内源性类氧化酶Pt@MXene复合材料(PMCs)纳米酶用于杀菌与伤口愈合
2022-04-19邵长胜郑鑫鑫
刘 超, 邵长胜, 郑鑫鑫, 黄 青
(1. 中国科学院合肥物质科学研究院智能机械研究所强磁场与离子束物理生物学重点实验室安徽省环境毒理学与污染控制技术重点实验室, 合肥230031; 2. 中国科学技术大学研究生院科学岛分院,合肥230026)
1 Introduction
Bacterial infection is one of the major public health threats that afflict millions of people[1-3]. To deal with this problem, various antibiotics and agents have been developed, which include antibiotics[4], metal ions[5], quaternary ammonium ions[6], etc. However, chronic abuse or uncontrolled use of antibiotics has facilitated the emergence of super-bacteria with multi-drug resistance, posing another potential hazard to public health and causing environmental pollution through food chains[7-8]. Apart from the bacterial drug resistance, the low antibacterial efficiency was also persist and poor biocompatibility. Hence, for combating bacterial infections, alternative regents and therapies are urgently needed. For this purpose, new agents and methods for antibacterial are currently proposed and developed, such as photocatalytic bacteria-killing[9], photothermal bacteria-killing[10]and the inhibition of bacterial adhesion[11]. In addition, recent researches have confirmed that the intrinsic defense system of the organisms could utilize biocatalysts such as peroxidase (POD)[12]and oxidase (OXD)[13]to suppress bacterial growth. Owing to the excellent activity and high selectivity towards special substrate, these enzymes are considered the preferred alternatives to current antibiotics.
However, the natural enzymes face challenges due to their high production cost, complex production conditions and low catalytic stability, which greatly limit their potential as antibacterial agents in actual applications[14-15]. Surprisingly, nanomaterials with enzyme-like activity (denoted as nanozymes) have attracted great attention in recent years, as they can catalyze the reactions of biologically relevant substrates under mild conditions. Nanozymes not only possess the same reaction and kinetic similarities to natural enzymes, but also show some unique properties such as high stability, low cost and efficient performance[16-17]. Therefore, nanozymes with peroxidase and oxidase-like properties are recommended to combat bacterial infection. Recently, benefiting from the fast development of nanomaterials, a series of nanomaterials such as noble metals[18], metal oxide[19]and carbon materials[20]have been synthesized, which are proved to possess excellent enzyme-like activity and display high antibacterial property. For example, Qu et al. reported that graphene quantum dots (GQDs) could catalyze the decomposition of H2O2into·OH through their peroxidase-like activity, which improved the antibacterial ability and avoiding the high toxicity of H2O2[21]. Wang et al. prepared the novel Co4S3/Co(OH)2hybrid nanotubes (HNTs) with outstanding oxidase-like activity, which could destroyEscherichiacoli,Pseudomonasaeruginosa,StaphylococcusandBacillus[22]. Mukherjee et al. designed a water-soluble Pd12nanocages with outstanding oxidase-like activity, which showed the photocatalytic antibacterial activity against methicillin-resistantStaphylococcusaureus(MRSA) strains[23].
Furthermore, at present, there are lots of reports of use of nanozymes with high antibacterial ability and good biocompatibility in the study of bacterial inactivation[24-25]. Alternatively, current mechanism studies mainly focus on the regulation of ROS levels of nanozymes, such as the promotion of HOBr/Cl generation and the scavenging of extracellular DNA[26]. Although the generation of ROS by nanozymes is one of promising strategies for designing and preparing antibacterial agents, the mentioned nanozymes may suffer from low antibacterial efficiency and potential side effects because of the high reactivity of ROS in biological systems and its limited diffusion distances[27], and the adverse effects of ROS on normal host cells may also require special consideration[28].
In this work, inspired by the principle that most oxidase-like reactions are similar to the redox reaction (ORR) process in electrocatalysis[29-31], and with the consideration that Pt/carbon-based composites not only become the most promising catalytic materials in the field of electrocatalysis[32-33]but also can be used as antibacterial materials[34], we therefore proposed a new type of nanozyme for inactivation of bacteria and infection suppression for wound healing.According to previous work, MXene could be selected as the Pt-supported substrate, as the graphene-like 2D sheet material possesses excellent electron transport property, which has been used as fascinating support in various aspects[35-36]. Herein, Pt@MXene composite (PMCs) nanomaterial was synthesized by one hydrothermal method and applied as an oxidase-like enzyme for antibacterial effect (Figure 1). A series of experimental results proved that Pt nanoparticles were uniformly dispersed on the two-dimensional MXene sheets, and the as-fabricated PMCs showed extremely high oxidase-like activity. Theinvitroantibacterial experiments indicated that PMCs had extremely over 90% inactivation effect on bothE.coliandS.aureus. Besides, bothinvivoandinvitroexperiments based on cytotoxicity experiments, hemolysis experiments and animal experiments further proved that the PMCs could effectively treat wound infection and accelerate wound healing. The involved mechanism, which may be related to the oxidizing of the bacterial cell membrane and cutting off the electron respiratory transfer chain, was also explored.

Figure 1 Preparation and bactericidal action of Pt@Mxenecomposite (PMCs)图1 Pt@Mxene复合材料的制备及其抑菌过程示意图
2 Materials and methods
2.1 Materials
K2PtCl4, LiF,3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China), HCl, Ti3AlC2were obtained from Sinopharm Chemical Reagent Co., Ltd. (China), SYTO 9, propidium iodide (PI), and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Thermo Scientific, CCCP, DiOC2(3) and CCK-8 were obtained from Maokang Biotechnology Co., Ltd. (China).
2.2 Methods
2.2.1 Preparation of Pt/MXene composites (PMCs)
Few-layer MXene (Ti3C2Tx) nanosheets were prepared according to the method reported in our group’s previous work with some modifications[37], as detailed in the supporting information. In order to obtain the Pt/MXene composite (PMCs), a certain amount of K2PtCl4(24.09 mmol/L) was added to the evenly dispersed few-layer MXene suspension, then the mixture was transferred to a Teflon-lined autoclave after sonication for 15 min, then reacted at 180 ℃ for 6 h and cooled to room temperature naturally. The products were washed at least three times with ddH2O followed by drying for 12 h.
2.2.2 Characterization
The morphology of PMCs was examined with the transmission electron microscope (TEM, Jeol F200, Japan). The absorbance was recorded with Ultraviolet-visible spectrum (UV-Vis, Shimadzu UV-2550, Japan). X-ray photoelectron spectroscopy (XPS) was performed for elemental analysis with a single Al Ka excitation instrument (XPS, Thermo Kalpha, USA), and X-ray diffraction (XRD) was used to analyze the crystal form of the obtained materials (PANalytical X’Pert, Netherlands). Flow cytometry (Cyto FLEX, BECKMAN COULTER, USA) was employed to monitor the change of fluorescence.
2.3 Assessment of oxidase-like activity
The oxidase-like activity of PMCs was measured using 3,3′,5,5′-Tetramethylbenzidine (TMB) as the standard substrate, with the details as follows: 0.5 mg/mL PMCs and TMB (500 μmol/L) were added to 0.5 mL HAc-NaAc buffer solution (pH=4.5), and the mixture was placed at room temperature for 10 min; next, the oxidase-like activity of the PMCs was investigated by measuring its UV-Vis absorption change at 652 nm. All the experiments were repeated 3 times.
2.4 Steady-state kinetic analysis
Steady-state kinetic studies were carried out by monitoring the absorbance change of oxidized products at 652 nm. The specific steps are as follows: under optimal experimental conditions, the absorbance was measured at 652 nm by changing the concentration of TMB. According to Lambert Beer’s law, the absorbance can be converted into the concentration of oxTMB, while A=εbC and ε is 39 000 L/(mol/cm), and so the Michaelis-Menten constant is calculated as:
V0=Vmax×[S]/(Km+[S])
whereV0is the initial velocity,Vmaxis the maximum reaction rate, [S] is the substrate concentration, andKmis the Michaelis constant[38].
2.5 Bacteria culture and antibacterial activity test
E.coliandS.aureusstrains (ACTT 8099 and ATCC 43300) were grown overnight at 37 ℃ in L-B liquid medium. The specific operation is as follows: single clone was picked and incubated in L-B liquid medium firstly, then cultured with standard bacterial liquid culture in bacterial shaking incubator at 180 r/min and 37 ℃ for 12 h. The bacteria solutions were diluted and added into fresh L-B medium at a ratio of 1∶100 and cultured at 180 r/min and 37 ℃ overnight. The obtained bacterial suspension was centrifuged for collection of bacterial pellets. For the antibacterial activity test, PMCs at different concentrations were added to the dispersed bacterial solution, and incubated together at 37 ℃ and 180 r/min for 2 h. The control group was treated with an equal volume of PBS instead of PMCs. Then, the obtained bacterial solution was diluted 104times, and 100 μL of the diluted bacterial suspension was transferred to the L-B argal plates and cultured at 37 ℃ for 14 h. Total clones were counted the next day. Before the plate sterilization experiment, all materials stayed under ultraviolet light for 30 min, and the experiment was repeated three times. The bacteriostatic rate (R) was calculated by the following formula:
R=A/A0×100%
whereA0andArepresent the number of single clones in the control group and the treated group, respectively[39].
2.6 Investigation of mechanisms of antibacterial effect
2.6.1 Morphological observation of bacteria
The bacterial suspensions treated with PMCs were centrifuged and re-dispersed in 2.5% of glutaraldehyde overnight in the dark. Then the cells were dehydrated with 30, 50, 70, 80, 90, and 100% ethanol for 15 min, respectively. The bacteria were collected by centrifugation, placed on the conductive tape and observed by SEM[40].
2.6.2 ROS detection
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was chosen to measure intracellular ROS content[41]. Bacteria treated with PMCs were stained with 10 μmol/L DCFH-DA for 30 min at room temperature in the dark, and then washed three times with PBS. Intracellular ROS levels were measured using a flow cytometer with Ex/Em of 488/525 nm respectively.
2.6.3 Interaction between Cytochrome C and PMCs
Cytochrome C (Cyt C) (100 μg/mL) was mixed with an equal volume of PMCs (500 μL), and then the UV-Vis spectrum of Cyt C at 200-800 nm was measured, with PBS instead of PMCs as the control group[42].
2.6.4 Analysis of cell membrane potential
The membrane potential of the 2 h treated bacteria was analyzed by 3,3′-diethyloxo-carbocyanine iodide (DiOC2(3))[43]. Briefly, 1 mL of bacterial suspension in PBS treated with different concentrations of PMCs was centrifuged and resuspended in 1 mL of PBS. 2 μL of 5 mmol/L DiOC2(3) was then added to the above suspension and incubated for 30 min at room temperature. Subsequently, the fluorescent intensity was monitored by flow cytometry with Ex/Em of 482/497 nm respectively. For the positive control group, 10 μL of 500 μmol/L carbonyl cyanide m-chlorophenyl hydrazone (CCCP) was added to the bacterial suspension.
2.6.5 Electron transport activity assay
Electron transport system activity was assessed by the reduction of 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyltetrazolium chloride (INT) to formazan (INF)[44]. In brief, bacterial cells were harvested by centrifugation, washed twice with PBS (0.1 mol/L, pH=7.2) and resuspended in 1 mL of PBS. Then 200 μL of INT (0.5%) and 1 mg of nicotinamide adenine dinucleotide (NADH) were added and the mixture was incubated at 30 ℃ for 30 min in the dark. After the reaction was terminated by adding 100 μL of formaldehyde, the bacterial cells were collected by centrifugation, and formazan was extracted from the cells with 500 μL of 96% methanol. The extraction process was repeated twice and measured its absorbance at 490 nm.
2.7 Mouse skin wound healing model
Bacterial infection wound model was established on the back of male mice (Balb/c, 6-8 weeks, 18-22 g). Briefly, after chemically removing the dorsal hair of the mice, wounds with a diameter of about 1 cm were created with surgical scissors, and after infection with 100 μL ofS.aureus(1×107CFU/mL) within 24 h, the 9 mice were divided into three groups. The mice with infected wounds were treated with PMCs for 7 d with continuous monitoring of wound healing. Meanwhile, PBS and MXene in PBS were used as control groups. The mice were sacrificed on day 7, and the wound tissues were placed in PBS and sonicated for 15 min in order to count the bacterial number in the wounds.
2.8 In vivo toxicity evaluation of PMCs
The cytotoxicity of PMCs to mammalian cells (Hela cells) was investigated by standard CCK-8 assay. Firstly, cells were cultured using a 96-well plate in the incubator (5% CO2) at 37 ℃ for 24 h and then incubated with different concentrations of PMCs. After another 24 h, CCK-8 solution (10 μL) was added and the mixture was incubated in a 37 ℃ incubator for another four hours before obtaining the absorbance at 450 nm on a microplate reader. Cell viability (CR) was assessed with the following equation:
CR=A/A0×100%
whereAandA0are the absorbance of the treated and control groups, respectively[45].
2.9 Hemolysis assay of PMCs

Figure 2 TEM image (a), HRTEM image (b), EDS mapping of C, O, Ti and Pt elements of PMCs (c) 图2 Pt@Mxene复合材料的透射电子显微镜结果(a);高分辨透射电子显微镜结果(b);C 、O、Ti、Pt元素的X射线能谱分析结果(c)
The samples were tested for hemolysis with fresh rat blood. Firstly, the collected whole blood was obtained from rat’s eyes and centrifuged at 3 000 r/min for 10 min to collect red blood cells (RBCs), washed three times with PBS, and then the collected red blood cells were made into a 5% storage solution for later use. Secondly, different concentrations of PMCs were incubated with an equal volume of RBCs stock solution at 37 ℃ for 3 h, and after centrifugation at 12 000 r/min for 10 min, the absorbance at 540 nm was measured. ddH2O was used as a positive control and PBS was used as a negative control. The hemolysis rate of the sample was calculated according to the following formula:
hemolysis ratio(%)=
(AS-AN)/(AP-AN)×100%
whereASrepresents the absorbance of RBCs incubated with PMCs, andANrepresents the absorbance of RBCs exposed to PBS,APdenotes the absorbance of RBCs exposed to ddH2O[46].
2.10 Histological analysis
The infected mice were sacrificed on the 7th day, and the wounds and major organs including heart, liver, spleen, lung, and kidney were harvested, fixed in 4% paraformaldehyde solution, paraffined, sectioned, and observed after H&E staining by microscope.
2.11 Statistical analysis
Each group of experiments was repeated thrice. Origin software was used to process the data, and the experimental results were expressed as Mean ± standard deviation.
3 Results and discussion
3.1 Characterization of PMCs
To demonstrate the successful synthesis of PMCs and explore their morphologies, the TEM and HRTEM images of PMCs were acquired. Figure 2(a) shows the flake-like structure of MXene of the as-prepared PMCs, and Figure 2(b) displays the HRTEM of PMCs. The resulting 0.21 nm lattice fringes in the insets demonstrate that most of the exposed facets are (111), although a small fraction may also be (110) and (100)[47]. In addition, the EDS-mappings of PMCs in Figure 2(c) also show the uniform distribution of the characteristic elements C, Ti, and O of MXene, and the mapping of Pt elements also proves the uniform distribution of Pt nanoparticles on the 2D sheets of MXene.

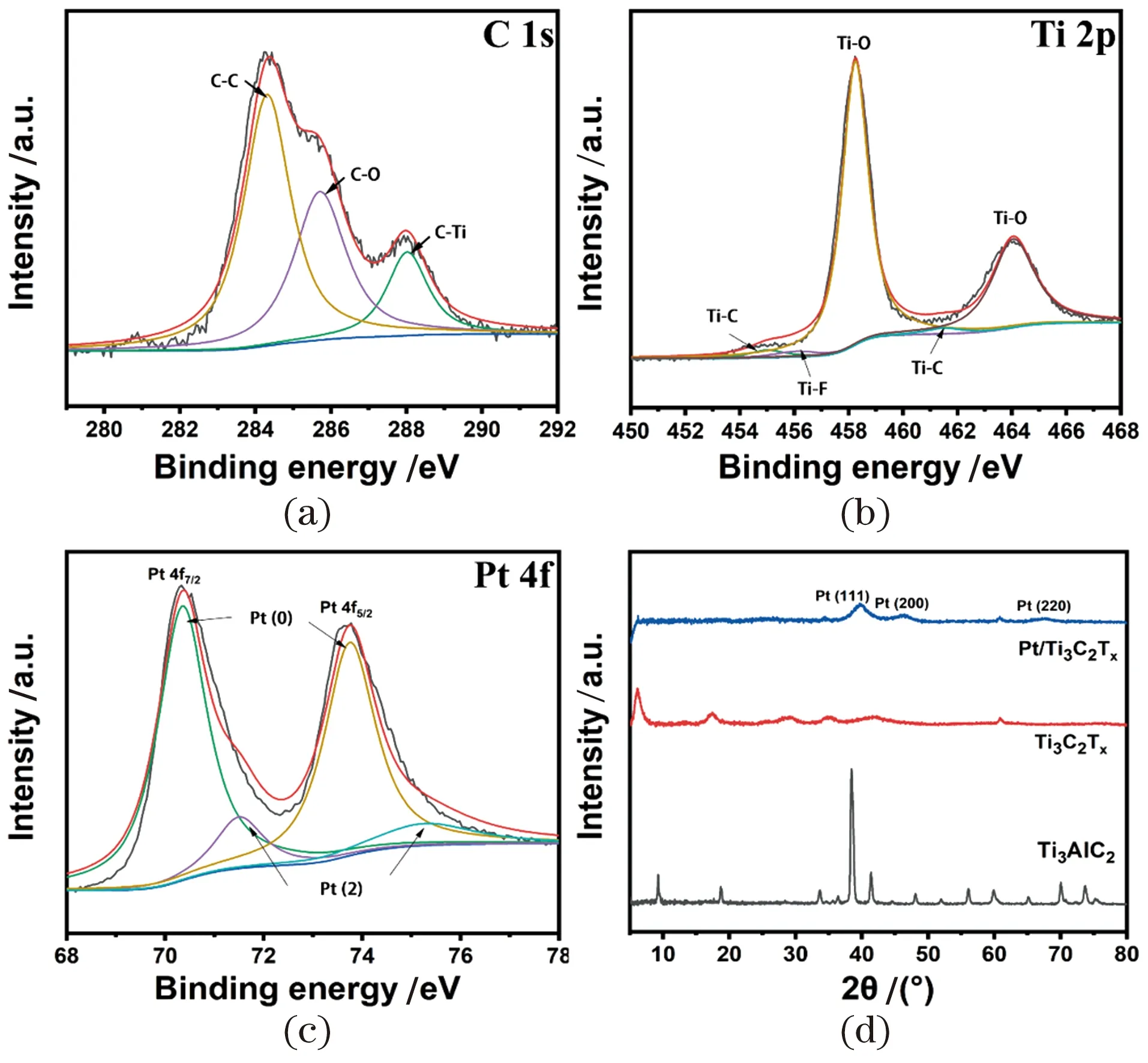
Figure 3 The XPS spectra of C 1s (a); Ti 2p (b); Pt 4f (c) ofPt@MXene composites (PMCs)图3 Pt@Mxene复合材料的X射线光电子能谱分析结果C 1s (a); Ti 2p (b); Pt 4f (c)
The structures of the samples before and after the reaction were also analyzed by XRD shown in the Figure 3(d). The (104) peak of Ti3AlC2disappears at 39° after etching and the peaks at 17.5°, 28.9°, 35.1°, 41.8° and 60.8° correspond to (006), (008), (0010), (0012) and (110) respectively, which are characteristic planes of Ti3C2layered caused by LiF and HCl, indicating the stability of MXene after exfoliation and modification[50]. For the recombined PMCs, the diffraction peaks at 2θ≈40°, 46.4° and 67.3° correspond to the (111), (200) and (220) Pt planes, all of which prove the successful loading of Pt nanoparticles onto MXene plane, which is also consistent with the previous HRTEM analysis results.
3.2 Oxidase-like catalytic activity of PMCs
For assessing the oxidase-like activity of PMCs systemically, the colorimetric experiment was carried out, in which TMB was employed as the representive substrate. The results are shown in Figure 4(a). The peak at 652 nm increased immediately when PMCs was mixed with TMB, suggesting the excellent oxidase-like activity of PMCs.
Furthermore, in order to explore the influencing factors of the oxidase-like reaction of PMCs and to simulate the conditions of its application in real biological conditions, the enzyme-like activity under different parameters (time, pH, temperature) was also investigated. As shown in Figure 4(b)-(d), PMCs nanozymes maintained certain level of enzyme-likeactivity over a wide range of pH and temperature, suggesting the promise for practical applications.
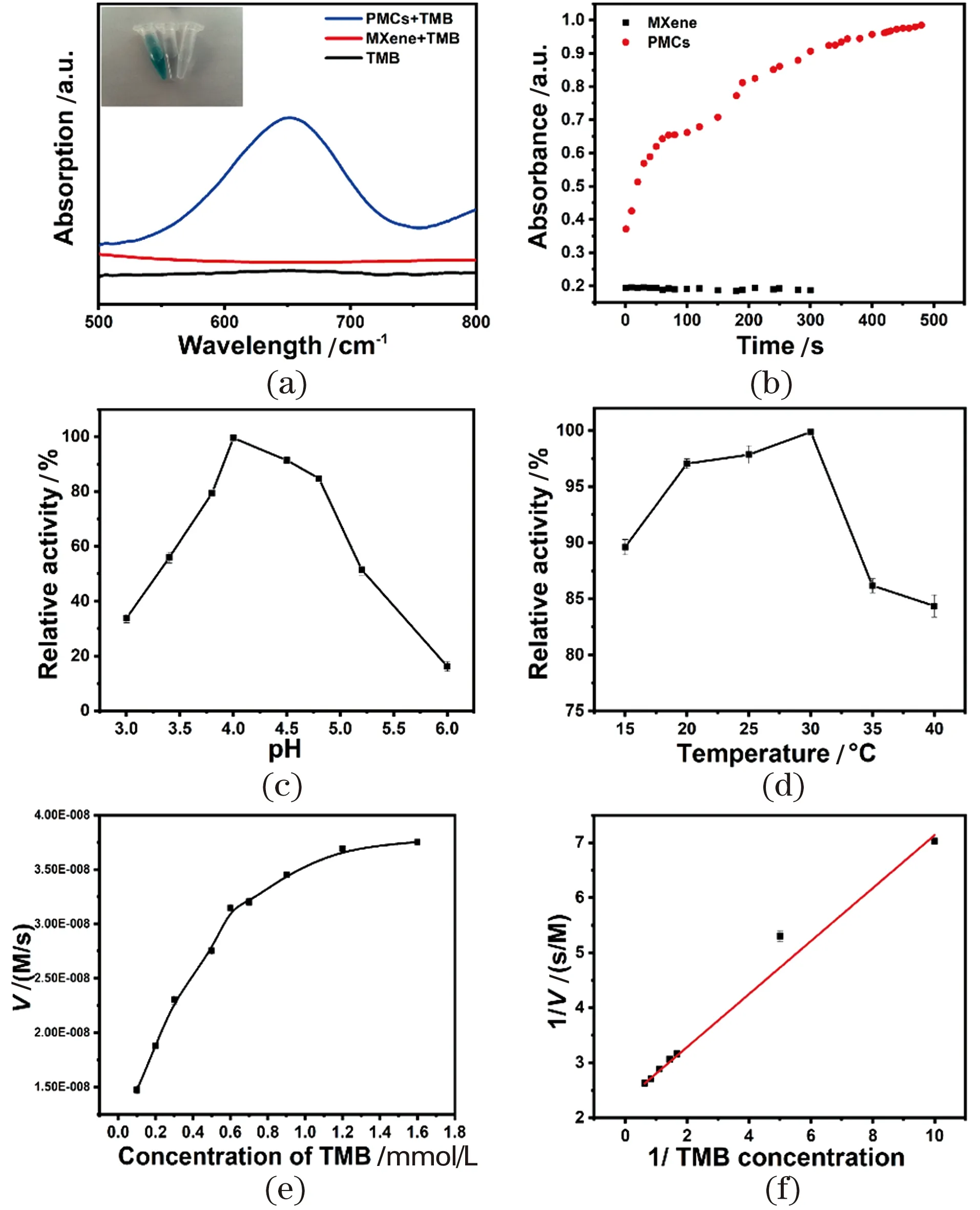
Figure 4 UV-vis spectra and color changes of different reaction system: PMCs + TMB (blue line), MXene + TMB (red line), TMB (black line) (inset: photographs of different groups) (a); influence of time, temperature and pH on the oxidase-like activity changes (b)-(d); steady-state kinetic curves of PMCs (e); Lineweaver-Burk plots of the reciprocals of initial rate vs TMB concentration (f)图4 Pt@Mxene复合材料+TMB(蓝线),MXene+TMB(红线),TMB(黑线)反应体系的光谱曲线(a);时间、反应温度和pH对氧化酶活性的影响(b)~(d);Pt@Mxene复合材料的稳态动力学 曲线(e);Lineweaver-Burk速率与浓度双倒数作图(f)
3.3 Steady-state kinetic assay and catalytic mechanism of PMCs
To calculate the relevant kinetic parameters, the Michaelis-Menten kinetics of PMCs nanozymes was evaluated by varying concentrations of TMB Figure 4(e)~(f). TheKmvalue represents the affinity of the substrate to the enzyme, and the smaller the value, the higher the affinity.Vmaxcorresponds to the maximum reaction rate at which the substrate reaches saturation of the active site of the enzyme[52]. In addition, the Michaelis-Menten constant (Km) of PMCs was determined to be 0.16 mmol/L, indicating that PMCs have a good affinity for TMB, which is far superior to that of several oxidase-like materials that have been reported (Table 1). Furthermore, to explore the role of O2in the reaction, two control experiments were performed in O2- and N2-saturated solutions, respectively (data not shown). Based on the absorbance at 652 nm of PMCs in air-saturated buffer, the reaction rate of TMB oxidation catalyzed by PMCs increased significantly under O2-saturated conditions, but decreased sharply under N2-saturated conditions. Therefore, the oxidation rate of TMB is highly dependent on the O2concentration, indicating that PMCs can effectively promote the dissociation of O2and enhance the catalytic oxidation of TMB[53].

Table 1 Comparison of the apparent Michaelis-Menten constants (Km)and maximum reaction rates (Vmax) of different nanozymes表1 不同纳米酶的米氏常数(Km)和最大反应速率(Vmax)的比较
3.4 Antibacterial activity of PMCs
Quantitative bacterial colony counting on agar plate method was used to evaluate the antibacterial activity of different materials againstE.coliandS.aureus. The antibacterial activities of the samples are shown in the Figure 5(a) and (b). The PMCs showed the highest antibacterial activity compared to the control materials. In order to illustrate the bactericidal activity of PMCs, the bacteria treated by PMCs were stained by mixing the appropriate proportion of SYTO 9 and PI and excited by the laser, the dead cells would present red fluorescence. Therefore, the results showed that the PMCs can effectively killE.coliandS.aureusas proved by red fluorescence staining Figure 5(d).
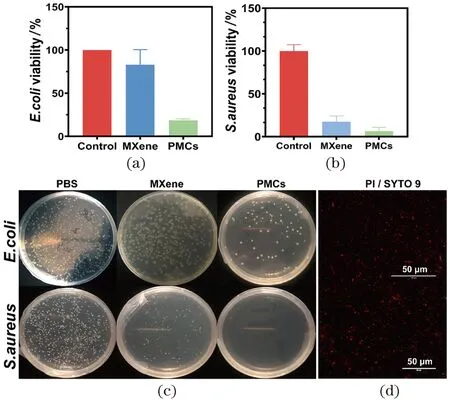
Figure 5 Viability of E. coli (a) and S. aureus (b) after treated by different materials PBS, MXene and PMCs; and the correspondingphotographs of bacterial colonies treated by relative materials (c): E. coli (upper) and S. aureus (below); fluorescence images (d)of E. coli (upper) and S. aureus (below) after treatment图5 经过不同材料处理的大肠杆菌(a)和金黄色葡萄球菌(b)的存活率和相应的细菌单克隆图片(c)以及荧光显微镜图片(d)
3.5 Effect of PMCs on skin wound healing
To further evaluate the practical application of PMCs in wound healing, wounds were created on their backs. At first, 107CFU/mL ofS.aureuswere chosen to induce bacterial infection in wounds.Figure 6(a) shows the results of wound healing after 7 d treated with PBS, MXene and PMCs respectively. Compared with the control groups, the mice treated with PMCs showed a strong wound healing ability on the third day and the wound healed completely after seven days.H&E staining showed that the healed wound treated with PMCs exhibited well with the differentiated epidermis, while the PBS or other control groups had different levels of fragmented epidermal layers,indicating infiltration of inflammatory cells[56-57].
In addition, the wound area was quantified on different days (1, 3, 5 and 7) with the size of the fresh wound on 1st day as the control (100%). PMCs exhibited the greatest wound healing ability among all the tested groups Figure 6(b). Moreover, for investigating the antibacterial effects,S.aureuscells collected from wound tissues, and the abundance ofS.aureuswas measured by the CFU method. As shown in Figure 6(c) and (d), 300-400 bacterial colonies were quantified in the group of PMCs. This result suggests that enhanced wound healing can be mediated by its antimicrobial activity of the PMCs system.
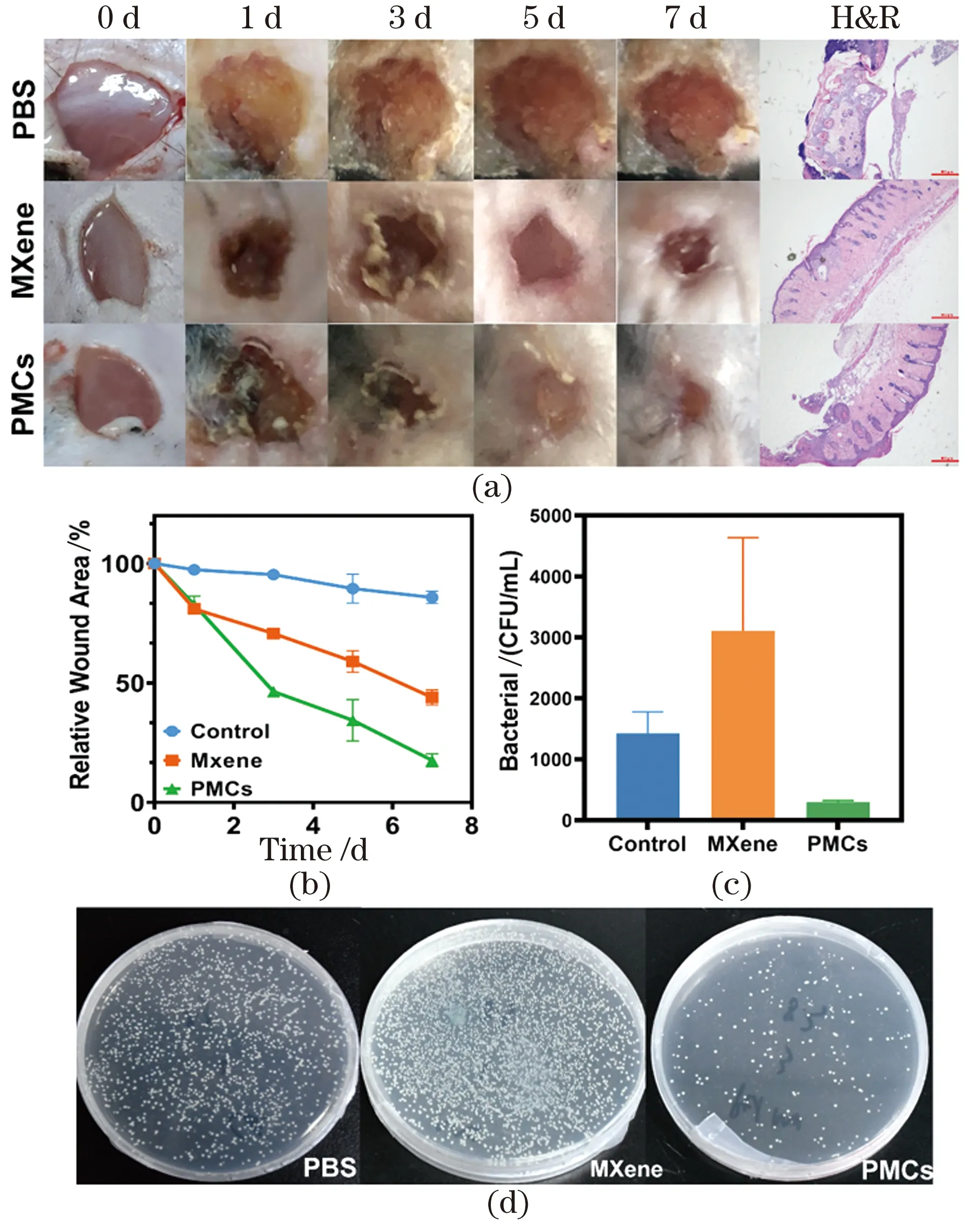
Figure 6 Pictures of S. aureus infected wounds treated by PBS, MXene and PMCs on day 0, 1, 3, 5, 7 and relative H&E staining (a); changes of wound area during treatment (b); number of the surviving bacteria in the infected wound tissues and relative photographs of bacterial colonies (c)(d)图6 经过不同材料处理的金黄色葡萄球菌感染的小鼠伤口图像以及对应的H&E染色结果(a);治疗过程中伤口大小的变化(b);感染伤口组织中存活细菌的数量和相对应的细菌单克隆照片(c)(d)
3.6 Mechanism investigation
Previously, the antibacterial effect of nanozymes focused on the regulation of ROS levels. Nowadays, more attention is shifted to the mechanism of charge transfer between microorganisms and nanomaterials[58]. In our case, we also noticed that the as-prepared nanozymes are essentially biocatalysts, as their enzymatic activity could accelerate the transfer of electrons, as verified by the following experiments.
3.6.1 Cell membrane integrity of bacteria
Although the high antibacterial activity has been proved according to the above results, the sterilization mechanism of PMCs is still not fully known. Considering that antibacterial mechanism of most nanozymes or 2D composites tends to due to the damage the cell membrane[59], SEM was firstly employed to observe the large intestine treated with PMCs. The integrity of the cell membranes ofE.coliandS.aureusare shown in the Figure 7(a) and (b). Unexpectedly, the treatedE.coliandS.aureusstill maintained relatively intact cell morphology, unlike reported studies[60]. It is worth noting that adherent lamellar bulk materials can be seen on their surfaces clearly, which may be due to the strong binding force of PMCs to bacteria. Previous studies showed that both Gram-positive and Gram-negative bacteria have peptidoglycan cell walls composed of amino acid residues, which may coordinate with metal ions in PMCs[61-62]. In addition, as a graphene-like two-dimensional material, MXene has an excellent ability to adhere to bacterial cell walls due to its abundant oxygen-containing functional groups on its surface, inherent sheet-like structure and large specific surface area[63-64].
Furthermore, in order to verify whether the PMCs nanozyme was killing bacteria by ROS during the antibacterial process, DCFH-DA was used to explore the role of ROS in the actual antibacterial process. The results ofE.coliFigure 7(c) andS.aureus(data not shown) treated with PMCs showed no significant ROS production, which may be due to the relative short lifetime and limited diffusion distance of ROS.
It was reported that aerobic microorganisms can transfer electrons which extracted from various electron sources such as NADH and FADH2to O2to produce H2O and pass through a series of respiratory proteins (including complexes I, II, III and IV, Coenzyme Q (CoQ) and Cyt C are embedded in microbial cell membranes[65]. Given that respiratory proteins on the respiratory electron transport chain are a series of electron carriers that can accept and donate electrons, direct contact of microorganisms with nanomaterials may also lead to electron transfer through these respiratory proteins, eventually leading to cell death[66-67]. In fact, several studies have also provided evidence for this antibacterial mechanism based on this charge transfer theory. Some membrane-bound components, such as Cyt C, can transport electrons from the respiratory chain to the outside of the membrane. PMCs as a highly active oxidase are capable of catalyzing the oxidation of substrates, whose essence is to extract electrons from the substrates. Herein, we hypothesized that PMCs might act as oxidase-like nanozymeto interact with Cyt C or other respiratory chain components to facilitate the electron transfer process. To verify this, we investigated the interaction between Cyt C, a transmembrane protein, and PMCs. As shown in Figure 7(d), the reduced form of Cyt C showed peaks at approximately 520 and 550 nm, and the protein showed a broad peak at about 530 nm (red line)after interacting with PMCs, proving that Cyt C was changed to its oxidized form. In addition, the direct contact of PMCs with bacterial cell membranes was observed, which is necessary for the reaction to proceed.
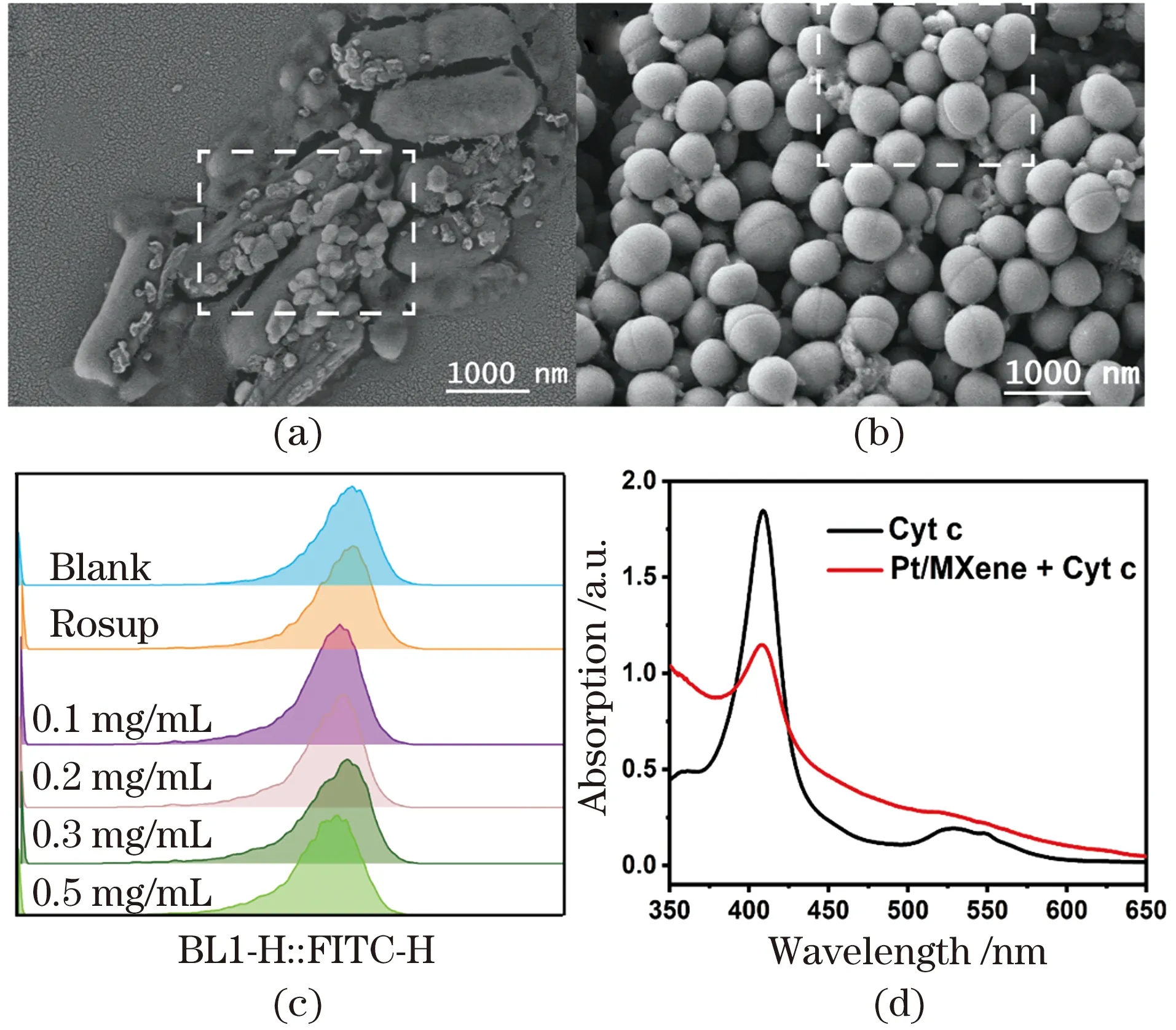
Figure 7 SEM images of E. coli (a) and S. aureus (b) treated by PMCs; ROS intensity in E. coli (c); UV-vis spectra of Cyt C during interaction with PMCs (d)图7 经过PMCs处理的大肠杆菌(a)和金黄色葡萄球菌(b)的扫描电子显微镜照片;大肠杆菌的ROS强度(c);PMCs与细胞色素c相互作用后的紫外-可见光谱结果(d)
3.6.2 Cell membrane potential and electron transport activity
Furthermore, the membrane potential ofE.coliafter PMCs treatment was measured by flow cytometry analysis with DiOC2(3) fluorescent probe to determine whether the interfacial electron transfer ofE.coli/PMCs would lead to the loss of membrane potential[68]. The DiOC2(3) fluorescence changing from red to green was observed. FCM gate P1 (black triangle) is plotted to indicate the positive control (+CCCP), and the percentage of P1 inE.coliafter PMCs treatment was obtained to reflect the degree of membrane depolarization. As shown in Figure 8(a), the percentage of P1 significantly increased from 3.66% to 93.5 with increasing PMCs concentration, indicating that PMCs could depolarize the membrane potential ofE.coli. The same effect was also demonstrated in PMCs treatedS.aureus(data not shown). These results therefore suggested that interfacial electron transfer led to the loss of bacterial membrane potential. Moreover, Figure 8(b) shows that the electron transport delivery ability indeed decreased significantly after treated by PMCs (P<0.001), confirming that the antibacterial ability is mainly attributed to the interruption of the respiratory chain in this case.
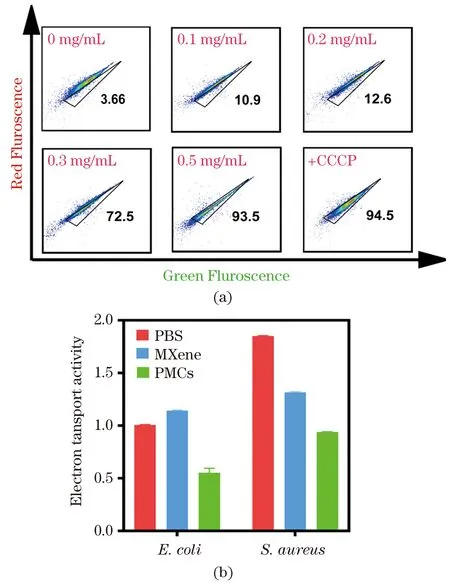
Figure 8 Membrane potential of E. coli treated by PMCs at different concentrations for 30 min via FCM analysis (a); the change of electron transport system activity (b)图8 不同浓度PMCs处理后的大肠杆菌膜电势的流式结果分析(a);电子传递系统活性的改变(b)
3.7 Biocompatibility of PMCs
Potential biological applications require satisfyinvivoandinvitrobiosafety, so the toxicity assessment of PMCs was conducted. Theinvitrocytotoxicity of PMCs was evaluated by the CCK-8 method, and HeLa cells, which are often used as a model cell line forinvitrotoxicity assessment, were selected to be incubated with PMCs for 24 h. The results showed the negligible cytotoxicity of PMCs to Hela cells in Figure 9(a). Briefly, the survival rate was about 100% and did not decrease significantly even under high concentration (500 μg/mL) treatment. In addition, the hemolysis rate of PMCs was investigated using rat erythrocytes, as shown in Figure 9(b), the hemolysis rate of PMCs was negligible even under the treatment of high concentrations of PMCs, indicating that PMCs have good biocompatibility. Forinvivobiocompatibility experiments, PMCs were injected intravenously into mice, and no significant body weight changes were observed, indicating that PMCs did not cause any toxic effects Figure 9(c). Meanwhile, no obvious organ abnormalities or inflammation were observed from H&E staining of different tissues, confirming the excellent biocompatibility of the designed nanozymes Figure 9(d).
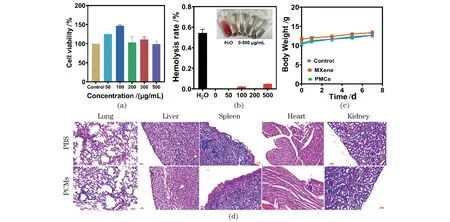
Figure 9 Toxicological effect of PMCs. cell viability determined by CCK-8 assay using Hela cells (a); hemolysis ratio of fresh rat red blood cells (RBCs) incubated with PMCs (b) (inset: RBCs incubated with different materials); the change of body weight during treatment (c); photomicrographs of the major organs (lung, liver, spleen, heart, and kidney) stained with hematoxylin and eosin (H&E) after treatment with PBS or PMCs (d) (Scale bar=50 μm)图9 PMCs的毒性影响。使用Hela细胞通过CCK-8测定法测定的细胞活力(a);用PMCs孵育的新鲜大鼠红细胞(RBCs)的溶血率(b)(插图:RBCs与不同材料孵育);治疗期间小鼠体重变化(c);用PBS或PMCs处理后用H&E染色的主要器官(肺、肝、脾、心脏和肾)的显微照片(d)(比例尺=50 μm)
4 Conclusions
In summary, we successfully synthesized Pt@MXene composites (PMCs) with Pt nanoparticles uniformly dispersed on lamellar-structured MXeneviaone-step hydrothermal method, and investigated their antibacterial effect and mechanism.Benefiting from the excellent electron transport ability of MXene and the inherent enzyme-like properties of Pt nanoparticles, the obtained PMCs have significantly enhanced oxidase-like activity. To the best of our knowledge, this is the first time that synthesized PMCs were used as oxidase-like nanozymes for antibacterial and wound healing enhancement. The improved oxidase-like activity endows it with excellent antibacterial activity, which can effectively inhibitE.coliandS.aureus, and mechanism study reveals that PMCs affect by capturing the electrons of the substrates on the surface of the bacterial membranes for cutting off their electron transport of electron respiration. Importantly, our experimental results have demonstrated that PMCs can effectively prevent bacterial infection and accelerate the wound healing process. Moreover, PMCs have satisfactory biocompatibility, as confirmed by the results ofinvitroandinvivotoxicity experiments such as CCK-8 assay, hemolysis test and H&E staining experiments. This study therefore not only provides an effective nanozyme with high oxidase-like activity and antibacterial efficiency, but also paves a new way for the design and development of low-toxicity and effective antibacterial materials.
