High-energy-density gelled fuels with high stability and shear thinning performance
2022-04-27YangLiuHongzhiZhangLunPanKangXueXiangwenZhangJiJunZou
Yang Liu,Hongzhi Zhang,Lun Pan,Kang Xue,Xiangwen Zhang,Ji-Jun Zou
Key Laboratory for Green Chemical Technology of the Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China Collaborative Innovative Center of Chemical Science and Engineering (Tianjin),Tianjin 300072,China
Zhejiang Institute of Tianjin University,Ningbo 315201,China
Keywords:High-performance fuel Gelled fuel Gellants Fuel properties
ABSTRACT Gelled fuels are the very promising propellants for new-generation rocket and ramjet propulsion.Here we report a new type of low-molecular mass organic gellant(Z),and prepared four kinds of stable gelled fuels based on HD-01,HD-03,RP-3 and QC liquid fuels,with the critical gellant concentration less than 1%(mass).The characterizations show that the gellant can form 3D network structure,via hydrogen bonding,π-π stacking and van der Waals forces,to fix fuel molecules during the formation of gelled fuels.So,the gelled fuels show high stability,with the remaining gel mass of 0.25%(mass)Z/HD-01 more than 90%even at high centrifugal speed of 7500 r.min-1,but the rheological property test shows that all gelled fuels have obvious shear thinning property,which benefits its storage in gelled state while supply in liquid state.The gelation of liquid fuels by gellant Z can increase the volumetric net heat of combustion(for HD-01,it increases from 39.58 MJ.L-1 to 40.76 MJ.L-1 with 1%(mass) Z),and liquefied gelled fuels show the comparable ignition delay time with the pristine liquid fuels.So,the gelled fuels made by gellant Z have better stability,shear thinning and combustion performances,which have great potential for the practical application.
1.Introduction
Compared with traditional refined fuels,high-energy-density(HED) hydrocarbon fuels can provide more propulsion energy,which can extend the flight range [1–5].Gelling the HED fuels makes them behave as solids (or gels) during storage and transportation,which makes them less volatile and less leaky than liquid fuels.Importantly,the gelled fuels in solid (or gel) state can change to liquid state under external force,so they can be atomized and combusted like conventional liquid fuels under shear flow [6].
The gelled fuels are formed by the network structure of gellants after adding them into liquid fuels[7].The type and content of gellants have significant influence on the properties of gelled fuels[8].For the same fuel,the more gellants are added,the more stable gelled fuels will be.However,adding too much gellants will greatly reduce the flow and combustion performance of fuels[9].The gellants used now are mainly divided into organic gellants and inorganic gellants [10].Organic gellants contain polymeric organic gellants and low-molecular mass organic gellants (LMOGs) [11].The former generally has large molecular weights,such as pectin,cellulose derivatives,agarose,etc.[10,12],while the latter has relatively small molecular weights,like amino acid derivatives,carbohydrate derivatives,organic acid-base complex,cholesterol derivatives,etc.[13,14]For the inorganic gellants,the mostly used material is nano silica.In order to form a stable gelled fuel,the amount of inorganic gellants is more than 5% (mass) of the total mass.In addition,inorganic gellants are generally nonflammable,and the excessive addition will greatly reduce the combustion energy of the fuel.
As flammable gellants,organic gellants have less influence on fuel performance than inorganic gellants [15].In recent years,LMOGs have received increasing interest because they have lower additive amount and better gelling property compared with polymeric organic gellants.Qiu et al.[16] reported a tris-urea gellants named HDIT-18 and found that it is a super gellant when preparing gelled fuel.Chen et al.[17] reported a series of glucose derivative gellants Gn for the preparation of gelled fuels,and found that the gelling ability of gellants increased with the length of side chain.E et al.[18]reported a gellant which can prepare gelled fuels with better rheological and thixotropy properties than SiO2,which is more conducive to pipeline transportation and atomization.Cao et al.[19,20] prepared gelled JP-10 containing nano-sized aluminum particles using a mannitol derivative gellant named LMWG and found that the gels still maintain good shear thinning performance after adding nanoparticles.However,the molecular structures of the above LMOGs are relatively complex and the gels prepared by them have poor stability.
In this work,we report a simple low-molecular mass organic gellants (Z) with better gelling ability compared with previously reported gellants.The stable gelled fuels were prepared based on HED fuel HD-01 (exo-tetrahydrodicyclopentadiene),HD-03 (exo-t etrahydrotricyclopentadiene),RP-3 (jet fuel in China),and QC(quadricyclane).Scanning electron microscopy studies and driving force analysis show that hydrogen bonding,π-π stalling and van der Waals forces are involved in the formation of gelled fuels.So,the gelled fuels show good stability,with the gelled HD-01 having the best centrifugal stability,and gelled RP-3 performing the best thermal stability.The rheological properties tests show that the gelled fuels prepared by gellant Z have better shear thinning performance than other gellants.The viscosity and mechanical strength of gelled HD-01 are the highest,followed by gelled RP-3,gelled QC and gelled HD-03.Importantly,the liquefied gelled fuel shows the similar combustion performance with pure fuel.This work provides an effective gellant for synthesis and application of gelled HED fuels.
2.Experimental
2.1.Materials
Nano-fumed silica(with surface area of 300 m2.g-1and average particle size of 7–40 nm),phenol,mercaptoacetic acid,hydrochloric acid,acetic acid,and cyclohexanone were obtained from Shanghai Aladdin Bio-ChemTechnology Corporation.Thixatrol ST gellant was purchased from Elementis Specialties.Pure liquid high-density fuels HD-01,HD-03,RP-3 and QC were synthesized according to the literatures [21–27].Typical properties of the liquid fuels are summarized in Table 1.
2.2.Synthesis of gallant and gelled fuels
Gallant synthesis:phenol (28.23 g,0.3 mol),mercaptoacetic acid (0.5 g) and a mixture of hydrochloric acid and acetic acid(75 ml,2:1 by volume) were added to a flask,and stirred at 50 °C for 5 min.Then cyclohexanone (9.82 g,0.1 mol) was added dropwise,and the reaction was kept for 10 h.Then the mixture was washed with deionized water to remove the excess phenol,with the crude product recrystallized in methanol aqueous solution.Then,the product was obtained by vacuum drying at 50 °C,which is named as gellant Z.Low-molecular mass organic gellants Gn and LMWG were synthesized according to the literatures[18,19].
Gelled fuels were obtained by mixing the gellant and fuels.Z/-fuel gel was prepared by heating the mixture of gellant and fuel to clear solution and then cooling it to room temperature.The preparation procedures of G/fuel gel and L/fuel gel were the same as that of Z/fuel gel,but the used organic gellants are Gn and LMWG,respectively.The preparation method of Thixatrol ST/fuel gel was the same as that of Z/fuel gel,while SiO2/fuel gel was prepared by mechanically stirring the mixture at room temperature until the gelled fuel was formed.
2.3.Characterization of gelled fuels
The density of the gelled fuel was measured by the specific gravity method.Centrifugal stability tests were carried out in a laboratory centrifuge.The phase transition temperature of the gelled fuel was measured by dropping ball method in a test tube.The temperature at which the glass ball (0.17 g) sinks to the tube bottom through gelled fuel was the phase transition temperature(TG).The heat of combustion was determined by ZDHW-300A oxygen bomb calorimeter.The weight of sample was kept same (0.5 g)before the combustion test in oxygen bomb.The morphology of xerogel were determined by Hitachi S-4800,and the rheological measurements were performed using a stress-controlled rheometer (TA Instruments,DHR).For comprehensive characterization of the thixotropy and recovery capability of the gelled fuels,the pre-shear step was not performed during the rheological measurements.X-ray diffraction(XRD)was collected on MiniFlex 600 X-ray diffraction,while Fourier transform infrared spectroscopy (FTIR)was collected using Bruker Vertex70.The self-assembly model was optimized with DFT method.The scheme of the hightemperature spontaneous combustion system is shown in Fig.1.During the high-temperature spontaneous combustion experiment process,each droplet is ca.15 μl and the drop height between the droplet and hot-plate surface was controlled at 110 mm.
3.Results and Discussion
3.1.Gelation properties of gelled fuels
The critical gellant concentration(CGC)directly reflects the gelling ability of the gellant,and the pursuit of a lower CGC is very important for the advanced gelled fuels.The gelled fuels prepared by gellant Z(Fig.2(a)) are thermal reversible which can change to liquid state from gel state after heating and then change to gel state after cooling (Fig.2(b)).We compared the CGC of gellant Z with the previously reported low-molecular mass organic gellants(Gn,LMWG),and the traditional gellants (SiO2,Thixatrol ST),with the results shown in Table 2.For all tested liquid fuels(HD-01,HD-03,RP-3,and QC),gellant Z has lower CGC compared with other gellants.Especially for gelled HD-01,the CGC of gellant Z is only 0.1% (mass),while it is 0.5% (mass),1.0% (mass),4.5% (mass) and 3%(mass)for LMWG(L),Gn(G),SiO2and Thixatrol ST,respectively.So,the application of gellant Z can significantly reduce the amount of gellant in the gelled fuels to minimize the effect on fuel properties.Meanwhile,the CGC of gellant Z is increased gradually for the liquid fuels from HD-01 to HD-03,RP-3 and QC.

Table 1 Properties of liquid fuels
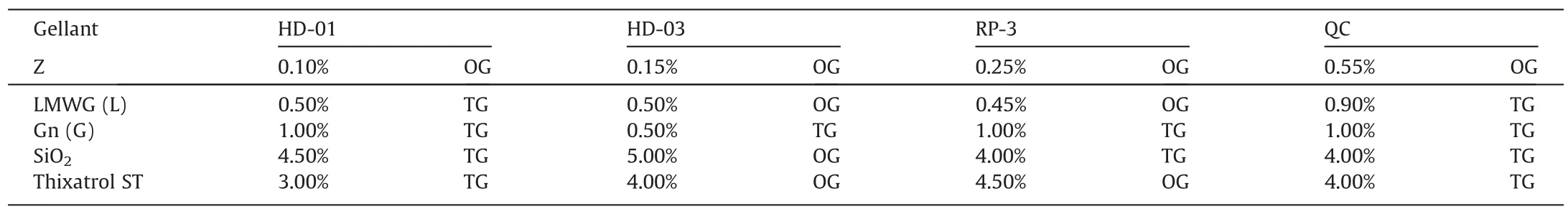
Table 2 The critical gellant concentration (CGC/% (mass)) and appearance of gelled fuels
The pictures of gelled fuels are shown in Fig.2(c)-(j).The 1%(mass) Z gelled HD-01,HD-03,RP-3 and QC fuels show the white color (translucence for HD-03),while Gn,LMWG,SiO2and Thixatrol ST gelled HD-01 fuels are transparent.

Fig.1.Schematic diagram of the high-temperature spontaneous combustion system.

Fig.2.(a)Molecular structure of gellant Z,where the white,gray,and red spheres are hydrogen,carbon,and oxygen atoms,respectively,(b)sol-gel transformation of Z/HD-01 gelled fuel,and photographs of(c)1%(mass)Z/HD-01,(d)1%(mass)Z/HD-03,(e)1%(mass)Z/RP-3,(f)1%(mass)Z/QC,(g)1%(mass)L/HD-01,(h)1%(mass)G/HD-01,(i)7% (mass) SiO2/HD-01 and (j) 5% (mass) Thixatrol ST/HD-01.
3.2.Morphology of gelled fuels
In order to understand the self-assembled structure of gelled fuels,the micro-morphology of xerogel (the xerogels were prepared by vacuum drying the gelled fuels) was tested by scanning electron microscopy (SEM),and the results are shown in Fig.3.It can be seen that gellant Z self-assembles into fibrous structure and forms a 3D network structure,which can entrap the fuel molecules into its interstices and form gelled fuels.However,the assembled structures of gellant Z in different fuels are totally different.As shown in Fig.3,the mean width of gelled fuel fibers is 0.40 μm,0.42 μm,2.00 μm and 0.45 μm for 1% (mass) Z/HD-01,1% (mass)Z/HD-03,1% (mass) Z/RP-3 and 1% (mass) Z/QC,respectively.The networks of 1% (mass) Z/HD-01 and 1% Z/HD-03 are dense and thin,while the fibers of 1% (mass) Z/RP-3 are large,corresponding to the low CGC of Z/HD-01 and high CGC of Z/RP-3.Specially,the fibers of 1% (mass) Z/QC are much shorter than others,which makes QC difficult to form gelled fuel with low gallant concentration.This indicates that the compatibility between gellant Z and fuels is in the order of HD-01 >HD-03 >RP-3 >QC.
3.3.Formation mechanisms of gelled fuels
FT-IR spectra of gallant Z in solution state and gel state with HD-01 as the solvent are shown in Fig.4(a).The -OH stretching vibration peak and two bending vibration peaks of gellant Z in HD-01 solution are at 3238 cm-1,1348 and 1369 cm-1,which shifts to 3199 cm-1,1340 cm-1and 1365 cm-1in Z/HD-01 gel.The stretching vibration peaks of aromatic phenyl ring at 1510 cm-1and 1546 cm-1in Z/HD-01 solution shift to 1508 cm-1and 1544 cm-1in Z/HD-01 gel.Besides,the stretching vibration peak of CH2at 2943 cm-1in Z/HD-01 solution shifts to 2937 cm-1for Z/HD-01 gel.These changes show the hydroxyl groups,aromatic phenyl ring and CH2in gellant Z take part in the formation of gelled fuels,indicating the existence of hydrogen bonding,π-π stacking and intermolecular van der Waals forces in the self-assembly of gelled fuels [28].
Fig.4(b) shows the XRD pattern of the Z/HD-01 xerogel.A distinct peak at 6.72° corresponds to the d spacing value of 1.314 nm (D).The diffraction peaks at 13.44° (D/2),19.98° (D/3),27.00°(D/4)and 33.40°(D/5)indicate the existence of one dimensional lamellar structure of gelled fuel[28].The value of the molecular layer spacing (1.314 nm) is larger than the size of a single Z molecule (0.83 nm) but smaller than the size of two molecules(Fig.4(c)).So the gellant molecules should stack cross each other to form the layer structure [29].
The diffraction peaks at 22.32° (0.398 nm) and 23.98°(0.371 nm) indicate the presence of hydrogen bonding forces between molecules[30],while the peak at 25.40°(0.35 nm)corresponds to π-π stacking distance of the aromatic phenyl ring [31],which correlates to the DFT calculation results (Fig.4(c)) that the gellants form one-dimensional assembly through hydrogen bonding and π-π stacking,and then cross stacking each other through van der Waals force to form a fiber structure.These fibers eventually form a 3D network to fix the fuel molecules to stable the gelled fuels.
3.4.Physicochemical properties of gelled fuels
The gelled fuel properties,i.e.,density,centrifugal stability,viscosity,gel transition temperature and net heat of combustion(NHOC),are then characterized.As shown in Fig.5(a),the density of gelled fuel is a little higher than pristine fuel,and it increases gradually with the rise of gellant Z concentration.For example,the density of HD-01 increases by nearly 3.6% (from the 0.935 g.ml-1to 0.969 g.ml-1)after adding 1%(mass)gellant,which is the largest improvement among the four gelled fuels.
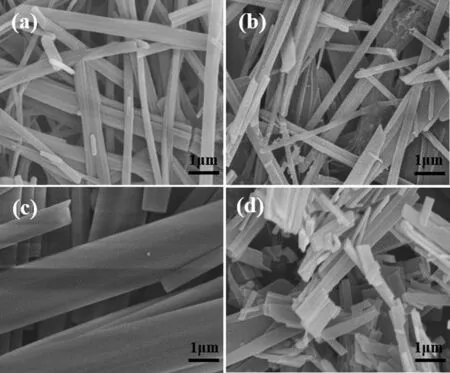
Fig.3.SEM images of (a) 1% (mass) Z/HD-01,(b) 1% (mass) Z/HD-03,(c) 1% (mass) Z/RP-3 and (d) 1% (mass) Z/QC gelled fuels.

Fig.4.(a) FT-IR spectra of gellant Z in solution state and gel state with HD-01 as the solvent,(b) XRD diffraction pattern of Z/HD-01 xerogel,and (c) the optimized selfassembly model of Z/HD-01 gel,where the white,gray,and red spheres are hydrogen,carbon,and oxygen atoms,respectively.
The gelled fuels were liquefied by mechanical stirring at 500 r.min-1for 5 minutes and the rotary viscometer was used to measure the viscosity(20°C)of the liquefied gelled fuels.As shown in Fig.5(b),when gellant Z concentration is lower than 0.5%(mass),the viscosity of the liquefied gelled fuels increases slowly compared with pure liquid fuel.As gellant Z concentration exceeds 0.5%(mass),the fuel viscosity increases obviously,which indicates the concentration gellant Z higher than 0.5% (mass) makes the gelled structure more stable and less likely to be damaged.This phenomenon is more obvious for Z/HD-03,following by Z/HD-01,Z/RP-3 and Z/QC.Specifically,the viscosity of liquefied 1% (mass)Z/RP-3 is 312 mPa.s (ca.251.76 times higher than pure RP-3,1.27 mPa.s),which shows the largest viscosity increase among the four gelled fuels,indicating the destruction of RP-3 gelled structure with larger size is more difficult than other gelled fuels under mechanical stirring.
High-speed centrifugation can simulate the mechanical interference during the storage and transportation of gelled fuels [19],which were used to determine the stability of the gelled fuels.The centrifugal stability test was conducted by centrifuging the gelled fuels at a fixed speed for 10 min.During the centrifugation,the liquid fuel will exude from gelled fuels,and the ratio(ωrem)of remaining gel mass to the initial gel mass is used to refer the physical stability of gelled fuels.As shown in Fig.5(c),the ωremvalue of Z/HD-01 gelled fuel is>90%at centrifugal speed from 2500 r.min-1to 7500 r.min-1,indicating Z/HD-01 has good centrifugal stability.With the increase of gellant Z concentration,the centrifugal stability of 1% (mass) Z/HD-01 gelled fuel is obviously enhanced.For example,the ωremvalue of Z/HD-01 gelled fuel increases from 95.46% to 99.05% with gellant Z concentration increased from 0.25% (mass) to 1.00% (mass) at 2500 r.min-1.As shown in Fig.5(d),the ωremvalues of Z/HD-03,Z/RP-3 and Z/QC gelled fuels are lower than Z/HD-01 gelled fuel.Especially for 1% (mass) Z/RP-3 gelled fuel,its ωremvalue at 5000 r.min-1is only 73.14%,much lower than other gelled fuels.The reason for this result should be that the self-assembled fibers of 1% (mass) Z/RP-3 have too large size (Fig.3(c)),which makes it difficult to fix fuel molecules very well and results in the large mass loss after centrifugation.
Different from traditional inorganic and polymer gellants,the gelled fuels prepared by low-molecular mass organic gellants are thermally reversible.When the temperature reaches phase transition point,the gelled fuels will turn into liquid phase.The gel-sol phase transition temperature (TG) of gelled fuels was measured by the falling-ball method and the results are shown in Fig.6(a).It can be seen that the TGvalue increases significantly with the rise of gellant concentration.For example,the TGof Z/HD-01 increases from 96°C to 123°C with the gellant concentration increased from 0.25%(mass)to 1%(mass),which is due to the enhanced stability of three-dimensional network structure by increasing gellant Z concentration.According to the relationship between TGand gellant concentration (cg),the gel-sol transition enthalpy (ΔHg) can be obtained according to the Van’t Hoff relation (Eq.(1)) [32–34].

where R is molar gas constant.
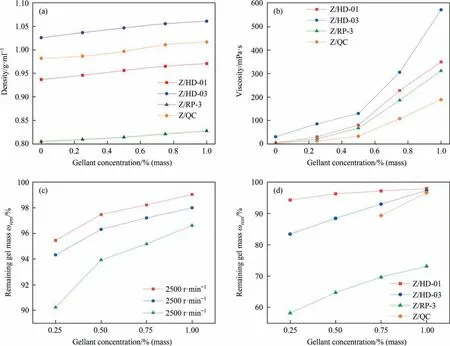
Fig.5.(a) Density and (b) viscosity of gelled fuels with different concentration of gellant Z at 20 °C,(c) stability of Z/HD-01 gelled fuels at different centrifugal speeds(2500 r.min-1,5000 r.min-1,and 7500 r.min-1),and (d) stability of four gelled fuels at centrifugal speed of 5000 r.min-1.

Fig.6.(a) Transition temperature and (b) the corresponding van’t Hoff plots of gelled fuels with different concentration of gellant Z.
Based on the data of Fig.6(a),the corresponding Van’t Hoff curves were plotted and exhibited in Fig.6(b).The ΔHgvalues of different gelled fuels obtained by fitting data can directly measure the thermodynamic stability of the gelled fuels and the gelled fuels with higher ΔHgwill have better thermodynamic stability.Z/RP-3 shows the highest ΔHgvalue of 95.31 kJ.mol-1,followed by Z/HD-03 (72.29 kJ.mol-1),HD-01 (63.44 kJ.mol-1) and Z/QC(63.07 kJ.mol-1),which indicate Z/RP-3 has better thermal stability for its large width size of skeleton structure.
Adding gellant in HED fuels also affects their net heat of combustion (NHOC) values.As shown in Fig.7(a),the mass NHOC of Z gelled fuels gradually reduces with the rise of gellant Z concentration.For instant,the mass NHOC of QC decreases from 43.95 MJ.kg-1to 43.54 MJ.kg-1after adding 1% (mass) gellant Z.Differently,the volumetric NHOC (mass NHOC multiplied by density)of the gelled fuels increases with the rise of gellant Z concentration (Fig.7(b)),owing to the larger increase degree of density than that of mass NHOC with the rise of Z gellant concentration.For the typical HED fuel HD-01,its volumetric NHOC increases from 39.58 MJ.L-1to 40.76 MJ.L-1with gellant Z concentration increasing from 0%(mass)to 1%(mass),which benefits the energy performance of HED fuels.

Fig.7.(a)Mass net heat of combustion and(b)volumetric net heat of combustion of gelled fuels with different concentration of gellant Z.Pressure change of oxygen bomb calorimeter for (c) Z/HD-01 gelled fuels with different gellant concentration and for (d) different fuels with 1% (mass) gellants Z.
In addition,the pressure change of oxygen bomb calorimeter have been recorded when testing the heat of combustion,with the results shown in Fig.7(c),(d).The liquefied gelled fuel shows the similar combustion performance with pure fuel.The pressure change at the start of combustion can be used to measure initial combustion speed of fuel.It can be seen from Fig.7(c)that the initial combustion pressure change during the combustion process has slowed slightly after adding gellant Z,due to the slow combustion rate of gellant Z.From Fig.7(d),the initial combustion speed of QC is higher than other three fuels and it also haven’t significant change after making into gelled fuel.These results show that the combustion performance of liquefied gelled fuel is decided by the performance of fuel and it will not change significantly after adding a small amount of gellants.
3.5.Rheological properties of gelled fuels
The rheological properties of gelled fuels are the most important index to evaluate the stability and usability of gelled fuels.The tests of rheological properties include shear thinning test,destroy and recovery test,strain sweep test and frequency-sweep test(Fig.8 and Fig.9).Shear thinning performance is the basic rheological property of gelled fuels.As shown in Fig.8(a),the viscosity of Z/HD-01 gelled fuels decreases obviously with the increase of shear rate from 0.01 s-1to 100 s-1,and the gelled fuels with higher gellant Z concentration shows larger viscosity under the same shear rate.1.00%(mass)Z/HD-01 gelled fuel shows higher viscosity than previously reported 1.00% (mass) L/HD-01 and 1.00% (mass)G/HD-01,indicating the high gel-forming performance of gellant Z.It is worth noting that 0.25% (mass) Z/HD-01 has much higher viscosity than 1.00% (mass) G/HD-01 and 1.00% (mass) L/HD-01 without shearing,but show almost the same viscosity with 1.00%(mass) G/HD-01 and 1.00% (mass) L/HD-01 when the shear rate reaches 100 s-1.This indicates the low-concentration gellant Z can realize highly stable HD-01 gelled fuels and excellent shear thinning properties.
Moreover,the Power-Law(P-L)model equation(Eq.(2))is used to fit the model to measure the shear thinning performance of gelled fuels show in Fig.8(a) and Fig.9(a)) [35],and the results are shown in Table 3.

where γ is the shear rate,η is non-Newtonian viscosity,K is the consistency index and n is the power index.
Gelled fuels with lower power index (n) have the better shear thinning performance.From Table 3,the shear thinning performance of Z/HD-01 gelled fuels decrease with the increase of the gellant concentration by comparing the n value of the P-L equation.The n values of gelled HD-01 with different gellants show that gelled fuels with gellant Z have better shear thinning performance compared with the previously reported gellants G and L.Moreover,for different type of HED fuels show in Fig.9(a),1% (mass) Z/QC exhibits the best shear thinning performance,followed by 1%(mass) Z/HD-01 and 1% (mass) Z/HD-03,while 1% (mass) Z/RP-3 shows the worst shear thinning performance.
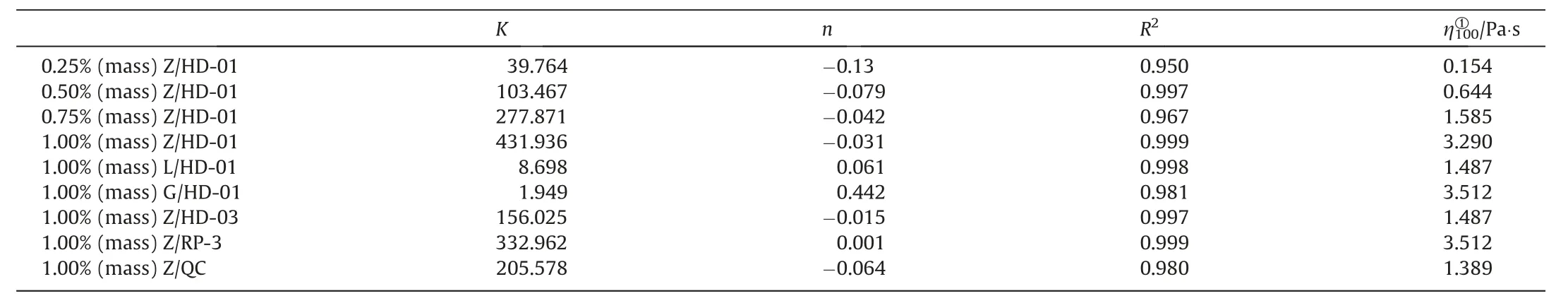
Table 3 Coefficient values of gelled fuels fitted with P-L mode equation

Fig.8.Rheological property of gelled HD-01 with different concentration of gellant Z and different type of gallants:(a)shear thinning test,(b)destroy and recovery test,(c)strain sweep test,(d) frequency-sweep test,where G′ is storage modulus and G′′ is loss modulus.
The thixotropy and recovery capability of the gelled fuels are tested by destroy and recovery test,which is a step experiment:1.low share rate step of 0.01 s-1for 120 s;2.high shear rate step of 60 s-1for 30 s;3.low share rate step of 0.01 s-1for 120 s;4.high shear rate step of 60 s-1for 30 s.The thixotropy is determined by the variation of viscosity with time at high shear rate,while the recovery capability is judged by the change of viscosity before and after shear.
From Fig.8(b) and Fig.9(b),the viscosity of all Z gelled fuels decreases rapidly when step 2 starts,indicating the gelled fuels have the property of thixotropy.This change becomes weak with the increase of gellant Z concentration (Fig.8(b)),indicating the thixotropy decreases with the increasing of gellant Z concentration.All gelled fuels have recovery capability,which is gradually enhanced with the increase of the gallant Z concentration by comparing with the viscosity after and before shear.From Fig.8(b),the viscosity of 1% (mass) Z/HD-01 after shear recovers to 65.39% of that before shear,while 72.22% for 1% (mass) G/HD-01 and 99.78% for 1% (mass) L/HD-01.So,gelled fuels made by gellant L has better recovery ability than others.By comparing the gelled fuels made from the four fuels,it can be seen from Fig.9(b) that the viscosity of 1% (mass) Z/RP-3 after shear recovers to 65.39%of that before shear,indicating it has the strongest recovery ability,followed by 1%(mass)Z/HD-01(65.39%),1%(mass)Z/QC (42.85%)and 1% (mass) Z/HD-03 (38.06%).Relatively weaker recovery ability can avoid blocking the pipeline due to regelation,which benefits the practical application of gelled fuels.
Then,the mechanical strength and stability of the gelled fuels were studied by strain sweep test (with test temperature of 298 K and the frequency of 1 Hz).As shown in Fig.8(c) and Fig.9(c),the range in which the storage modulus (G′) is independent of strain is linear viscoelastic (LVE) region.The mechanical strength of gelled fuel can be characterized by its storage modulus(G′)in the LVE region and gelled fuels with higher G′have stronger mechanical strength and stability.In Fig.8(c),the G′of Z/HD-01 gelled fuel increases significantly with the rise of gellant Z concentration but the critical strain value of LVE region decreases from 0.19%(0.25% (mass)Z) to 0.07% (1.00%(mass)Z) with the increasing of gallant concentration.This indicate the stability of the gel network is improved effectively with the increase of gallant concentration but the gel network structure becomes easier to be affected by strain.Moreover,the G′of 1.00%(mass)Z/HD-01 is significantly higher than 1.00% (mass) G/HD-01 and 1.00% (mass) L/HD-01,indicating gellant Z can prepare gelled fuels with good stability.Fig.9(c) shows the strain sweep results of four kind gelled fuels with 1% (mass) gellant Z,and the critical strain value of LVE region of the gelled fuels is around 0.06%–0.09%.However,the value of the G′is obviously different,which makes the gelled fuels show different stability.

Fig.9.Rheological property of gelled fuels with different HED fuels:(a)shear thinning test,(b)destroy and recovery test,(c)strain sweep test and(d)frequency-sweep test,where G′ is storage modulus and G′′ is loss modulus.
Moreover,the dependence of storage modulus (G′) and loss modulus (G′′) on frequency was studied by frequency-sweep test.,which can simulate the situations of gelled fuels in storage or loading (low frequencies) and injection or impact (high frequencies)[36].The value of strain was kept at 0.01%,which is in the LVE region of gelled fuels,and the scanning range was 0.1–100 rad.s-1.As depicted in Fig.8(d),the G′of Z/HD-01 is higher than G′′,indicating they exhibit typical solid-like behavior[6].With the increasing of gallant Z concentration,the frequency dependence of the G′′becomes weaker,indicating the gelled fuels become more stable.The G′′of 1%(mass)G/HD-01 become higher than G′when the frequency is higher than 2.9 rad.s-1,indicating G/HD-01 will be liquefied at higher frequencies.As shown in Fig.9(d),the G′′of 1%(mass)Z/HD-03 changes most obviously with the change of frequency among the four different gelled fuels indicating that 1% (mass) Z/HD-03 is more likely to be destoried by external forces at high frequencies [8].
3.6.Ignition and combustion properties of gelled fuels
The high-temperature spontaneous ignition in air was performed to test the ignition delay time of gelled fuel.The ignitiondelay time is defined as the time interval between the initial droplet with contacting hot plate and the appearance of flame [37–39].The ignition delay times of pure HD-01,1% (mass) Z/HD-01 gelled fuel and liquefied 1%(mass)Z/HD-01 gelled fuel were tested at 430 °C,which is a relatively higher temperature than HD-01(410 °C) to make sure the successful ignition of the gelled fuel).
As shown in Fig.10,the ignition delay time of HD-01 increases from 2128 ms to 2995 ms after the gelled fuel is formed.After the gelled fuel is liquefied,the ignition delay time decreases for 500 ms,because the network structure of gelled fuel has been destroyed making its combustion properties closer to pure fuel.These results suggest the Z gelled fuels can maintain the gel state in the storage state and transfer to liquid state under mechanical shearing,which shows the similar combustion performance with the pristine liquid fuel.

Fig.10.Photographs of fuel combustion flame images obtained by high-speed camera for(a)HD-01,(b)1%(mass) Z/HD-01 gelled fuel and(c) liquefied 1%(mass)Z/HD-01 gelled fuel at 430 °C.
4.Conclusions
In this work,we synthesized a phenolic gellant Z via one-step electrophilic addition reactions,and prepared four kinds of gelled fuels based on HD-01,HD-03,RP-3 and QC liquid fuels.Compared with previously reported LMOGs and SiO2gellants,gellant Z shows higher gelling ability in high density fuels,and the CGC of gellant Z is less than 1%(mass)for the four liquid fuels.Especially for HD-01,the CGC is as low as 0.1%(mass).The thermal stability of the gelled fuels increases with the increasing of gallant concentration,and the gelation of liquid fuels by gellant Z increases the volumetric net heat of combustion and density of the gelled fuels.Through the analysis of rheological properties,the prepared gelled fuels have great shear thinning performance,and the recovery ability of gelled fuels made by gellant Z is weaker than that of the reported LMOGs,which can effectively reduce the possibility of pipeline blockage.Meanwhile,the ignition and combustion tests show that the liquefied gelled fuel shows the similar ignition performance with the pristine liquid fuel.So,the gelled fuels made by gellant Z which have better stability,shear thinning and combustion performances have great potential for the practical application.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the support from the National Natural Science Foundation of China (21978200) and the Scientific Research Projects of the Ministry of Education of China(6141A02033522).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
