,ion exchange,wetness impregnation,mechanical mixing,etc.[16–21].These different preparation methods impact the existing states of Zn and the catalytic performance in OTA[17,22–25].Currently,the common preparation method of Zn/HZSM-5 catalysts is the wet impregnation method.In the aqueous solution,the hydrated zinc ions can transfer a hydrogen ion to water molecule by acting as an acid (+H2O ⇌[ZnOH(H2O)5]++H3O+).The ZnOH+species is formed by stepwise removing water molecules during calcination[26],which is recognized as the active site for the formation of aromatics.The temperature-programmed surface reaction with CO as probe molecule(TPSR/CO)was used to confirm the presence of ZnOH+species by Bernt et al.[24].The reaction of Zn/HZSM-5 with CO produced the equimolar amounts of H2in addition to CO2.The authors proposed the following reaction Eq.(1):

While Biscardi et al.inditified the hermodynamic instability of ZnOH+species by using X-ray adsorption experiment [23].Especially in the Zn/HZSM-5 prepared by wetness impregnation and ion exchange,the exchange usually can achieve 60%.So,the ZnOH+species would decompose into ZnO and restore the Brønsted acid sites of the zeolite during the calcination (as shown in Eq.(2))[27].Niu [17] proposed that the Zn/HZSM-5 catalysts prepared by mechanical mixing and wet impregnation method contain two states of Zn species:ZnO and ZnOH+species.The zinc species mainly exist in the state of macro ZnO particles accompanied by a small amount of ZnOH+species in the catalyst prepared by mechanical mixing.Whereas in the catalyst prepared by impregnation,the ZnOH+species are the main existing states of zinc.Long and co-workers [28] introduced P and La into Zn/HZSM-5 to increase the ratio of the amount of ZnOH+to ZnO.Despite the nature of active zinc sites over Zn/HZSM-5 in OTA is still controversial,the ZnOH+seems more active than ZnO.The aromatics selectivity increased with increasing with the amount of ZnOH+in the formation of aromatics from methanol [22],propane [29],and butene[12].Gabrienko and co-workers[12] analyzed the n-butene aromatization process on different Zn/H-BEA catalysts by using13C solid-state nuclear magnetic resonance.The results suggested that the combination of ZnO species and n-butene is too weak to achieve dehydrogenation.This means the states of zinc have a significant influence on the OTA process.The studies on the effect of zinc species on olefin aromatization have been performing for many years.Despite these studies,how the states of zinc species affect the two main reaction paths in OTA(hydrogen-transfer path and the dehydrogenation path)are still not reported,especially for the formation of aromatics from hexene(67.8%in olefin of middle fraction FCC gasoline[30]).To clarify the influence of different zinc species on the reaction paths and increase the zinc species with higher aromatization activity in a targeted manner can greatly improve the aromatic selectivity of the catalyst.
This study concentrates on the influence of the states of zinc species on the two reaction pathways of OTA.Preparing a series of catalysts with a certain amount of bulk zinc content and different amounts of ZnOH+species,an optimized impregnation method for Zn/HZSM-5 zeolite combines traditional impregnation and mechanical mixing was used in this work.The catalyst efficiency was evaluated by 1-hexene aromatization.The dehydrogenation abilities of Zn/HZSM-5 catalysts were tested through cyclohexane dehydrogenation.
2.Experimental
2.1.Preparation of the catalysts
Nanosized HZSM-5 (Nankai University Catalyst Plant,China)was chosen as the parent catalyst.The preparation of Zn/HZSM-5 with Zn(NO3)2solution by the traditional incipient wetness impregnation [31].Under constant stirring,Zn(NO3)2solution was added to the parent zeolite.Then the sample was aged for 6 h and dried at 120°C overnight.Finally,the sample was calcined at 550 °C for 4 h and named as IMP/Z5.Before calcination,a mechanical stirring step was added via planetary high-energy ball mill under 200 r.min-1for 30 min.Then keep the other steps consistent with the traditional incipient wetness impregnation method.The obtained sample was named IMX/Z5.For mechanical mixing Zn into HZSM-5.Thoroughly mixing nanosized ZnO and the parent zeolite by planetary high-energy ball mill under 200 r.min-1for 30 min,followed by calcining at 550 °C for 4 h [32],the prepared sample was named as MIX/Z5.The theoretical contents of zinc were all 3% in the three Zn/HZSM-5 catalysts.
2.2.Characterization of the catalysts
The crystallographic structures of the samples were determined from X-ray diffraction (XRD) patterns (2θ ranging from 5° to 50°).The Bruker D8 advance-X-ray diffractometer with Cu Kα irradiation worked at a fixed power source (40 kV,30 mA).
The nitrogen adsorption/desorption isotherms were obtained by the BET method at -196 °C by using Micromeritics ASAP 2460 and then used to calculate the specific surface areas of the catalysts.The volumes and specific surface areas of micropores were measured by t-plot analysis [33].
The surface compositions of the Zn/HZSM-5 catalysts were determined from X-ray photoelectron spectroscopy (XPS) spectra collected by Thermo ESCALAB 250Xi.The C 1 s line at 284.8 eV belong to adventitious carbon was used as a reference for calculating binding energies.The bulk contents of Si,Al,and Zn elements of the samples were obtained by an inductively coupled plasma-optic emission spectrometer (ICP-OES,Agilent 5110).
Diffuse reflectance ultraviolet–visible spectra (UV–vis DRS)were obtained from UV-1800-spectrophotometer (Shanghai Meiji Instrument Co.Ltd.) to identify the state of zinc in Zn/HZSM-5.
Solid-state NMR measurements of the samples were acquired at v0(27Al)=130.4 MHz with a magic-angle spinning(MAS)frequency of 14 kHz by using a JNM-ECZ600R spectrometer (JEOL RESONANCE Inc.,Japan) [34].
The NH3temperature-programmed desorption (NH3-TPD) was performed on a DAS-7200 chemical adsorber instrument (Huasi Instrument Co.,Ltd.,China).For NH3-TPD,the sample was pretreated at 600 °C for 30 min by nitrogen flow,then cooled to 100 °C and kept in flowing NH3for 30 min.Purge with nitrogen to remove the physically adsorbed NH3at 100°C until the baseline was unchanged.The other NH3was desorbed from catalysts by heating from 100 to 650°C at a rate of 10°C.min-1.A thermal conductivity detector (TCD) was used to monitor the NH3desorption rate of the effluent stream continuously [35].
Py-FTIR (pyridine-Fourier transform infrared) characterization was used to test the types of acid sites by a Nicolet Avatar 360 spectrometer.The samples were dried at 120 °C for 6 h.20 mg of the samples were weighed to prepare circular laminates with a radius of 0.65 cm and then placed in a quartz IR cell with CaF2windows.Firstly,the sample was pretreated at 400 °C for 1 h under a vacuum 10-4Pa and then cooled to 35°C[31].The recorded spectrum was taken as background.Then add pyridine vapor to allow the sample to adsorb for 10 min.Finally,the IR spectra of pyridine were recorded after desorption at 200 °C and 350 °C,respectively[36].The absorption coefficients of BAS were 1.67 cm.μmol-1for bands around 1545 cm-1and the absorption coefficients of LAS was 2.22 cm.μmol-1for bands around 1455 cm-1.The formulas for calculating the concentration of acid sites were as follows[37].

where CBand CLare the concentration of Brønsted acid sites and Lewis acid sites (mmol.g-1),respectively.ABand ALrepresent the peak area of the absorption peaks of B acid and L acid,respectively.R is the radius (cm) of the sample tablet,and m is the mass of the sample (mg).
2.3.Catalyst tests and analysis methods
The conversion of 1-hexene to aromatics was tested in a continuous flow fixed-bed reactor(13 mm I.D.).In a typical reaction,5.0 g of catalyst(sieve fraction 40–60 mesh)was packed in the intermediary zone of the reactor between quartz sand.Before the aromatization reaction,the packed catalyst was pretreated at 500°C under an N2flow of 150 ml.min-1for 1 h.In a typical reaction,1-hexen was pumped with the liquid hourly space velocity (LHSV) of 1 h-1and reacted over the catalyst at 350 °C under atmospheric pressure.The liquid and gas products of olefin aromatization were separated with a cold trap and collected every hour.The liquid product composition was measured using an Agilent 7890B gas chromatograph with a 50 m×0.25 mm×0.25 μm capillary column.An Agilent 6890 gas chromatograph equipped with FID and TCD detectors was used to analyze the gas products,following similar procedures described previously.
The dehydrogenation of cyclohexane was carried out in pulse reaction online with an Agilent 8890B gas chromatograph(with FID,TCD,and an OV-101 capillary column:50 m × 0.2 mm×0.50 μm)at atmospheric pressure.The pulse reactions were carried out in a fixed-bed microreactor with an inner diameter of 13 mm (100 mg of the catalyst with the particle size of 0.250–0.425 mm) at 350 °C.The injection volume of cyclohexane was 2 ml for each experiment.Space-time was altered by flow rate while keeping the same amount of catalyst (0.05–15 gmol.h-1).Under the reaction conditions,internal and external limitations have been eliminated (small particle size and high gas velocity).
3.Results and Discussion
3.1.Physicochemical properties of the catalysts
Fig.1 shows the XRD patterns of H-ZSM-5,IMP/Z5,IMX/Z5,and MIX/Z5 catalysts.The diffraction peaks at 2θ=8°–10°and 22°–25°showed all catalysts maintain typical MFI structured.[38,39].Table 1 shows the crystallinity of all catalysts prepared by different methods decreased slightly.The relative crystallinity of MIX/Z5 was reduced from 95% to 93% due to the introduction of extraframework ZnO.For IMP/Z5 and IMX/Z5,the relative crystallinities are reduced due to the slight framework distortion caused by the replacement of Zn species.Moreover,the characteristic diffraction peaks (2θ=22°–25°) shifted to lower angles,indicating the different preparation methods changed the unit cell parameters of Zn/HZSM-5 [17].

Fig.1.XRD patterns of HZSM-5 and Zn/HZSM-5 catalysts.
The unit cell parameters and volumes of all catalysts obtained by the Rietveld refinement method were shown in Table 1 [40].Compared with the HZSM-5,the unit cell parameters of MIX/Z5 did not change obviously.The phenomenon indicated that the interaction between the framework of zeolite and the Zn species introduced by the mechanical mixing method enlarges the unit cell parameters slightly.The unit cell parameters of IMP/Z5 and IMX/Z5 had an inevitable growth,especially the unit cell volume of IMX/Z5 increased most significantly.This may be caused by increasing the lattice spacing by substituting Zn species into the cation site or entering zeolite channels [41],and the effect is more substantial in IMX/Z5.The unit cell volumes of HZSM-5,MIX/Z5,IMP/Z5,and IMX/Z5 catalysts were 5.3512,5.3552,5.3755,and 5.3982 nm3,respectively,indicating they increase with the degree of substitution of Zn species.
Furthermore,the crystal sizes of catalysts were calculated via the Debye-Scherrer formula according to the five characteristic diffraction peaks.As shown in Table 1,the MIX/Z5 showed a similar size to HZSM-5.In contrast,there has been a marked decline in the crystal sizes of IMP/Z5 and IMX/Z5,especially the IMX/Z5 showed the most considerable reduction from 68.62 to 57.01 nm.Gao et al.[42,43] found that introducing the Zn species into the zeolites during the synthesis process would cause the crystal size to decrease.The lower the SiO2/ZnO ratio of the skeleton,the smaller the crystal size.Therefore,more Zn species were substituted at cationic sites or entered into the channels of zeolites in IMX/Z5.These results are in line with the conclusion of the unit cell parameters results.
The texture properties of catalysts are presented in Table 2 and Fig.S1.Compare with the parent HZSM-5,the introduction of Zn leads to a decrease in both the pore volumes and specifics surface areas.For MIX/Z5,the pore volumes and specific surface areas were slightly reduced due to a small fraction of extra-framework ZnO species are unevenly dispersed.[44,45].Compared with MIX/Z5,the micropore volume and surface areas of the catalysts prepared by the impregnation method were reduced more significantly.Especially the micropore volume and surface areas of IMX/Z5 reduced to 0.120 cm3.g-1and 234 m2.g-1,respectively.This means more Zn species entered into the micropores of zeolite for IMX/Z5.According to these data,it could be inferred that different introducing methods alter the position of Zn species in catalysts,and more Zn species are introduced into micropores or skeleton of zeolite for IMX/Z5.

Table 1 Unit Cell Parameters,crystal sizes and relative crystallinity of HZSM-5 and Zn/HZSM-5 catalysts

Table 2 Texture Properties of HZSM-5 and Zn/HZSM-5 catalysts
3.2.Acidic properties of catalysts
Table 3 presents the acid sites concentration of HZSM-5 and Zn/HZSM-5 obtained by analyzing the NH3-TPD.Generally,there are two desorption peaks on HZSM-5 in the NH3-TPD profile.The peak around 200 °C is ascribed to weak acid sites,whereas the peak above 300 °C is attributed to strong acid sites [46].In this work,the desorption peaks are integrated into three different peaks at about 180–220,220–320,and 320–550°C,designated as the weak,medium,and strong acid sites according to the work by Niu and Pan [17,35,47–49],respectively (Fig.S3).
It is apparent from Table 3 that introducing Zn species into HZSM-5 has an obvious effect on the acidic strength distribution of acid sites.Compared with the pristine HZSM-5 zeolite,the acid strength of the Zn/HZSM-5 is decreased.The degree of temperature drops of ammonia desorption are followed by IMX/Z5 >IMP/Z5 >MIX/Z5.What is striking in the table is the variability of acid amounts of different catalysts.Compared to the pristine HZSM-5 zeolite,there is a marked rise in the amount of medium acid sites due to the sharp decline of weak and strong acid sites[17,50].Significantly,the medium acid sites amount in IMX/Z5 increased to 0.209 mmol.g-1,the largest amount of the three catalysts,followed by IMP/Z5 and MIX/Z5 (0.184 and 0.144 mmol.g-1,respectively).The zinc species reacted with the acid sites in the pristine HZSM-5 zeolite to produce the medium acid sites.The phenomenon is more pronounced in the Zn/HZSM-5 prepared by the incipient wetness impregnation method than the mechanical mixing method.Combine the two preparation methods can further increase the amount of the interacting Zn species.
In order to obtain more information about the types of acid sites,the Py-IR spectra were shown in Fig.2.The characteristic band at 1545 cm-1is associated with pyridinium ions transformed from pyridine chemisorbed on the Brønsted acid sites of HZSM-5.Meanwhile,the characteristic band at 1455 cm-1is related to Lewis sites.The Py-IR spectra scanned after degassing at 200 °C were used to measure the amount of total Lewis acid and total Brønsted acid sites.Similarly,strong Lewis and Brønsted acid were measured according to the spectra scanned after degassing at 350 °C.Table 4 shows the most considerable total amount of acid sites(552 μmol.g-1)belong to HZSM-5.By introducing the Zn species,the total amount of acid sites declined significantly.The extent of the decrease for the total amount of acid sites follows the sequence of IMX/Z5 >IMP/Z5 >MIX/Z5,based on pure HZSM-5 (18.3%,18.1%,and 13.0%,respectively).But IMX/Z5 has the highest proportion of strong acid sites (57%) among the three catalysts.The proportions of strong acid sites in IMP/Z5 and MIX/Z5 are 55%and 44%,respectively.The results of the amount of acid sites concluded by the Py-IR are similar to the NH3-TPD.Introducing Zn species into HZSM-5 also exhibits a significant influence on the distribution of acid types.The Brønsted acid sites were used to generate more Lewis acid sites [50].Moreover,the preparation methods of Zn/HZSM-5 have different influences on the redistribution of Lewis and Brønsted acid sites.Compared with MIX/Z5,IMP/Z5 shows a higher ratio of the Lewis acid site to the Brønsted acid site (L/B) at 4.3 (versus 3.7).And IMX/Z5 presents the highest L/B ratio at 4.6,which is slightly higher than IMP/Z5.The result indicates that more Brønsted acid sites interacted with the Zn species in IMX/Z5,consistent with previous characterization results.It was worth noting that because the kinetic diameter of NH3(0.26 nm)was smaller than that of pyridine (0.534 nm) [51],NH3diffused more easily to the internal acidic sites.The total acid amount of samples obtained by NH3-TPD were higher than those measured by Py-IR.

Fig.2.Pyridine-FTIR profiles of HZSM-5 and Zn/HZSM-5 catalysts.

Table 3 Acid sites concentrations of HZSM-5 and Zn/HZSM-5 catalysts

Table 4 Acid types and acidic properties of HZSM-5 and Zn/HZSM-5 catalysts

Fig.3.UV–vis–DRS spectra of HZSM-5 and Zn/HZSM-5 catalysts.
3.3.The state of zinc in catalysts
The UV–vis DRS spectra of the HZSM-5 and Zn/HZSM-5 catalysts are shown in Fig.3.Compared to HZSM-5,there are three prominent absorption bands in the UV–vis DRS spectra of the Zn/HZSM-5 at about 220,275,and 368 nm,respectively.The band at about 368 nm is ascribed to the macro-crystalline ZnO particles[50,52,53],which is particularly evident in MIX/Z5.In the spectra of three Zn/HZSM-5 catalysts,the band at 275 nm is ascribed to small ZnO particles (about 1 nm diameter) [54].This means there are ZnO particles that enter into the channels of these catalysts.The band at 220 nm should be attributed to the tight interaction between the Zn species and the pristine HZSM-5.Although the adsorption bands below 230 nm are usually ascribed to the framework Zn species,it is more sensible to reckoned as the charge transfer transition of Zn species with lattice O2–,by considering the preparation methods of Zn/HZSM-5 [46].The most notable aspect was that the intensity of the ZnOH+species in MIX/Z5 was significantly weaker than that of other Zn/HZSM-5 catalysts.In addition,the27Al MAS NMR spectra for HZSM-5 and Zn/HZSM-5 were shown in Fig.S4.The peaks at 0 and 55 were assigned to the six-coordinated extra-framework aluminum (EFAl) and the four-coordinated framework aluminum (FAl) in HZSM-5,respectively [55,56].The disappearance of the peak at 0 after the introduction of Zn into HZSM-5 by the three ways indicated that all three introductions leaded to the reaction of EFAl and Zn species[34].To further compare the differences of the Zn species among the three catalysts.The XPS characterization was used to analyze the distribution of Zn species.
For the Zn/HZSM-5 catalysts,the amount of bulk zinc species determined by ICP are lower than the surface amounts determined by XPS,indicating that the zinc species in the catalysts are concentrated on the surface of HZSM-5 (Table 5).Moreover,the IMX/Z5 shows the lowest surface zinc content (4.19%),followed by IMP/Z5 and MIX/Z5 (4.22% and 6.60%).The data from BET also shows the surface area decrease of micropore follows the sequence of IMX/Z5 >IMP/Z5 >MIX/Z5.Such phenomena were attributed to more zinc in the zeolite channels for IMX/Z5 than in IMP/Z5 and MIX/Z5.
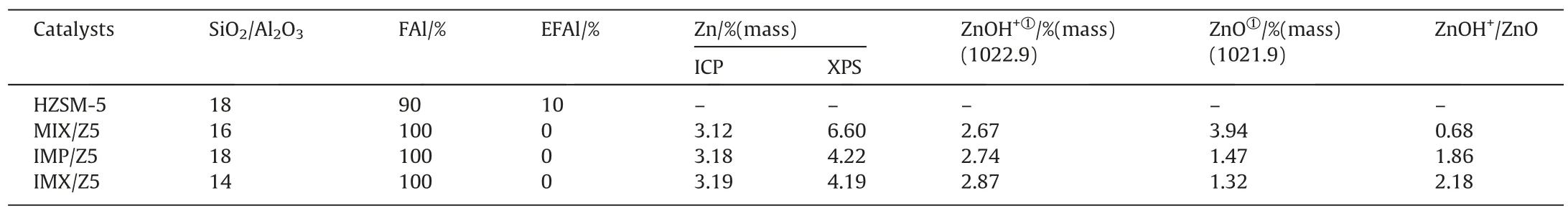
Table 5 Composition of HZSM-5 and Zn/HZSM-5 catalysts
To further analyze the amount of different types of Zn species.The Zn(2p3/2) XPS spectra of Zn/HZSM-5 catalysts are illustrated in Fig.4.Depending on the preparation method,the XPS spectra varied distinctly in shape and position.This demonstrated that varying the methods of introducing zinc significantly influence the distribution of Zn with different states.By fitting the XPS spectra,there are two apparent peaks at 1021.9 and 1022.9 eV,respectively.The peaks at low binding energy around 1021.9 are attributed to ZnO,and the peaks at higher biding energy are ascribed to ZnOH+species [19,57].The binding energy argument is attributed to zinc species affected by the pristine HZSM-5 framework,which has stronger electronegativity than O2-[58].For MIX/Z5,the peak area ratio at 1022.9 to the peak at 1021.9 is 0.68.This means the majority of Zn states are ZnO in MIX/Z5.However,significant differences in the distribution of Zn states are observed between MIX/Z5 and IMP/Z5,the area ratio of peak increases to 1.86.In other words,the introduction of Zn species by impregnation is mainly ZnOH+.With the combination of impregnation and mechanical mixing,the ratio further increases to 2.18,indicating the formation of more ZnOH+in IMX/Z5.As shown in Fig.5,the Zn species dispersed more evenly on the surface of IMX/Z5 resulting in more ZnOH+and few ZnO clusters.The main reason is that the stirring is not uniform enough during the traditional incipient wetness impregnation.And the loading content of zinc species is not low.The formed ZnOH+may decompose into ZnO because of the instability.The poorly dispersed ZnO will further sinter into ZnO clusters during the calcination [27].But add a mechanical mixing step before calcination could increase the dispersity of ZnOH+and reduce the sintering of ZnO.
In conclusion,the impregnation combined with mechanical mixing effectively distributes Zn species in the micropores of HZSM-5 to form ZnOH+.Compared to IMP/Z5,the IMX/Z5 possessed more medium acid sites and a higher ZnOH+/ZnO ratio,which may cause a different catalytic performance in OTA (see Section 3.4).
3.4.Catalyst performances in 1-hexene aromatization
Fig.6 displays the conversions of 1-hexene as a function of time on stream(TOS)over the pristine HZSM-5 and different Zn/HZSM-5 catalysts.The 1-hexene conversions are above 99%over all catalysts,indicating that 1-hexene is extremely active under the process condition.Despite the minor differences among the 1-hexene conversions,significant discrepancy could be found in the product distribution over the catalysts.Considering the reaction procedure is espicially complex,the hundreds of products are classified into C0-C4fragments (including H2,CH4,C2H4,C2H6,C3,and C4components),aromatics (C6-C10aromatics),olefines (C4-C10cycloolefins,diolefins,straight-chain olefins,and branched olefins).The relationships between the different product components and TOS are shown in this section to study the effect of ZnOH+upon the 1-hexene aromatization.

Fig.4.XPS spectra of Zn/HZSM-5 catalysts.
Firstly,from Fig.7,the selectivities of C0-C4fragments decreased slightly over the four catalysts with the TOS prolonging.However,the proportions of C0-C4fragments on different catalysts were remarkably different.The product belongs to pristine HZSM-5 had the highest proportion of C0-C4fragment above 40%,followed by MIX/Z5 (around 30%).By contrast,the IMP/Z5 had a lower proportion of C0-C4fragment,which decreased from 27%to 24%,and the IMX/Z5 showed the most diminutive proportions,which were consistently below 25% during the period.The data from Py-IR illustrates the amount of Brønsted acid sites in HZSM-5 (435 μmol.g-1) are far more than those in Zn/HZSM-5 catalysts(below 105 μmol.g-1).Moreover,those in MIX/Z5 17 μmol.g-1are more than those in IMP/Z5.The least amount,81 μmol.g-1,belongs to IMX/Z5.This is because the amount of acid sites,especially the Brønsted acid sites where the cracking reaction happens,significantly influences the proportion of C0-C4fragments,in agreement with the work of Zhang [59].

Fig.5.The dispersion of zinc species over Zn/HZSM-5 zeolites by different preparation method.

Fig.6.The conversion of 1-Hexen over HZSM-5 and Zn/HZSM-5 catalysts.

Fig.7.C0-C4 fragments yield of OTA products over HZSM-5 and Zn/HZSM-5 catalysts.
Table 6 showed the previously reported results of ZSM-5-based catalysts for hexene aromatization.It can be easily found the IMX/Z5 catalyst in this work shows the best aromatization ability under the same reaction condition.Although the aromatics content in the product over 5Zn/Z5-AT reached 47.8%,the reaction temperature was 130 °C higher than that in our work.Increasing reaction temperature could greatly improve aromatization ability because olefin aromatization is an endothermic reaction.Comparing the aromatic content of the products at different reaction temperatures is not meaningful,while the aromatics increment could better indicate the difference in the effect of the modification method.The aromatics increment was only 15.5% in 5Zn/Z5-AT,compared with the largest aromatics increment (19.8%) by the new preparation method in our work.This means the impregnation combined with mechanical mixing is a more effective preparation method for modifying olefin aromatization catalysts.Fig.8 compares the yields of aromatics over different catalysts in this work during the TOS from 4 to 10 h.The aromatics yields of IMX/Z5 were the highest,with 36.9% at 4 h TOS.It sharply decreased to 17.8% at 10 h TOS.The aromatics yields of IMP/Z5 were lower throughout the period:they declined from 30.5% to 15.4%.The proportions of aromatics over MIX/Z5 were lower.There was a similar trend in the proportion for aromatics from 29.5% at 4 h TOS to 14.0% at 10 h TOS.And the aromatics yields that belonged to the pristine HZSM-5 zeolite remained at the lowest level during the period(consistently below 20%).Fig.9 shows the olefin yields in the product distribution of different catalysts over the TOS period from 4 to 10 h.Contrary to the variation trend of aromatics yield,the olefin yield increased steadily during the period.For the pristine HZSM-5 zeolite,the olefin yields were the highest and expanded to 54.2% at 10 h TOS.By contrast,there were the lowest proportions of olefin in IMX/Z5,only 32.5% at the same TOS.The MIX/Z5 showed little more olefin yields than IMP/Z5,although it is a percentage point lower at 10 h TOS.
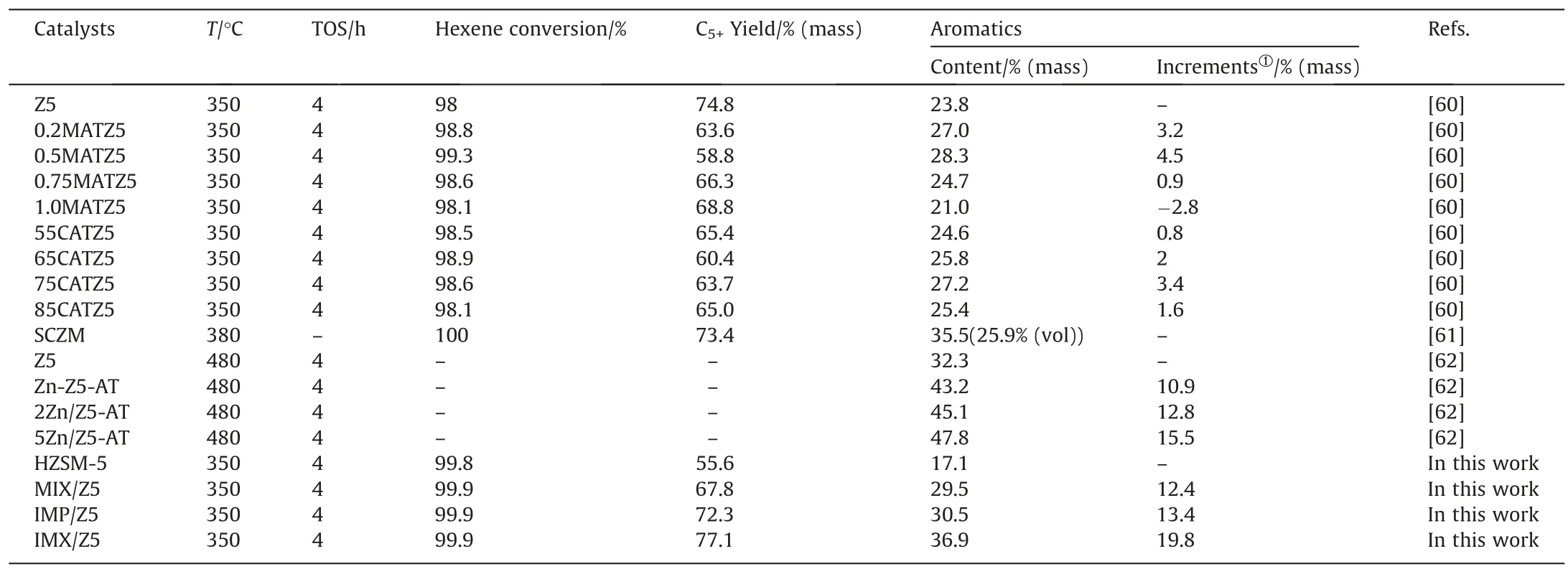
Table 6 Representative catalysts for hexene aromatization
These results indicated the high ratio of ZnOH+in the catalysts with the same Zn content was beneficial to the 1-hexene aromatization.The reaction pathway for converting the olefins to aromatics over HZSM-5 via carbenium ion mechanisms involves cracking,polymerization,cyclization,and hydrogen-transfer steps[11,63,64].Considering the proposed olefin conjunct polymerization mechanisms,one can conclude that hydrogen-transfer capacity plays an essential role in the process of OTA.To clarify the effect of ZnOH+species for 1-hexene aromatization,we further analyzed the hydrogen-transfer ability on catalysts with different amounts of ZnOH+.
3.5.The role of ZnOH+ in the hydrogen-transfer step
We have observed that increasing the proportion of ZnOH+when keeping the same bulk Zn content in Zn/HZSM-5 results in significant variations of the distribution of catalytic products.For all samples,the introduction of Zn enhanced the aromatization activity significantly.And the olefins yield decreased dramatically,especially the amount in IMX/Z5 was only 32.50% which was the lowest one,versus 54.20% in the pristine HZSM-5 at 10 h TOS as shown in Fig.9.The most surprising aspect of the data is that the olefins yields are similar in IMP/Z5 and MIX/Z5.From the olefin aromatization pathway,it is clear that the formation of aromatics needs the formation of cycloolefins (CO) and diolefins (DO) [11].

Fig.8.Aromatics yield of OTA products over HZSM-5 and Zn/HZSM-5 catalysts.
Moreover,CO and DO are formatted by the hydrogen transfer of chain olefins and belonged to the olefins series in the PONA results.As shown in Fig.10,the detailed distributions of olefins product over catalysts were analyzed to study the influence of ZnOH+on hydrogen transfer.The proportion of CO+DO saw steady growth in the period from 4 to 7 h TOS,and then they remain at this level in the rest of 4 h.This is suggested to be caused by the intense activities of the catalysts at the beginning of the reaction.The hydron transfer step between olefins for generating DO was the rate-controlling step.By contrast,the DO cyclization and the formation of the aromatics from CO were 10–100 times higher than the DO formation rate [11].So the formed DO was quickly converted to CO.Therefore,the analysis of the amount of CO+DO can better compare the effect of different catalysts on the hydrogen transfer process than the analysis of only the amount of DO.DO and CO quickly turned into aromatics under the substantial activities,their proportion gradually increased as the activity decreases at the beginning of the reaction.And the proportions of CO+DO for IMP/Z5 and IMX/Z5 are around 2.5% higher than those for MIX/Z5.The lowest proportions belonged to the pristine HZSM-5 zeolite were only 5.78% at 10 h TOS.These results show that although the olefin contents in IMP/Z5 and MIX/Z5 are similar,the proportions of CO+DO are higher in the IMP/Z5,which possesses more ZnOH+.And the olefin contents of products in IMX/Z5 with the most ZnOH+are significantly lower than other catalysts.These results suggested the proportion of ZnOH+affects the hydrogen-transfer ability.

Fig.9.Olefins yield of OTA products over HZSM-5 and Zn/HZSM-5 catalysts.

Fig.10.The proportion of CO+DO of Olefins products over HZSM-5 and Zn/HZSM-5 catalysts.
To further analyze the relationship between the amount of ZnOH+and hydrogen-transfer steps more intuitively,the ratio of C4alkanes to C4olefins (the HTI for C4hydrocarbons) was used to define the hydrogen-transfer ability [9,16].A higher HTI would mean a better hydrogen-transfer ability.Fig.11 shows the relationship between the HTI and the amount of surface ZnOH+species in Zn/HZSM-5 catalysts.The HTI increases with increasing amount of ZnOH+.At the same TOS,the HTI nearly linearly increase with the surface amount of ZnOH+species.In addition,the ratio of HTI to the surface ZnOH+decreases with the extension of TOS (Fig.S4 and Table S2).These data show that the ZnOH+species are accounted for the high hydrogen-transfer ability of the Zn/HZSM-5 catalysts.A linear relationship could be found between the HTI and the amount of surface ZnOH+.

Fig.11.Relationship of the HTI to the amount of surface ZnOH+species and TOS in the Zn/HZSM-5 catalysts.
3.6.The role of ZnOH+ in the dehydrogenation step
Ono and co-workers[63]proposed that light olefins could convert to aromatics through dehydrogenation on Zn modified zeolites directly without transforming into alkanes.Therefore,the ZnOH+species may also influence the dehydrogenation step.The cyclohexane is an intermediate of olefin aromatization and is hardly observed among the products owing to its high reactivity[14].But cyclohexane could be used as feed to characterize the dehydrogenation ability of the Zn/HZSM-5 catalysts.To study the relationship between the ZnOH+species and dehydrogenation ability,cyclohexane was chosen as feed the dehydrogenation ability was tested by pulse reaction.The cyclohexane dehydrogenation is performed at the same reaction temperature as 1-hexene aromatization (350 °C).
Fig.12 displays the aromatization yield and H2yield as functions of the cyclohexane conversion.It is clear that the aromatization yield increases with the cyclohexane conversion and there is a linear relationship between them on the same catalyst.A similar relationship can be found between H2yield and cyclohexane conversion.

Fig.12.Correlation between the H2 yield and the cyclohexane conversion Zn/HZSM-5 catalysts.
Interestingly,despite the ratios of the change in aromatization yield to the change in cyclohexane conversion (dA/dC) and the ratios of the change in H2yield to the change in cyclohexane conversion (dH/dC) are different in different catalysts,they both decrease following the sequence of IMX/Z5 >IMP/Z5 >MIX/Z5.Considering that dehydrogenation is a new reaction path on the Zn/HZSM-5 catalysts compared to HZSM-5.The dehydrogenation ability may be related to zinc states.Fig.13 shows that the ratios of dA/dC are well correlated with the amount of surface ZnOH+species.And a similar linear relationship could be observed in the ratio of dH/dC to the amount of surface ZnOH+species.These data show that the amount of surface ZnOH+species are accounted for the high dehydrogenation performance of catalysts,and there is a linear relationship between them.According to the influence of the ZnOH+species in the hydrogen-transfer step and dehydrogenation step,the simple hexene aromatization reaction pathway was shown in Fig.14.

Fig.13.Relationship of the ratio of dA/dC and dH/dC to the amount of surface ZnOH+ species.

Fig.14.The simple reaction pathway of hexene aromatization over Zn/HZSM-5.
4.Conclusions
The work presented here investigates the aromatization of 1-hexene and cyclohexane over Zn/HZSM-5 catalysts.And a new two-step incipient impregnation method was developed to prepare IMX/Z5 catalyst,which possessed more OTA active sites (ZnOH+species)than by the traditional impregnation method.Three kinds of Zn/HZSM-5 catalysts with identical bulk Zn content and different amounts of the surface ZnOH+species were designed by optimized impregnation,traditional impregnation,and mechanical mixing.Interestingly,they showed different catalytic abilities in both 1-hexene aromatization and cyclohexane dehydrogenation.The main reaction paths are hydrogen transfer and dehydrogenation in the 1-hexene aromatization.The amount of surface ZnOH+species in the Zn/HZSM-5 zeolites is decisive to both the hydrogen-transfer ability and dehydrogenation ability.For the first time,a linear correlation between the amount of surface ZnOH+species and the HTI for the 1-hexene aromatization over Zn/HZSM-5 catalysts was found.Moreover,there are similar linear relationships of the amount of surface ZnOH+species to the ratios of dA/dC and dH/dC for cyclohexane dehydrogenation.Hence,the ZnOH+species may act as active sites for the hydrogen-transfer step and dehydrogenation step.The aromatics yields of OTA process increase with the amount of surface ZnOH+species when the catalysts possess the exact content of bulk Zn.Compared to IMP/Z5,IMX/Z5 shows a larger amount of surface ZnOH+species(2.87% versus 2.74%),a higher proportion of medium-strong acid sites (42.4% versus 36.9%),and the yield of aromatics on 1-hexene increases by 6.3%(at 5 h TOS).This work would be instructive to enhance olefin aromatization catalysts for upgrading FCC gasoline quality.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (21838011).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2022.01.005.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
