Regulatory Difficulties and Governance Paths of Telemedicine Platform
2022-04-26HuoJingyu,ZhangMingjian
Huo Jingyu, Zhang Mingjian

Abstract: As an emerging medical model integrating medicine and communications, telemedicine, which carries virtualized services of "Observation, Auscultation, and Olfaction, Interrogation, Pulse Feeling and Palpation" has become the preferred solution for digital technology to reshape the medical system. In this process, the telemedicine platform is no longer just a single role of the technical service provider, but also involves many functions such as medical service pricing, medical information transmission and storage, and telemedicine service standard setting. Patients' health, safety, and privacy information are also facing unknown risks such as damage or infringement on the platform. The government needs to timely adjust the regulatory concept and regulatory framework, change the traditional coping regulatory tools, pay attention to the telemedicine platform as an independent subject, set legal norms such as unified platform access standards, data privacy protection, and medical responsibility identification, and ensure that new technologies and methods are smoothly embedded in the diagnosis and treatment scheme, to promote the scientific, standardized and orderly development of telemedicine.
Keywords: telemedicine; telemedicine platform; risk regulation; medical disputes
CLC: D 923 DC: A Article:2096?9783(2022)02⁃0108⁃08
1 Introduction
As an emerging medical model integrating the fields of medicine and communication, the cross-regional connectivity and cross-temporal interaction of telemedicine are conducive to releasing medical dividends, promoting the sinking of advantageous medical resources, and realizing the accessibility of medical resources in grassroots areas. It plays a unique role in cross-regional diagnosis and treatment of difficult diseases and response to public health emergencies. However, the telemedicine platform carrying" Observation, Auscultation and Olfaction, Interrogation, Pulse Feeling and Palpation" virtualization services is different from the commercial platform sharing products or information services. It not only has the link function of hospitals, doctors, and patients but also has the screening power to collect, store and send protected health information. Therefore, it is more likely to face network security and data risks. With the rapid development of 5G, big data, VR, and 3D printing technology, the number of telemedicine platforms in China has increased rapidly (as shown in Figure 1), but the corresponding regulatory concept and measures are stagnant in the traditional medical regulatory thinking of medical resources superimposed on Internet technology and do not pay attention to telemedicine platforms as an independent subject. Due to the lack of legal norms such as unified platform access standards, data privacy protection, and medical responsibility identification, the government needs to timely adjust the regulatory concept and regulatory framework, and establish a full cycle regulatory system for endpoint security, data security, and border security of telemedicine platform, to promote the scientific, standardized and orderly development of telemedicine.
2 Basic Definition of Telemedicine Platform
2.1 Definition of Telemedicine and Telemedicine Platform
Based on the different understanding of the subject, object, and access mode of high-tech applications, there are some differences in the definition of telemedicine in different institutions and regions. In the Opinions of the National Health and Family Planning Commission on Promoting Telemedicine Services in Medical Institutions issued by the former National Health and Family Planning Commission in 2014, telemedicine is defined as a medical activity in which a medical institution invites another medical institution to use a computer, communication and network technology to provide technical support for the patients receiving diagnosis and treatment in this medical institution. The telemedicine platform is a third-party platform independent of hospitals and patients. The platform brings together many functions including personnel, equipment, communications, finance, medical treatment and expert data compilation, time and space preparation, and makes reasonable arrangements for both parties of telemedicine to ensure the smooth progress of telemedicine activities.
2.2 Types of Telemedicine Platforms in China
Telemedicine in China started in the 1980s and 1990s[1], with the development of technology renewal and iteration, the number of telemedicine consultations increases rapidly. In May 2012, the 12th Five Year National Government Informatization Project Construction Plan proposed to promote the pilot of telemedicine, In February 2015, the National Development and Reform Commission and the former Health and Family Planning Commission issued the Notice on Approving the Pilot Program of Telemedicine in Five Provinces including Ningxia and Yunnan, At present, there have been 17 provinces and cities in the 14th Five-Year Plan outline of the construction of telemedicine, nine provinces and cities have enacted telemedicine into the 14th Five-Year Plan. As the basic support for the development of telemedicine, the construction of telemedicine platforms is also diversified.
2.2.1 Classification by Business Model
Telemedicine platforms can be classified into the following three models according to their business models:(1) B2B (Business to Business) model refers to a medical activity in which one medical institution (the inviter) invites other medical institutions (the inviter) to provide technical support for the inviter's patients by using a computer, communication, and network technologies. In this mode, the platform is only instrumental and mainly undertakes the technical support of information interchange, but does not directly participate in the screening and decision-making of diagnosis and treatment plans; (2) B2C (Business to Customer) model, which involves the same subjects as the traditional doctor-patient relationship, highlights medical institutions as application subjects and provides off-site diagnosis and treatment services directly to patients outside the institutions through information technology. The independent function of the platform has not yet emerged; (3) In B2B2C (Business to Business to Customer) model, telemedicine institutions take the platform and equipment required for telemedicine services as the premise, manage these devices and platforms, and directly participate in the control of telemedicine related information. Integrate with physical medical institutions to provide telemedicine services for patients[2].
2.2.2 Classification by Initiator
The telemedicine platform is classified according to the initiator, and can be divided into the following three modes:(1)Provincial unified platform initiated by the government, this type of platform is planned by the government to integrate the health resources in the region, with the regional health information platform as the core, building a unified remote service platform to carry out telemedicine services, covering medical institutions connected to the information platform. Taking Guangdong Province as an example, the telemedicine platform project in Guangdong Province is composed of a telemedicine policy management system, business service system, information technology system, and operation and maintenance system, covering remote services such as remote consultation, remote outpatient service, and remote operation teaching, radiating 20 provincial hospitals, 56 county-level people's hospitals and more than 1 000 town and village health institutions. Through the construction of a telemedicine interconnection network at the provincial, municipal, county, town, and village levels, hospital diagnosis, treatment resources, and electronic medical record data sharing can be realized in the same province[3]. By the end of 2018, 19 provinces nationwide had established provincial telemedicine platforms, and the national telemedicine collaboration network covered more than 24 000 medical institutions in all prefecture-level cities and 1 808 counties (county-level cities), including all national-level poor counties[4]. (2)Hospital united community platform, this type of platform takes large third-class hospitals or influential hospitals in the region as the leading hospitals and forms a hospital joint community with grassroots hospitals to make full use of high-quality medical resources, carry out telemedicine services, and realize information sharing among patients and collaborative work among doctors in cross-hospital areas. For example, the telemedicine network constructed by the Telemedicine Center of the PLA General Hospital covers different aspects such as telemedicine consultation, telemedicine education, telemedicine emergency treatment, difficult cases, multi-disciplinary telemedicine discussion, telemedicine health management, and telemedicine academic exchange. By 2020, there are 900 Internet hospitals in China, the telemedicine collaboration network covers more than 24 000 medical institutions in all prefecture-level cities, and more than 5 500 hospitals above level II can provide online services[5]. (3)Civil mutual aid type platform, this type of platform is mainly formed by enterprises, individuals, and associations, and is a voluntary mutual assistance platform formed by civilians. However, due to the inconsistent supporting policies of telemedicine in different regions, unified pricing and mutual recognition of medical insurance reimbursement have not been formed. The development of mutual assistance telemedicine platforms is quite different, and the frequency of use by doctors and patients is not high.
3 Difficulties in Regulatory of Telemedicine Platform
3.1 The Access Mechanism of the Telemedicine Platform Is Not Clear
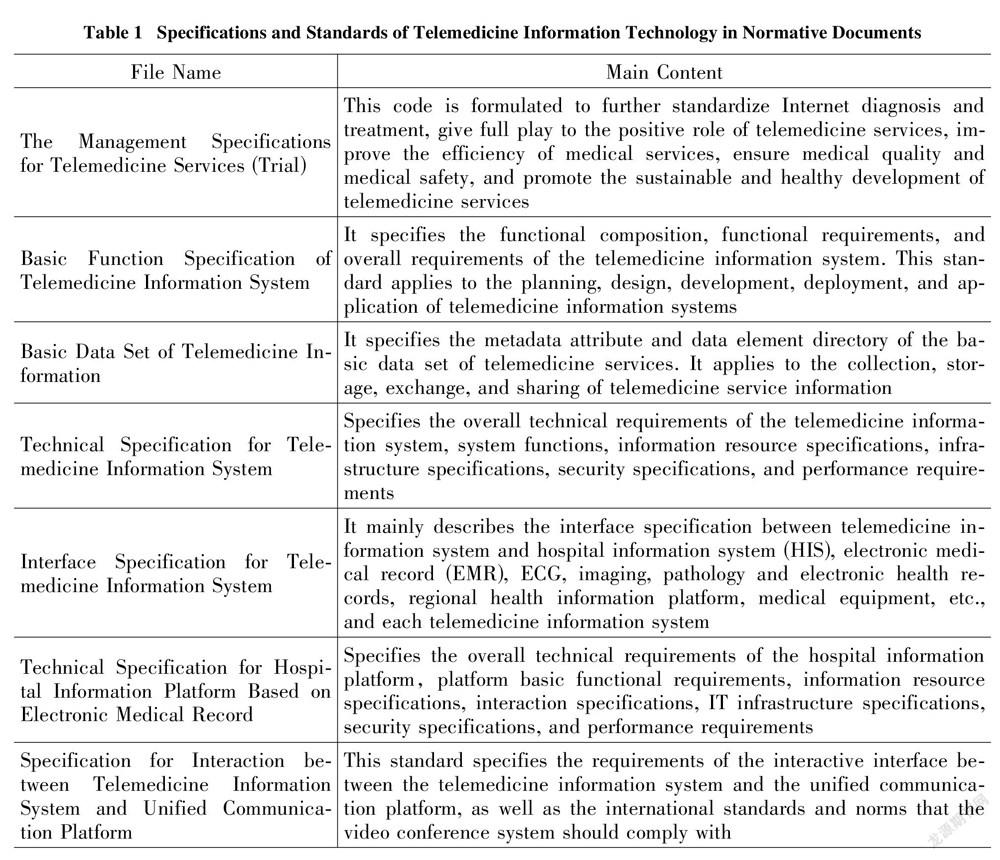
Source regulation of platform qualification and behavior is an important part of telemedicine risk regulation. The telemedicine platform is not the direct provider of diagnosis and treatment services, and it is not a qualified body that sets relevant health information technical norms and standards for medical and health institutions in the Regulations on the Management of Medical Institutions. The Measures for the Administration of Internet Medical and Health Information Services also did not mention the application and establishment standards for medical and health platforms such as medical and health websites. In practice, the establishment of the platform is mainly based on the Management Specifications for Telemedicine Services (Trial) and the Notice on Strengthening the Management of Telemedicine Consultation: "Approval and permission from provincial and above health authorities are required, and the application, qualification certificate of the sponsoring organization and information security measures should be submitted to the health authorities. After approval, the platform can provide services for the hospital." There is little mention of telemedicine image quality standardization and privacy protection of patients (as shown in Table 1). Each user can only self-regulate the maintenance and management of telemedicine service equipment, facilities, and information system, as well as the liability for medical malpractice and privacy disclosure through civil contracts. Policy tools that constrain each other will directly cause policy effects to offset each other, and the target groups will be at a loss as to what to do[6].
3.2 The Regulatory Mode of Telemedicine Platform Is Simplified
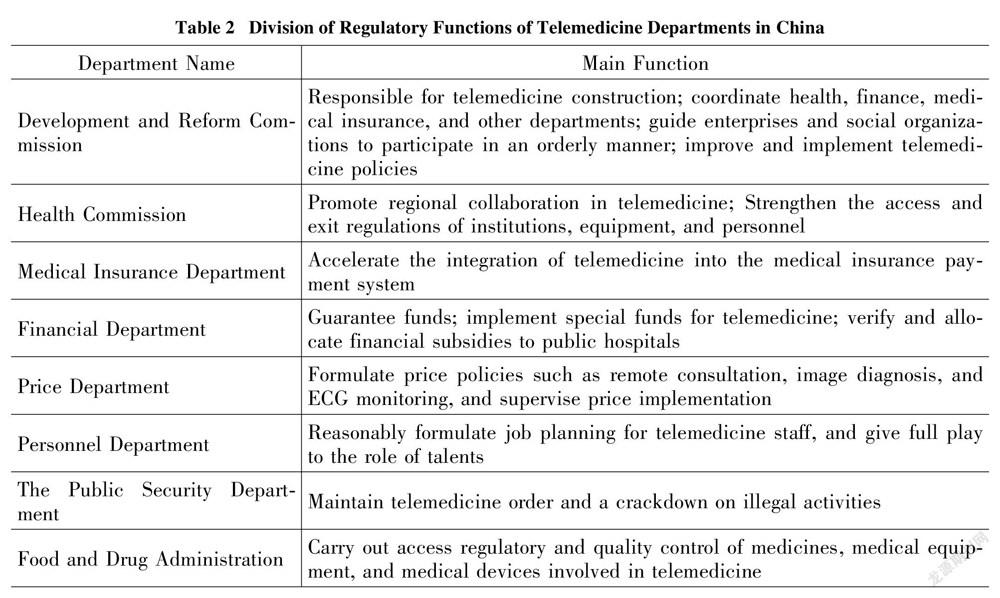
Different from the traditional one-way medical regulatory mode, the participants of the telemedicine platform dynamically point to the multidimensional governance structure of connecting hospitals, doctors, and platforms. At present, the regulatory model of telemedicine platforms in China is still dominated by the government, with less participation from diversified subjects such as industry institutions and the public. Among them, the government-led model has not been fully developed in different departments and regions. The Development and Reform Commission, the Health Commission, the Administration for Market Regulation, the Public Security, and other departments implement prior and subsequent regulations on the establishment of the telemedicine platform (as shown in Table 2). The Development and Reform Commission is responsible for the overall regulation of telemedicine. Health authorities review the compliance of hospitals, doctors and platform practitioners, the market regulatory department shall check whether the hospital and platform have matching equipment conditions, the public security department handles criminal cases of telemedicine. However, there is neither a mechanism for sharing platform qualification information between the entities nor a specific regulatory process division. The non-professional power setting of heavy industry guidance and light platform regulation has caused the regulation of telemedicine to lag, and more regulatory resources are consumed in dispute resolution. From the perspective of the regulatory path of telemedicine development in foreign countries, the government mainly uses its administrative expertise to conduct macro regulatory on the public interests involved, while the industry associations of Non-Governmental organizations undertake standard formulation and technical work mode.
3.3 Remote Medical Dispute Resolution Mechanism Is Not Sound
In the traditional medical model, the subject of medical malpractice liability is relatively clear, and medical institutions and doctors bear the corresponding legal liability for their scope of liability. However, with the intervention of the telemedicine platform, the bidirectional structure of the doctor-patient relationship has been transformed. When a telemedicine accident occurs, there is no employment relationship between the platform and the medical staff. How to define the platform due to diligence disclaimer boundary and other issues need to be clarified. In addition, the platform is different from medical institutions, and its establishment standard is technical configuration level rather than medical cognition level, and there is no professional basis for reviewing specific diagnosis and treatment plans and medical personnel's compliance with the code of ethics. With the rapid growth in the number of medical institutions and personnel stationed on the platform, questions such as whether it is necessary to double-check the conditions of practice approved by the health authorities are challenging the traditional medical management rules. For example, in the case of ma's medical malpractice dispute, the patient's family believed that the hospital's remote consultation through communication could not achieve the effect of offline consultation in the process of emergency treatment, which delayed the treatment of the patient and led to the patient's final death. Therefore, the hospital should bear the responsibility for the death of the patient. After the trial, the court thought that the use of communication technology for remote consultation is not improper, the judge rejected the lawsuit request.
4 Governance Path of Telemedicine Platform
4.1 Change the Regulatory Concept: Inclusive and Prudent Regulation
At present, telemedicine in China has gradually expanded into multi-level and wide group application scenarios such as remote consultation, remote appointment and registration, remote consultation, telemedicine assistance, and telemedicine education. In this process, the telemedicine platform is no longer only a technical service provider, but also involves many functions such as medical service pricing, medical information transmission and storage, and telemedicine service standard setting. Government regulatory departments, as representatives of public interests[7], should adopt an inclusive and prudent regulatory concept, and consider the compatibility between technological innovation and traditional regulatory strategies in the field of Internet intervention in diagnosis and treatment, as well as the connection between the rights and obligations of the platform as a subject of civil and administrative legal relations.
Even if the patient expresses the initial intention to use, such as agreeing to implant medical devices, or installing fall sensors and other auxiliary devices for telemedicine at home, in addition to receiving fall signals or other necessary medical information, these devices may also collect when the patient is at home, receive interaction with his family, or detect other non-medical activities and data such as other activities the patient is participating in. According to the Personal Information Protection Law of the People's Republic of China, the willingness to use does not represent the authorization to open private information, the platform must also comply with the requirements of the Cybersecurity Law of the People's Republic of China and be able to take necessary technical measures and measures to maintain the integrity, confidentiality, and availability of user data. At the same time, we should avoid the confusion between platform regulatory responsibility and government regulatory responsibility, and weaken the credibility and validity of regulations. For example, the platform's review of the qualifications of medical personnel is only based on the qualifications of doctors as stipulated in the Law of the Peoples Republic of China on Medical Practitioners, and it is difficult to determine whether they have the qualifications to operate telemedicine equipment. Under the framework of non-prohibition by law, the qualifications of medical personnel cannot be denied. Only after the relevant access conditions are formulated and evaluated by the health authority, the platform can set the requirements for licensed medical practice.
4.2 Improving Regulatory Capacity: Accurate and Scientific Regulation
Accurate and scientific regulations of the telemedicine platform will become a key link in the realization of the effectiveness of telemedicine. The telemedicine platform takes public welfare as the priority, but the judgment of public welfare is often dynamically adjusted with the occurrence of public health emergencies and other situations. According to a report from the Centers for Disease Control and Prevention (CDC), in the first quarter of 2020, the use of telemedicine increased by 50% compared to the same period in 2019[8]. The rapid increase in the number of interviews and usage of the platform sometimes leads to a decline in security and privacy. On March 8, 2020, the U.S. Civil Rights Office announced that it would not penalize providers who use telemedicine platforms for violations of HIPAA compliance. This change allows platform providers to use untraditional, easily accessible video conferencing programs, video software such as Zoom and Skype does not have an additional layer of security, but the health information (HPI) of the medical visitor faces the risk of being collected by a third party or sold to a third party. While the platform remains open and expands, control risks also increase[9], which may lead to severe legal challenges and even threaten the survival of the platform. Therefore, the government should uphold the concept of pre-regulation, and take preventive regulatory measures against the risks that may be caused during the use of the platform, as a necessary condition for the platform's online operation. First of all, the platform sets up a medical consent form on the user interface, including the name, certificate, organization affiliation, and location of the health professionals involved, the name and description of the recommendation procedure, potential benefits and risks, possible alternatives, and the emergency plan in case of problems in the procedure. Second, the platform should prominently inform the medical institutions, medical personnel and patients may hinder the ability to communication and operation hazards inherent technology, including fiber optic line damage, damage to the satellite system, or a hardware failure, may cause the failure of transmission is incomplete or damaged during transmission, file, lead to information or image to receive incomplete, unclear, or inaccurate, and natural disasters, such as hurricanes, tornadoes, and floods, may disrupt operations and compromise computer networks. At the same time, governments should mandate that platforms develop technical failure plans and ensure that they are communicated to all platform users before telemedicine contact. When implementing telemedicine practice on the platform, medical institutions and medical personnel should be informed of the unique risks of telemedicine in advance, and can only carry out diagnosis and treatment activities after verifying the authorization of legitimate telemedicine implementation, ensuring compliance with the protection of patients' data, and complying with binding rules such as privacy regulations and disclosure.
4.3 Expanding Regulatory Bodies: Diversified and Coordinated Regulation
The social governance pattern of co-construction, co-governance, and sharing needs to realize the collaborative governance of multiple subjects. Let all subjects give full play to their roles, integrate their forces, and effectively resolve conflicts and mistrust[10], to continuously promote the modernization of national governance capacity [11]. The intervention of the telemedicine platform makes the public more sensitive to non-medical risks such as information exposure. Static regulatory tools set up with the function of danger defense cannot integrate the cooperative mechanism of government, platform, and social multi-subjects. Therefore, the distribution of government regulatory functions can no longer take medical institutions and doctors as a single regulatory center. Instead, it should develop from standard governance to technological governance, from data-based regulatory to digital regulatory, to integrate scattered powers guided by technological empowerment, from fragmented regulatory to holistic regulatory, and from segmented regulatory to full-process regulatory. And actively introduce the third party to participate in the development of platform technical standards and industry evaluation, to increase the administrative needs of platform supervision for public welfare.
5 Conclusion
The traditional trial-and-error supervision model in the field of danger defense and technological regulation cannot effectively respond to the needs of the modern risk society[12]. As the carrier of remote multi-agent interactive diagnosis and treatment methods, the telemedicine platform not only has higher security requirements than the general internet platform but also attracts attention because it directly involves the right to personal health. With the continuous extension of telemedicine to primary medical institutions and remote areas, the defensive regulatory concept that only focuses on the prevention of the "attack surface" of data and equipment security cannot fundamentally solve the contradiction between platform development and innovation and medical disputes. Government departments need to break through the overlapping of existing regulatory responsibilities, establish a long-term mechanism between departments and a comprehensive crisis management model, and constantly optimize the platform's regulatory tools for informed consent, medical resource certification, remote prescription, and other core elements in various application scenarios.
References:
[1] BAO Y R, JIANG L L. Retrospect and prospect of telemedicine development in china[J]. China Digital Medicine,2019, 14(5): 99⁃102.
[2] XIE Y R. Research on legal relationship and civil liability of telemedicine—taking "B-B, B-C, and B-B-C terminal" service mode as examples[J]. Medicine and Jurisprudence,2017, 9(4): 80?82.
[3] LEI A X.Guangdong telemedicine platform has been launched[N]. Guangming Daily, 2018-12⁃19.
[4] National Health Commission of the People's Republic of China. Response to proposal No. 6789 of the second session of the thirteenth national people's congress[EB/OL]. (2020-7-13)[2021-04-11]. http://www.nhc.gov.cn/wjw/jiany/202007/9607789f88424536b014c0a7f86141dd.shtml.
[5] National Health Commission of the People's Republic of China. There are 900 Internet-based hospitals in China, and telemedicine covers more than 24 000 medical institutions [EB/OL]. (2020-10-28)[2021-04-29]. https://baijiahao.baidu.com/s?id=1681796823574076709&wfr=spider&for=pc.
[6] WEIBLE C M, HEIKKIL T. Policy conflict framework[J]. Policy Sciences, 2017, 50(1): 23⁃40.
[7] PAHNKE E C, KATILA R, EISENHARDT K M. Who takes you to the dance? How partners' institutional logics influence innovation in young firms[J]. Administrative Science Quarterly,2015(4).
[8] US Centers for Disease Control and Prevention[EB/OL]. (2021-05-27)[2021-08-11]. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm.
[9] BUEGER O, MOSER F. Toward an optimal degree of openness in IT innovation projects[J]. R&D Management,2019(2).
[10] ANSELL C, GASH A. Collaborative governance in theory and practice[J]. Journal of Public Administration,Research and Theory,2008,18(4).
[11] ZANG X W, SI W J. Local practice examples of collaborative governance of multiple subjects[J]. Governance,2019(29): 8.
[12] ZHAO P. The administrative law's response to risk society[M]. Beijing: China University of Political Science and Law Press,2018: 101.
远程医疗平台的监管难点与治理路径
霍敬裕,张明健
(合肥工业大学 文法学院,合肥 230011)
摘 要:作为融合医学与通信等领域的新兴医疗模式,承载 "望闻问切"虚拟化服务的远程医疗成为数字技术重塑医疗系统的优选方案。在此过程中,远程医疗平台不再仅是技术服务提供商的单一角色,还会涉及医疗服务定价、医疗信息传递储存,以及远程医疗服务标准制定等诸多功能。就医者的健康、安全和隐私信息亦在平台中面临损害或侵害等未知风险,需要政府适时调整监管理念和规制架构,改变传统应对型的监管工具,将远程医疗平台作为独立主体予以关注,设置统一的平台准入标准、数据隐私保护,以及医疗责任认定等法律规范,确保新技术、新方法平稳嵌入诊疗方案中,以推动远程医疗科学、规范、有序发展。
关键词:远程医疗;远程医疗平台;风险规制;医疗纠纷
猜你喜欢
杂志排行
科技与法律的其它文章
- Research on the Development Issues of Intellectual Property and Related Services as a Strategic Emerging Industry
- The Development of Digital Monetary Encryption in China and Its Application in Artificial Intelligence Information Security
- Tort Liability for Autonomous Vehicles in the Human-Computer Collaboration Scenario
- 我国录制录音制品法定许可的制度定位、完善思路与建议
- 聚焦“5G+智能”时代:数字出版著作权法治理困境及应对
- 请求权基础下个人信息权益保护的规范体系
