CO2 mineralization of carbide slag for the production of light calcium carbonates
2022-04-27TongyangZhangGuanrunChuJunlinLyuYongdaCaoWentaoXuKuiMaLeiSongHairongYueBinLiang
Tongyang Zhang ,Guanrun Chu ,Junlin Lyu ,Yongda Cao ,Wentao Xu ,Kui Ma ,Lei Song,Hairong Yue,2,*,Bin Liang,2
1 Low Carbon Technology and Chemical Reaction Engineering Laboratory,School of Chemical Engineering,Sichuan University,Chengdu 610065,China
2 Institute of New Energy and Low-Carbon Technology,Sichuan University,Chengdu 610207,China
Keywords:CO2 mineralization Calcium carbide slag Light calcium carbonate Process simulation Life-cycle assessment
ABSTRACT The production of polyvinyl chloride by calcium carbide method is a typical chemical process with high coal consumption,leading to massive flue gas and carbide slag emissions.Currently,the carbide slag with high CaO content is usually stacked in residue field,easily draining away with the rain and corroding the soil.In this work,we coupled the treatment of flue gas and carbide slag to propose a facile CO2 mineralization route to prepare light calcium carbonate.And the route feasibility was comprehensively evaluated via experiments and simulation.Through experimental investigation,the Ca2+ leaching and mineralization reaction parameters were determined.Based on the experiment,a process was built and optimized through Aspen Plus,and the energy was integrated to obtain the overall process energy and material consumption.Finally,the net CO2 emission reduction rate of the entire process through the life-cycle assessment method was analyzed.Moreover,the relationship between the parameters and the CO2 emission life-cycle assessment was established.The final optimization results showed that the mineralization process required 1154.69 kW.h.(t CO2)-1 of energy(including heat energy of 979.32 kW.h.(t CO2)-1 and electrical energy of 175.37 kW.h.(t CO2)-1),and the net CO2 emission reduction rate was 35.8%.The light CaCO3 product can be sold as a high value-added product.According to preliminary economic analysis,the profit of mineralizing can reach more than 2,100 CNY.(t CO2)-1.
1.Introduction
The chlor-alkali industry,one of the most basic chemical processes,is operated with high coal consumption and high emissions[1–3].Polyvinyl chloride (PVC) is a main product of chlor-alkali industry,widely used in our daily life and can be prepared through calcium carbide method.As a raw material for PVC production,calcium carbide can be generated by high temperature (>2000 K)melting reaction of lime and coal in calcium carbide furnace[4,5],with high energy consumption and massive CO2emissions.Additionally,the process also produces a large amount of carbide slag(CS),which is soluble in water and presenting strongly alkaline(pH >13).Currently,the treatment of CS is still facing intractable problems,and the CS is usually stacked in residue field,easily draining away with the rain and corroding the soil [6].At present,CS can only be used in the production of cement,the utilization rate of which is less than 40%.Worse still,it is also waste of abundant Ca resources [7].
Mineralization is an effective approach of carbon capture [8].Because the products produced by mineralization are very stable,which gives leakage-free,permanent storage that will not require poststorage monitoring [9,10].CO2mineralization and sequestration technology seeks to mimic the natural weathering process in which calcium or magnesium silicates are transformed into carbonates via reaction with gaseous and/or aqueous CO2[11].In terms of reaction types,mineralization can also be divided into direct mineralization and indirect mineralization [12].When energy input is in the form of pressure and heat and the mineral ores are reacted directly with carbon dioxide,it is called direct carbonation.When chemicals are used for acid leaching of the mineral ores and/or as a means of CO2carrier,the process is called indirect carbonation [13].In terms of reaction raw materials,the materials used for include natural minerals and industrial solid wastes containing Ca and Mg[14].Compared with natural minerals,the mineralization of industrial solid wastes can couple the treatment of the flue gas and solid wastes,possessing higher economic and social benefits [15].Until now,many kinds of solid wastes have been investigated for CO2mineralization,e.g.,blast furnace slag(BFS) [16–18],desulfurized gypsum [19–21],high calcium fly ash[22,23],and steelmaking slag [24,25].These solid wastes exhibit excellent CO2mineralization capacity.Moreover,high valueadded by-product are obtained to further improve the process economy.
Compared with other solid wastes,CS has higher Ca content,better activity,and higher storage capacity.There are several reports on the technology of using CS and CO2to generate CaCO3.Gao et al.[26]used hydrochloric acid to leach CS to prepare spherical ultra-fine CaCO3.However,the cost of hydrochloric acid is relatively high.Moreover,it requires high-quality equipment,and difficult to recycle,leading to pollution on the environment.Altiner et al.[27] focused on the conversion of CS and flue gas into CaCO3particles via an accelerated mineral carbonation process.Lyu et al.[28],Yang et al.[29]used NH4Cl aqueous solution and CS for leaching reaction,and then used NH4HCO3or Na2CO3for carbonization reaction to prepare CaCO3.Li et al.[30,31] investigated the CO2capture behavior of CS in calcination/carbonation cycles,and prepared a new CO2sorbent from CS,aluminum nitrate hydrate and glycerol water solution by combustion synthesis method.Wang et al.[32]used recyclable citrate extractant to prepare uniform calcite micro/nanorods from CS.Yang et al.[33] used CS to capture CO2at low or even ambient temperatures via a gas-liquid-solid three-phase fluidization system.At present,these studies mainly focus on the leaching agent and crystal structure of CaCO3,and pure CO2is usually used as carbon source.There is no comprehensive research on the whole technological route,such as process optimization,energy consumption calculation,CO2emission reduction assessment and cost estimation.Aspen plus is a typical process simulation software,which can be used to calculate and evaluate process materials and energy consumption.Keith et al.[34]used Aspen plus to simulate a process for capturing CO2from the atmosphere.Nduagu et al.[35–38]used Aspen Plus to simulate the mineralization process of natural minerals and improved energy economy by process integration.If carbon dioxide mineralization could combine with experiment and simulation calculation,it could not only obtain reliable process parameters and propose a new process route,but also would help obtain data on process energy consumption and materials,so as to facilitate the overall evaluation.
Despite numerous CO2mineralization methods have been proposed in the laboratory,it still lack relevant research in process route,energy consumption calculation and life cycle assessment.In the work,a new process route was proposed to couple the treatment of flue gas and CS to prepare light calcium carbide.The route feasibility was evaluated via experiments and modeling.Operation net including CS leaching,CO2mineralization,NH3escape capture,solid-liquid separation,leaching agent circulation,and solid washing,were tested through experiments and Aspen Plus.Then the energy in the process was optimized to reduce the CO2emissions,and the materials were recycled to reduce the material consumption.Finally,the entire process was evaluated by LCA to obtain the fixed efficiency,and evaluate the economic benefits.This work comprehensively investigates the preparation of light calcium carbonate from CS by CO2mineralization,which will push forward the industrialization of CO2mineralization.
2.Experimental
2.1.Materials
CS comes from chlor-alkali industrial tailings of Inner Mongolia Wuhai Chemical Co.,Ltd.Ammonium chloride (NH4Cl),ammonia(NH3.H2O,25%-28%) and other chemicals were analytical reagent and purchased from Chengdu Cologne Chemical Co.,Ltd.Pure carbon dioxide (CO2,99.9%) and nitrogen (N2,99.9%) were obtained from Tianyi Gas Co.,Ltd.
2.2.Experimental methods
2.2.1.Leaching experiment of CS
NH4Cl was used as leaching agent to react with a certain amount of CS under the set reaction temperature,reaction time,liquid-to-CS ratio and molar ratio,and then the insoluble impurities were removed through filtration.As shown in Eq.(1).Ca2+in the filtrate was detected by ethylenediaminetetraacetic acid(EDTA) method,the obtained filtrate was the leaching solution of CS.During the leaching experiment,NH4Cl was dissolved in water at a certain molar ratio under water bath and magnetic stir.The weighed CS was added after the temperature elevated to the set temperature.After the reaction,the obtained slurry was filtrated under vacuum environment and the supernatant liquid was the CS leaching solution.

2.2.2.CO2absorption and mineralization experiments
In this experiment,100 ml of CS leaching solution was poured into a 250 ml three-mouth flask,and ammonia was added to the leaching solution to adjust the pH value.Fig.1 shows the CO2absorption and mineralization device.First,check the air tightness of the device before the experiment to ensure that the air outlet is unblocked.Then turn on the water bath heating and the magnetic stirring rotor,waiting for the reaction temperature to reach 30 °C,40°C,50°C,and 60°C,respectively.N2was passed into the system for purge until the CO2IR analyzer showed 0.00%at the outlet.Two separate GFC were employed to provide constant CO2(0–100 ml.min-1)and N2(0–1 L.min-1)flow rates and adjusted the total flow rate(300,350,400,450,and 500 ml.min-1)and the concentration of CO2(5%,10%,12.5%,15%,20%).At the same time,the change of outlet CO2concentration was recorded during the absorption process.When the CO2concentration at the outlet had no significant change,the reaction was finished,as shown in Eq.(2).

2.3.Analysis and characterization
The crystal phase composition of CS and mineralized products were determined by X-ray diffraction (XRD-6100,Shimadzu,Japan) within the range 2θ=10°–80°.A combined energydispersive X-ray spectrometer(EDS,IS250,Oxford,Japan)was used to analyze the relative elemental content of the CS.The surface morphology of the CS and light CaCO3was observed by scanning electron microscopy (SEM,JSM-7500F,JEOL) at an accelerating voltage of 5 kV.The mass fraction of elements in CS was analyzed by X-ray fluorescence (XRF-1800,Shimadzu,Japan).The thermal decomposition range of compounds in CS was determined by thermogravimetric analyzer (TGA,HTG-2,Beijing Hengjiu Experimental Equipment Co.,Ltd.).The TGA operated in nitrogen atmosphere (100 ml.min-1),the program temperature range was 25 °C to 1000 °C,and the heating rate was 10 °C.min-1.The concentrations of various metal ions,such as Fe3+,Al3+,in the leached and filtered solution were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES,Spectro ARCOS ICP,Germany),in which the concentration of Ca2+was determined by EDTA method.
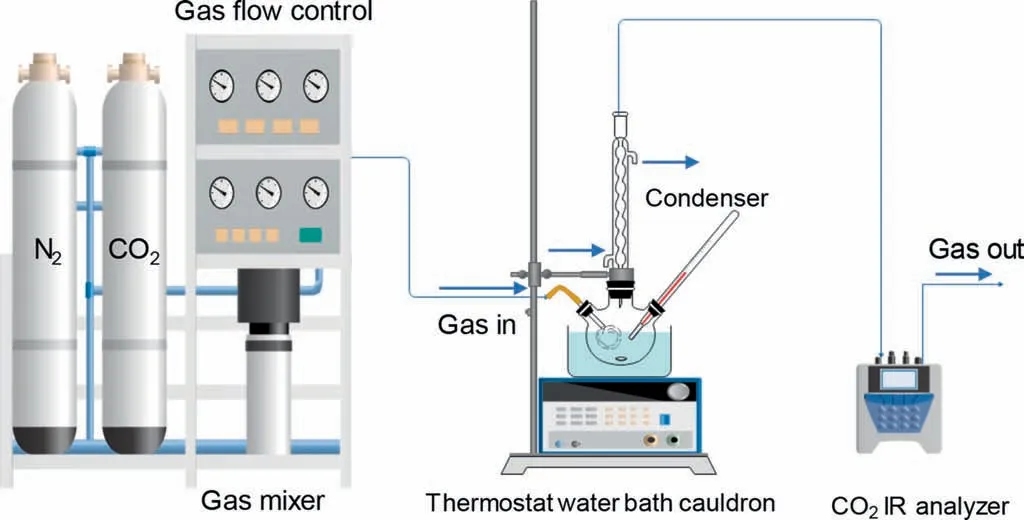
Fig.1.Schematic diagram of the CO2 mineralization device.
2.4.Aspen plus process modeling
2.4.1.Basis of simulation
The whole process of mineralization was simulated by Aspen Plus V11.The physical property data of chemical substances involved in the simulation were retrieved from the database(APV110.PURE37,APV110.AQUEOUS,APV110.ASPENPCD,APV110.SOLIDS).Electrolyte ions were involved in the process.In electrolytes,physical and chemical molecule–molecule interactions,ionic reactions,and interactions occurred(molecule-ion and ion-ion) [39].What’s more,the reaction and fluid transport processes were carried out under atmospheric pressure,so ELECNRTL-RK activity coefficient model was chose as the thermodynamic data model to calculate the temperature,pressure,viscosity,specific heat capacity,and other parameters of the process.N2,CO2and NH3were set as Henry coefficients in the simulation,and the Henry’s law constants of these species were retrieved from the Aspen electrolyte package of H2O-NH3-CO2[40].Reactions and related parameters involved in process were determined with an ELECNRTL-RK rate-based model (Eq.(S1),Table S2) [41].Chemical equilibrium was assumed with all the ionic reactions.In order to simplify the simulation,the following assumptions were put forward:
(1) The composition of CS was determined by experiment and characterization,ignoring the effects of the unstable composition of CS materials,and its simulated chemical composition mass fraction (% (mass)) data was shown in Table 1;

Table 1 Mass fraction of each component in the CS in simulation.
(2) According to the industrial flue gas data provided by Inner Mongolia Autonomous Region Wuhai Chemical Co.,Ltd.,the volume fraction of CO2was 12%,and the remaining inert components were simplified to nitrogen.In the leaching reaction section,only Ca(OH)2participates in the reaction.
(3) The absorption rate was affected by equipment size,mass transfer efficiency,etc.
2.4.2.NH4Cl cycle process
NH4Cl was used as the leaching agent and its acidity was used to leach Ca(OH)2in CS into Ca2+(aq).Since C and SiO2were insoluble in water,Al3+and Fe3+would react to form solid precipitation under alkaline conditions [29],and removed large amounts of impurities in the CS to obtain a high-purity Ca2+(aq) solution(Fig.2).
2.4.3.CS leaching
In the leaching section,the leaching reactor was simulated.Since the temperature had little effect on the reaction leaching rate,35 °C was chose as the reaction temperature.The conversion rate model RSTOIC was selected for simulation and the leaching reaction within a certain range of liquid-to-CS ratio was considered to simulate.After the reaction,the reaction liquid and precipitation were filtered and separated,the filtrate entered the next section,and the solid was discharged from the system as waste residue.Combined with the experimental and simulation results,the main components of the leaching residue were calcium carbonate,some insoluble silica and carbon residue.Due to the high content of calcium carbonate,the leaching residue could be used as raw material to calcine calcium carbide and returned to the calcium carbide production process.
2.4.4.CO2absorption mineralization and ammonia capture
In this section,the leaching liquid of the previous section mainly included Ca2+(aq),Cl-(aq),NH4+(aq) and OH-(aq).Prior to the mineralization reaction,ammonia aqueous was added to enhance the CO2capture capacity [42,43].In the simulation,Rad-Frac was used for modeling.Considering that the spray process had a mass transfer effect without trays,two trays were set to simulate the mass transfer effect of the spraying process in the tower.As CO2in the flue gas was absorbed after the reaction,a large amount of nitrogen removal caused the escape of ammonia.Inorder to reduce environmental pollution and material loss,an ammonia absorption tower was set up to recover the escaped ammonia.Similarly,RadFrac was used for modeling.The water produced by washing CaCO3was used to capture the escaped ammonia.Finally,the captured ammonia solution was recycled back to the mineralization tower.

Fig.2.CO2 mineralization of carbide slag for light calcium carbonates by ammonium chloride circulation route.
2.4.5.Calcium carbonate purification
In this section,Ca2+(aq)in the solution was precipitated by CO2through mineralization reaction to form light CaCO3products and the leaching agent.The mineralized slurry was filtered to obtain mineralized mother liquor which was concentrated,and CaCO3products were unwashed.Three stage washing was adopted for CaCO3to remove the ions carried in calcium carbonate.The washing water was used for ammonia capture in the mineralization section.Finally,light CaCO3products were obtained by filtration and flue gas drying.
2.4.6.Ammonium chloride cycle
In this section,since the mineralized mother liquor contained a large amount of NH4Cl which should be recovered,it was necessary to remove the excess water in the solution by evaporation.Herein,double effect evaporation was introduced.The secondary steam generated during evaporation in the previous evaporator was used as the heating steam of the subsequent evaporator.Generally,the first effect evaporator operated under a certain pressure.The pressure of the second effect evaporator decreased,resulting in an appropriate temperature difference,so that the liquid in the second effect evaporator could be evaporated [44].Multi effect evaporation significantly reduced the heat energy consumption in the evaporation process.Meanwhile,the heat exchange network was optimized.The steam after evaporation contained a lot of latent heat,but the steam temperature could not reach the temperature required for heat exchange.Therefore,heat pump technology was used to increase the steam temperature for mother liquor preheating.
2.5.LCA analysis method
Life-cycle Analysis (LCA) is a method that quantifies the environmental impacts associated with a product or service [45].The purpose of life cycle analysis is to evaluate the energy consumption and environmental load of products,and the product life cycle includes manufacturing (starting from raw material),using and wasting (reusing) [46].LCA can help identify opportunities to improve environmental performance at all stages of the product life cycle.According to the provisions of ISO 140440 and GBT 24040 standards,LCA includes four stages(Fig.3)[47,48]:(1)Goal and scope definition;(2)Life cycle inventory;(3)Life cycle impact assessment;4) Result and reporting.
2.5.1.Goal and scope definition
The determination of purpose and scope in LCA can be divided into the following four parts:purpose,system boundary,research object and functional unit [49].In this study,the purpose of LCA was to evaluate the CO2emission and cost analysis of the whole process,judge the contribution of the main factors affecting CO2emission,and explore the influence of liquid-to-CS ratio on the whole LCA process.According to the definition of LCA,the following aspects should be considered when setting the system boundary (Fig.3):acquisition of raw materials,manufacturing and processing,distribution/transportation,use and maintenance of products,disposal of process wastes and products,etc [50].In order to simplify the LCA calculation process,the following assumptions were made:
(1) Ignore the impact of equipment construction period and abandonment period;
(2) Ignore the impact of equipment damage and maintenance during operation;
(3) Ignore the influence caused by uncertain environmental factors during operation.
The research object was the CS mineralization project with an annual treatment of 1000 tons CO2,and the functional unit was based on the fixed 1 ton of CO2.
2.5.2.Life-cycle inventory
Since the process was in the development process of small-scale experiment and process amplification,the evaluation data in LCA were based on laboratory data and Aspen Plus model.To analyze the net CO2emission,it was necessary to know the direct and indirect CO2emission factors of materials,products and energy.
The data here came from CLCD-China v0.6 and Ecoinvent 3.1.The CS treatment compensation was based on the CO2emission factor produced by the production of cement with CS.Actually,the CS treatment would compensate more CO2.Based on the LCA evaluation method,the energy and material standard CO2emissions were converted to obtain the net storage and utilization rate for 1 ton of CO2absorbed in the process (Table S1,Supplementary Material).The net CO2emission was calculated from Eq.(3)[51].In terms of cost estimation,the cost arising from process construction and scrapping were not considered,and the materials were provided by the mother plant,which reduced the cost price(Table S2).The profit of the process absorbing 1 ton of CO2was calculated by Eq.(4).
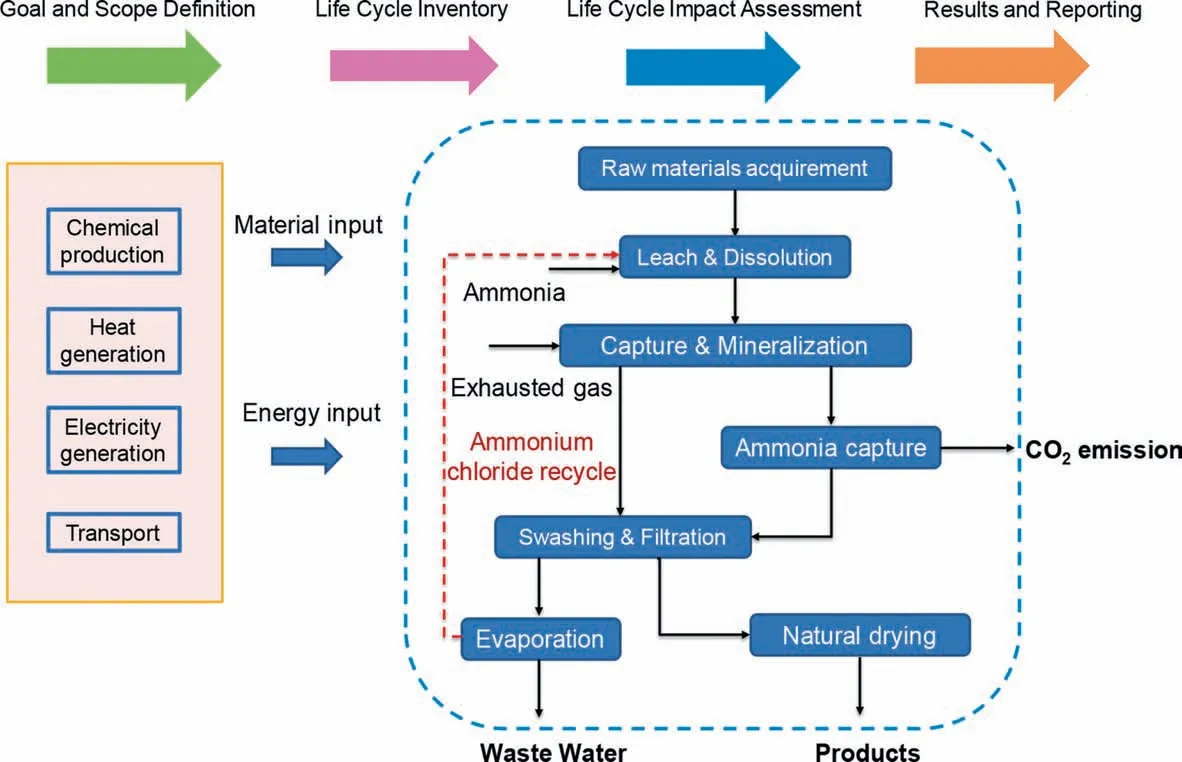
Fig.3.LCA evaluation process and system boundary of the carbide slag mineralization technology.

3.Results and Discussion
3.1.Results of CS and CaCO3 characterization
The composition of CS was analyzed by XRF and chemical titration and the result was listed in Table 2.Notably,the CS possesses high CaO content,which has the potential of efficient CO2capture.The impurities are mainly SiO2,Al2O3,Fe2O3,and carbon slag,which is insoluble in water and can be easily removed.

Table 2 Composition analysis of the carbide slag.
The XRD results from Fig.4(a) indicated that the Ca(OH)2and calcite were main crystalline component of CS.The SEM images showed that the micro structures of CS were particles with different sizes and amorphous morphology (Fig.5(a)).The TGA results showed three stages of weight loss (Fig.4(b)).The EDS results showed the relative elemental content of CS(Fig.5(b)).The weight loss in the three stage could be attributed to the water evaporation,decomposition of Ca(OH)2,and decomposition of CaCO3,respectively.The corresponding equations were shown in Eq.(5) to Eq.(7) [52–55].Based on the TGA result,the mass fraction of Ca(OH)2(~77.93% (mass)) and CaCO3(~15.26% (mass)) contents were calculated,the value of which was consisted with the XRF results.

Fig.4.(a) XRD patterns and (b) Thermogravimetric analysis curve of the CS.
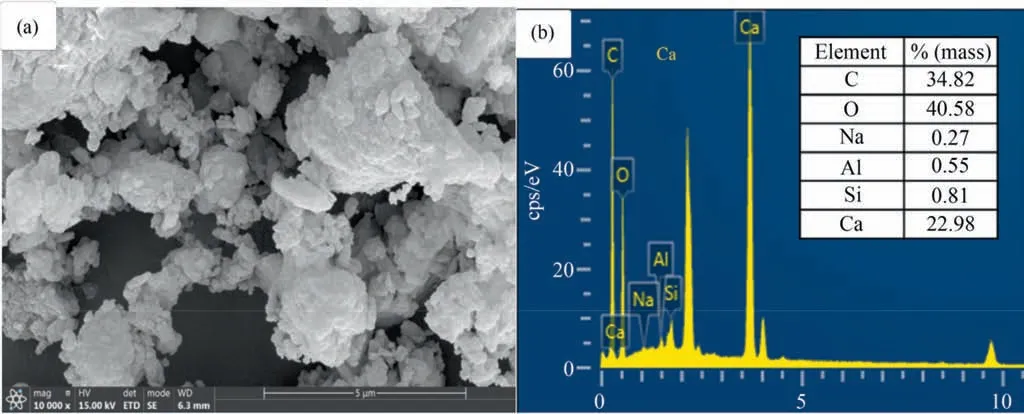
Fig.5.(a) SEM image of the CS and (b) EDS profile of the CS.

3.2.Experimental results of CS leaching
Fig.S1 shows the effect of reaction temperature.The calcium extraction efficiency would increase significantly with the elevated temperature.From the kinetic point of view,the elevated temperature would increase the reaction rate and aggravate the volatilization of ammonia,which was conducive to the calcium extraction.However,the volatilization of ammonia would affect the balanceof the entire tower absorption,leading to more loads of the ammonia absorption tower,thereby increasing both the water amount and the cost.
The liquid-to-CS ratio must be moderate for sufficient diffusion and contact between CS and ammonium chloride,which made the solid dispersion well and the reaction complete [27].However,excessive water reduced the concentration of reactants,decreasing the reaction rate and increasing the ammonia solubility,leading to high alkalinity of the solution.Thus,too much water would not be used rationally.
Considering about ensuring both the complete reaction and the extraction rate of calcium ions,an excess of ammonium chloride was selected.Moreover,the recycle of ammonium chloride lowered the cost,and the increase of ammonium chloride addition would make calcium hydroxide and ammonium chloride react completely,resulting in an increase of calcium extraction rate.
From the results of the orthogonal analysis,the optimum condition was reaction time of 50 min,reaction temperature of 40 °C,molar ratio of 2.2:1,and liquid-to-CS ratio of 10:1.The effect on the extraction rate of calcium ions was ranked in the following order of significance by polar difference analysis:Molar ratio>liquid to solid ratio >reaction time >reaction temperature (Fig.6).

Fig.6.Results of the CS leaching orthogonal experiments.
3.3.Mineralization experimental results
Fig.7 shows the effect of temperature on mineralization reaction.Under the principle of absorption,the lower the temperature,the better the absorption,however,the effect of temperature on the solubility of CO2was weakened by the high basicity of the experimental liquid.As the CO2flow rate and concentration fluctuated,the CO2was more easily detected at the outlet,which meant that CO2can easily penetrate the absorbing liquid,while the CO2concentration at the outlet increased faster due to the same volume and pH of the solution for each reaction,suggesting a faster absorption rate.In the end,the effects of pH on the mineralization effect were explored by adding ammonia.Due to the addition of ammonia,the hydroxide of the solution increased a large amount and thus increased the CO2capture efficiency and volume of the whole leaching solution.It can be seen from the Fig.7 that after the ammonia addition,the outlet CO2concentration decreased significantly at the same time in compared to no ammonia addition,which indicated that the addition of ammonia was more favorable to the CO2capture.At the same time,it could be seen from the time to reach the exit equilibrium of CO2that the carbon capture capacity of the whole solution was increased with the addition of ammonia.The mineralized product was cleaned and dried to obtain a lightweight calcium carbonate (Figs.S2 &S3).
3.4.Process simulation and energy optimization
Diverse technical routes and process parameters had a great impact on the raw material and energy consumption of mineralization[56,57].The process model of Aspen Plus simulation is shown in Fig.8.The escape of NH3was captured and recovered in the simulation.In the NH4Cl cycle process,double-effect evaporation and heat pump technology were combined to achieve efficient utilization of energy.As for water,the method of multiple utilization was adopted to decrease the use of fresh water.During the process,the outlet water contained 0.3% ammonia.It was combined with carbonate and bicarbonate in the liquid in the form of ammonium.Therefore,outlet water could be sent to the sewage treatment plant for aerobic biological nitrification degradation.Interestingly,ammonia carbonate and ammonium bicarbonate are commonly used as fertilizers,and outlet water could be used as fertilizer for irrigation of green plants in the park.
The whole process was designed to run 8000 h per year.Fig.9 shows the material balance of the process in 1 h.In order to achieve the goal of fixing 1000 t CO2per year,the consumption of CS was 450 kg.h-1.During the process,a low liquid-to-CS ratio was selected to decrease the energy consumption by heating in subsequent process,in which the circulating amount of water was 1923.8 kg.h-1.The conversion in the simulation was set at 80%,which matched the results of leaching test under the same condition.In addition,ammonia was added before mineralization to supplement the loss of NH3in the process.During the mineralization process,CO2and N2were not fully absorbed in the processed tail gas and ammonia volatilization due to the reduction of partial pressure,which would cause great pollution and lose a lot of ammonia.Therefore,the captured ammonia was recycled back to the leaching solution to realize the circulation of ammonia by recovering and capturing ammonia using the water of washing products process.For the circulation of NH4Cl solution,the method of evaporation was used to remove the excess water to realize the circulation of NH4Cl.In the treatment of mineralized products,three-stage washing method was chosen to remove ions and other impurities on the surface of calcium carbonate.After that,using pressure filtration,natural air drying or flue gas drying to remove the water in the products,and finally 343.5 kg.h-1products was obtained.
Fig.10 shows the distribution of energy consumption in the process.Notably,the energy consumption by evaporation was major in the process.Therefore,the optimal design of energy of water evaporation and NH4Cl cycle process was carried out.After sedimentation liquid-solid separation,the mother liquor contained H2O,NH3,NH4Cl and several other substances were obtained.Since washing water was added to recover the ammonia in the gas,water accumulation would occur.Therefore,it was necessary to evaporate the excess water.In order to maximize the energy utilization of the process,two aspects were designed:(1) The optimization of evaporation process.The differences of energy consumption and investment among direct evaporation,doubleeffect evaporation and three-effect evaporation were compared;(2) Combining with heat exchange integration.The underutilized steam energy was increased by heat pump technology to achieve the minimum heat transfer temperature difference,and countercurrent heat exchange with mineralized mother liquor,recovering a large amount of heat and achieving the purpose of energy saving.
In single-effect evaporation,the mineralized mother liquor was heated to 110.4 °C under the pressure of 0.12 MPa,in which the gasification rate of mother liquor was 43.13%,and then remove the steam by flash evaporation.Similarly,based on this,a double evaporator was connected in series,and the steam of the first evaporator was used as the heating steam of the second evaporator to form a double effect evaporation process.In the double-effect evaporation,the mother liquor was pressurized to 0.2 MPa by using a pump,and then heated to 124°C,in which the gasification rate of the mother liquor was 20.63%.The steam was also removed by flash evaporation.And using the secondary steam as the heat source to evaporate again under the pressure of 0.15 MPa,resulting in temperature difference through pressure drop.As for threeeffect evaporation,the pressure was increased to 0.3 MPa,the temperature was heated to 130.6 °C,and the three-effect evaporation was carried out by using the temperature difference generated by the pressure difference from 0.3 MPa to 0.2 MPa and then to 0.15 MPa.
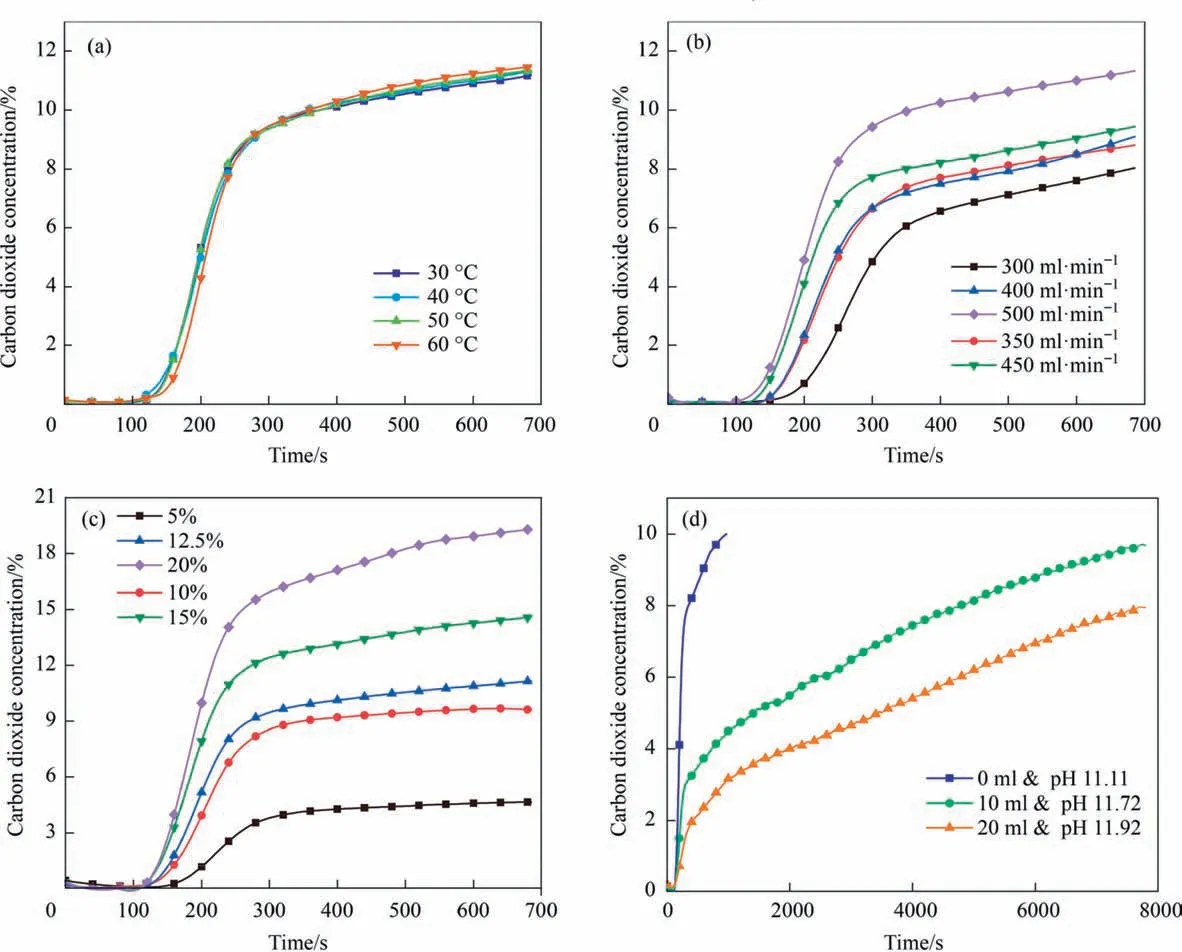
Fig.7.Effect of the(a)Temperature(°C),(b)CO2 flow rate(ml.min-1),(c)CO2 volume concentration,(d)Ammonia addition(ml)under the following conditions:A.CO2 flow rate=500 ml.min-1,CO2 volume concentration=12%,Ammonia addition=0 ml;B.T0=30°C,CO2 volume concentration=12%,Ammonia addition=0 ml;C.T0=30°C,CO2 flow rate=500 ml.min-1,Ammonia addition=0 ml;D.T0=30 °C,CO2 flow rate=500 ml.min-1,CO2 volume concentration=12%.

Fig.8.Schematic of CS mineralization to produce light calcium carbonate in Aspen Plus.
As shown in Fig.11(a),the energy consumption decreased with the increase of evaporation times while the equipment investment would increase.38.5%of energy was saved from single-effect evaporation to double effect evaporation,while three-effect evaporation only saved 52.0%.Thus,after comprehensive assessment,double-effect evaporation was selected.

Fig.9.Material balance of the CS mineralization process.

Fig.10.Process energy consumption of CS mineralization.

Fig.11.(a)Comparison of multi effect evaporation energy and investment cost.(b)Comparison of evaporation energy and investment cost combined with heat pump heat exchange network.
In order to achieve the maximum energy recovery,we carried out pinch analysis and optimization of heat exchange network.As shown in Fig.12,it is the temperature/enthalpy composite curve of this process,where QCis the energy to be removed,QHis the energy to be added,and QRrepresents the energy that can be recovered.Where ΔTminis the pinch temperature difference of the process.The lower the pinch temperature is,the greater the energy that can be recovered.However,the lower the pinch temperature also means that the heat exchange area increases,so we control ΔTmin>5 °C in this process.
The optimization results of heat exchange network are shown in Fig.13.S32is tertiary steam with 119.0°C and 0.16 MPa.In order to fully recover the latent heat in the steam,the tertiary steam was pressurized to 166.1 °C and 0.22 MPa through the compressor to exchange heat with the mineralized mother liquor S24.After heat exchange,the temperature of S24was 123.5 °C,0.2 MPa.It was heated to 124.1°C by steam and entered the flash tank.After flashing,secondary steam S30and concentrated liquid S31were obtained.The secondary steam S30was pressurized to 136.9 °C and 0.22 MPa by the compressor,and heat exchange was carried out with the concentrated liquid S31under reduced pressure to obtain the tertiary steam S32.

Fig.12.The temperature/enthalpy composite curve of process.
In Fig.11(b),the process combined with heat exchange saved 62.6% of energy consumption compared with that only using double-effect evaporation.Furthermore,coupling with heat pump technology,it could save 86.6% of energy consumption.Although the investment would increase,the use of heat pump doubleeffect evaporation can save much energy consumption,and reduce the carbon emission during the process.Combined with energy consumption and equipment investment,the use of heat pump double-effect evaporation was selected.
3.5.Life-cycle assessment
3.5.1.Carbon emission analysis
The LCA of the process was shown in Fig.14(a).The negative value represented the fixed amount of CO2,and the positive value represented the amount of CO2emissions.In this process,the fixed amount of CO2was 1000 kg.t-1,and the compensation for treating CS was 36.55 kg.(t CO2)-1.In terms of CO2production,313.38 kg.(t CO2)-1emissions were generated by heating,and 179.28 kg.(t CO2)-1emissions were generated by the compressor power consumption which used the electrical energy,indicating that the evaporation of water produced vast CO2emissions.Meanwhile,117.61 kg.(t CO2)-1resulted from the loss of ammonia.The net CO2emission was 357.95 kg.(t CO2)-1,leading to 35.8%CO2storage efficiency.
3.5.2.Cost profit analysis
In Fig.14(b),negative values represented process costs,and positive values indicated process revenue.2844.52 CNY.(t CO2)-1was the value of the product in this process.The cost of CS and the income from the treatment of CS were not considered.In the process cost,the heater’s power consumption was the major of 144.90 CNY.(t CO2)-1,followed by the ammonia escape of 111.48 CNY.(t CO2)-1.Herein,the labor and transportation costs were calculated at 100 CNY.(t CO2)-1.Therefore,the net income of the process was 2147.61 CNY.(t CO2)-1,bringing about high economic return.
3.5.3.Sensitivity analysis of liquid-to-CS ratio
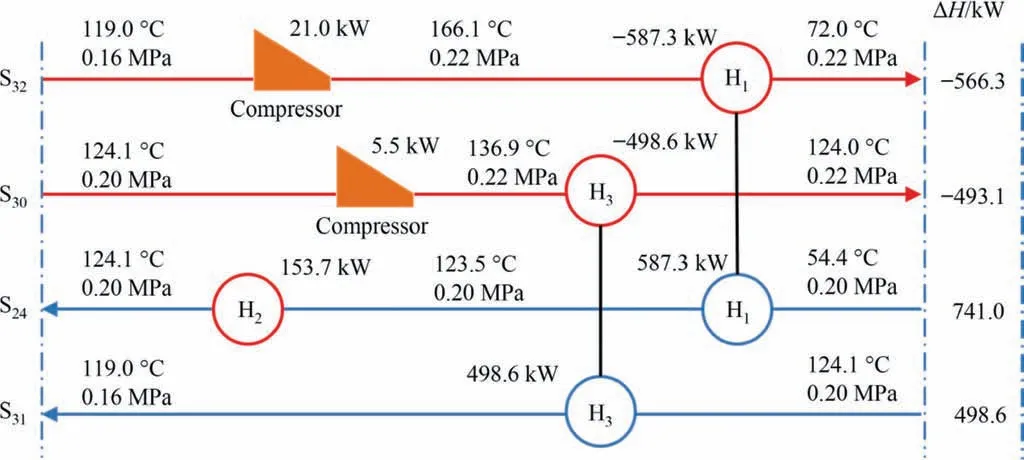
Fig.13.Heat exchange network of CS mineralization process.
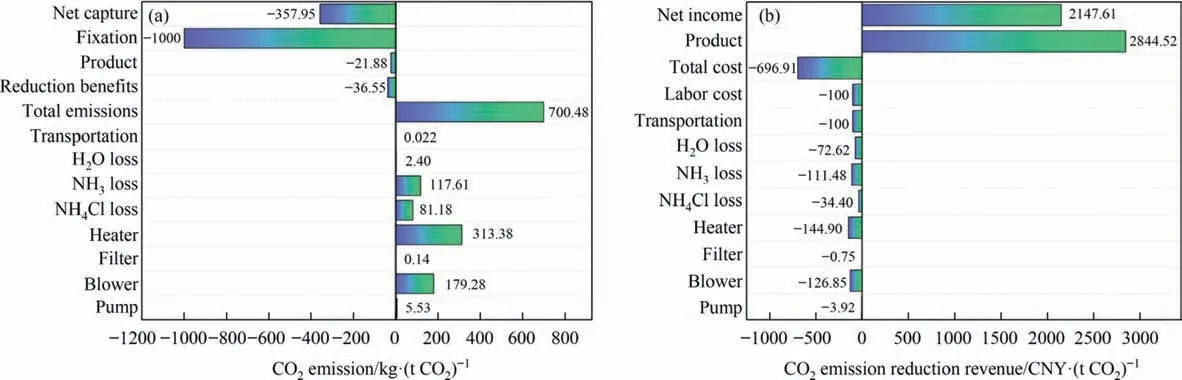
Fig.14.(a) LCA of CO2 emission through CS mineralization.(b) Revenue of CO2 emission reduction through CS mineralization.

Fig.15.Sensitivity analysis result of liquid-to-CS ratio on energy consumption,CO2 emission and cost.
Since the liquid-to-CS ratio in the leaching process would affect the water circulation in the whole process,experiment and simulation were used to analyze the energy consumption,CO2emission and cost.It was concluded from the experiment that as the liquidto-CS ratio increases,the leaching rate would be faster,which was brought into the simulation (Fig.15).In CO2emissions,as the leaching rate increased,the Ca2+would also increase,enhancing the CO2captured.However,due to the increased water,energy consumption was also increased.Interestingly,it was found that the increase in water increased the capacity of ammonia and reduced the loss of ammonia.Simultaneously,the consumption of materials was reduced,and the CO2emission caused by the loss of materials was reduced.Based on various influencing factors,it could be seen that as the liquid-to-CS ratio increased,the net CO2emissions would continue to decrease until it reached a positive value that could not be captured.In terms of energy consumption,as the liquid-to-CS ratio increased,the energy required for heating increased,resulting in larger energy consumption.In terms of cost,the overall change was small due to less energy consumption in the process and low raw material cost.However,the increase of water reduced the loss of ammonia,and the cost of heat energy was high.When the liquid-to-CS ratio was 2:1,the maximum profit was 2147.61 CNY.(t CO2)-1.Followed by the liquidto-CS ratio of 4:1,the profit was 2011.40 CNY.(t CO2)-1,and the liquid-to-CS ratio of 6:1 had the lowest profit of 1897.09 CNY.(t CO2)-1.
4.Conclusions
In this work,a process based on CS mineralization to produce light CaCO3from flue gas was proposed,which achieved the purpose of treating waste with waste.In the experimental part,through range analysis,the significance level of the influence on Ca leaching rate was as follows:molar ratio>liquid-to-CS ratio>reaction time >reaction temperature.In mineralization experiment,a light calcium carbonate of less than 10 μm was obtained at a CO2concentration of 12%.Based on the experiment,Aspen Plus was used to model the whole process.In order to achieve a fixed 1000 t CO2per year,it’s necessary to consume 450 kg of CS per hour and produce 343.5 kg.h-1of product.In terms of energy,it was focused on optimizing the leaching liquid circulation part.The double-effect evaporation,combined with the process of heat exchange materials and heat pump technology,saved 86.6% of energy consumption compared with ordinary evaporation.In terms of CO2production,the net fixed amount of CO2in the process was 357.95 kg.(t CO2)-1,leading to 35.8% CO2storage efficiency.In terms of CO2production,it was found that the 313.38 kg.(t CO2)-1emissions were generated by heating,and 179.28 kg.(t CO2)-1emissions were generated by the compressor power consumption which used the electrical energy,indicating that the evaporation of water in the process produced a large amount of CO2emissions.The net income of the process was 2147.61 CNY,which had a relatively high profit.In general,the CS mineralization process of capturing CO2in flue gas to generate light CaCO3had significant environmental and economic benefits.Meanwhile,it realizes the treatment of CS and flue gas in chloralkali industry and generates high value-added products,which will push forward the industrialization of CO2mineralization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for the support from National Natural Science Foundation of China (22078208),and the Major Science and Technology Projects of Inner Mongolia Autonomous Region(2020ZD0025).This work also acknowledges China Chengda Engineering Co.,Ltd.for its software support.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2022.02.011.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Green hydrogen:A promising way to the carbon-free society
- Electrochemical CO2 mineralization for red mud treatment driven by hydrogen-cycled membrane electrolysis
- Fabrication of azobenzene-functionalized porous polymers for selective CO2 capture
- Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution
- CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases
- Methane hydrate crystal growth on shell substrate
