Structures,Photoluminescent and Magnetic Properties of Three 2D Lanthanide Complexes
2022-04-18LIUShuangHUMingFeiLILeiLeiWANGWenZhen
LIU Shuang HU Ming⁃Fei LI Lei⁃Lei WANG Wen⁃Zhen
(1School of Chemistry and Chemical Engineering,Xi′an Shiyou University,Xi′an 710065,China)
(2Key Laboratory of Advanced Energy Materials Chemistry(Ministry of Education),Nankai University,Tianjin 300071,China)
Abstract:Three new 2D lanthanide complexes with the formula[Ln2(dmpa)4]Cl2,where Ln=Eu(1),Tb(2),Dy(3),have been prepared based on the ligand 2,2⁃bis(hydroxymethyl)propionic acid(Hdmpa)under solvothermal condi⁃tions and characterized by IR spectrum,elemental analysis,powder X⁃ray diffraction,thermogravimetric analysis,and single⁃crystal X⁃ray diffraction.These complexes are isostructural in which individual Ln(Ⅲ) ions are linked by carboxyl groups into 2D layers which are further assembled into 3D supramolecular structures by intermolecular interactions.Magnetic investigations on Dy complex indicate the presence of weak ferromagnetic interactions trans⁃mitted by carboxyl groups.And photoluminescence studies reveal that the Eu(Ⅲ) and Tb(Ⅲ) complexes exhibit charac⁃teristic red⁃light and green⁃light emissions,respectively.CCDC:1940310,1;1940311,2;1940312,3.
Keywords:lanthanide complex;crystal structure;magnetic property;photoluminescent property
0 Introduction
The study of trivalent lanthanide(Ln3+)complexes has attracted great attention owing to their potential applications in many fields,especially for photolumi⁃nescence[1⁃7]and molecular magnets[8⁃13].Different from transition metal ions,4felectrons in Ln(Ⅲ)ions are greatly shielded by the outer 5sand 5pelectrons mak⁃ing that the electronic structures of Ln(Ⅲ) ions are main⁃ly decided by the inherent 4felectron interactions and spin⁃orbit couplings than the ligand fields(LFs).Based on such fact,the luminescence of Ln(Ⅲ)ions,originat⁃ing fromf⁃ftransition,has the advantages of narrow line⁃like emissions,long lifetimes,and high quantum yields,which make them good candidates in building luminescent systems toward applications in molecule and ion recognition[1],display materials[14],fluorescence labeling[15⁃16],etc.While in the field of molecular mag⁃nets,the large shielding effects on 4felectrons lead to large unquenched orbital moments and strong spin⁃orbit couplings which means strong magnetic anisotro⁃py for most lanthanide ions[17⁃19].This makes Ln(Ⅲ) com⁃plexes also outstanding systems for building functional magnetic materials,especially for single⁃molecule mag⁃nets[8⁃13].In addition,lanthanide complexes as bifunc⁃tional materials with both single⁃molecule magnet prop⁃erty and luminescent property are now attracting more and more attention[20⁃22].
The key to building functional Ln(Ⅲ)⁃based coordi⁃nation systems is the design and choosing of organic ligands for they decide the crystal structures of the systems including the coordination environments of individual Ln(Ⅲ)ions and the linking modes among them.As Ln(Ⅲ)ions are more inclined to bond with O⁃containing ligands than N⁃containing ligands,carboxylate⁃based and hydroxyl⁃based ligands are wildly employed resulting in lots of Ln(Ⅲ)⁃complexes with fascinating structures and properties.Carboxyl⁃ates have rich coordination modes as bridging ligands and can therefore lead to various structures by modulat⁃ing the synthesis conditions.Compared with carboxyl⁃ates,hydroxyl⁃based ligands as single⁃atom bridging ligands can transmit more effective exchange couplings and lead to systems with interesting magnetic proper⁃ties,including magnetic ordering and/or slow relax⁃ation of magnetization(SRM)behaviors[23⁃24].Driven by such facts,2,2⁃bis(hydroxymethyl)propionic(Hdmpa)containing two hydroxyl groups and one carboxyl group was chosen as the ligand in our research to acquire new complexes with interesting structures and lumines⁃cent/magnetic properties.The rich coordination modes and effectiveness in transmitting exchange couplings of Hdmpa leading to fascinating cluster⁃based magnetic materials have been demonstrated in several reports[24⁃26].And there was also one report claiming the Hdmap ligand could well sensitize lanthanide ions to give strong luminescence[27],making it more attractive in functional coordination chemistry.However,to the best of our knowledge,almost all reported lanthanide com⁃plexes based on Hdmpa ligand were limited to zero⁃dimension structure,i.e.,mononuclear or polynuclear clusters.Therefore,the present work aims to investi⁃gate the potential of Hdmpa in constructing non⁃zero dimensional lanthanide complexes to expand the coor⁃dination chemistry based on Hdmpa ligand beyond the state of the art.And three novel Ln(Ⅲ)complexes[Ln2(dmpa)4]Cl2,where Ln=Eu(1),Tb(2),and Dy(3),were obtained by the solvothermal procedure.Single crystal X⁃ray tests show that all three complexes are isostructural with a 2D layer structure.Luminescent spectra of 1 and 2 exhibited characteristic red⁃light and green⁃light emissions,respectively.And magnetic measurements on 3 show the presence of weak ferro⁃magneticinteractionstransmittedbycarboxylatebridges.
1 Experimental
1.1 Materials and methods
All chemicals and solvents used are of analytical grade and were used as received without further treat⁃ment.Infrared spectra on powdered samples were recorded with a Bruker Tensor 27 Spectrometer with samples as KBr disks in the 4 000 ⁃400 cm−1region.Elemental analyses(C,H,and N)were carried out by using a Perkin⁃Elmer analyzer.Powder X⁃ray diffrac⁃tion(PXRD)data were collected on a Bruker D8 advance diffractometer with CuKαradiation(λ=0.154 18 nm)in a range of 3°≤2θ≤50°,operated under a voltage of 40 kV and a current of 30 mA.Simulation of PXRD pattern was derived from the Mercury program avail⁃able free of charge via the Internet at http://www.iucr.org.Thermogravimetric(TG)analyses were conducted on a Labsys NETZSCH TG 209 Setaram apparatus with a heating rate of 10 ℃·min−1from room temperature to 800 ℃.Direct⁃current(dc)magnetic susceptibility data from 300 to 2 K was obtained using crushed crystals of the sample on a Quantum Design MPMS XL⁃7 magne⁃tometer.The data was corrected using Pascal′s con⁃stants to calculate the diamagnetic susceptibility and an experimental correction for the sample holder was applied.Alternating⁃current(ac)magnetic susceptibili⁃ty data was collected on the same instrument employ⁃ing a 5.0 Oe oscillating field.Photoluminescence emis⁃sion spectra tests were carried out on powdered sam⁃ples in a Thermo FL spectrometer.
1.2 Synthesis of[Ln2(dmpa)4]Cl2
A mixture of Hdmpa(0.080 4 g,0.6 mmol),LnCl3·6H2O(Ln=Eu(1),0.2 mmol,0.073 3 g;Tb(2),0.2 mmol,0.074 7 g;Dy(3),0.2 mmol,0.075 4 g),and 6 mL CH3CN in the present of one drop(about 0.05 mL)trimethylamine(TEA)were sealed in a 25 mL Teflon⁃lined stainless steel container and heated at 120℃for 3 d under autogenous pressure.Then the container was slowly cooled to room temperature at 2 ℃·h−1.Color⁃less rod⁃like crystals were obtained by filtration and washed with CH3CN three times.The yields(based on Ln)were 22%,35%,and 54% for 1,2,and 3,respec⁃tively.Elemental Anal.Calcd.(%)for C20H36Cl2Eu2O16(1):C,26.47;H,4.00.Found(%):C,26.36;H,3.95.Elemental Anal.Calcd.(%)for C20H36Cl2Tb2O16(2):C,26.07;H,3.94.Found(%):C,25.98;H,3.85.Elemen⁃tal Anal.Calcd.(%)for C20H36Cl2Dy2O16(3):C,25.87;H,3.91.Found(%):C,25.75;H,3.85.FT⁃IR(KBr pellet,cm−1):3 443(br),1 601(vs),1 357(w),1 069(w),1 000(w),857(w),773(w),623(m)for 1;3 443(br),1 600(vs),1 385(w),1 356(m),1 138(m),1 068(w),1 000(w),859(w),772(w),619(m)for 2;3 446(br),1 606(vs),1 385(w),1 350(m),1 134(m),1 074(w),995(w),945(w),851(w),770(w),619(m)for 3.
1.3 Crystal structure determination
X⁃ray single⁃crystal diffraction data for complexes 1⁃3 were collected using theω⁃φscan technique on a Bruker Single⁃Crystal Diffractometer equipped with a graphite⁃monochromated MoKαradiation source(λ=0.071 073 nm).The structures were solved by direct methods with the SHELXS⁃2008program of the SHELXTL package and refined by full⁃matrix least⁃squares methods onF2with Olex2 program[28⁃29].All the non⁃hydrogen atoms were refined with anisotropic ther⁃mal parameters.The hydrogen atoms of the ligands were located geometrically and refined by using a rid⁃ing model.The crystallographic data and the selected bond distances and bond angles for complexes 1⁃3 are listed in Table 1 and Table S1⁃S3(Supporting informa⁃tion),respectively.
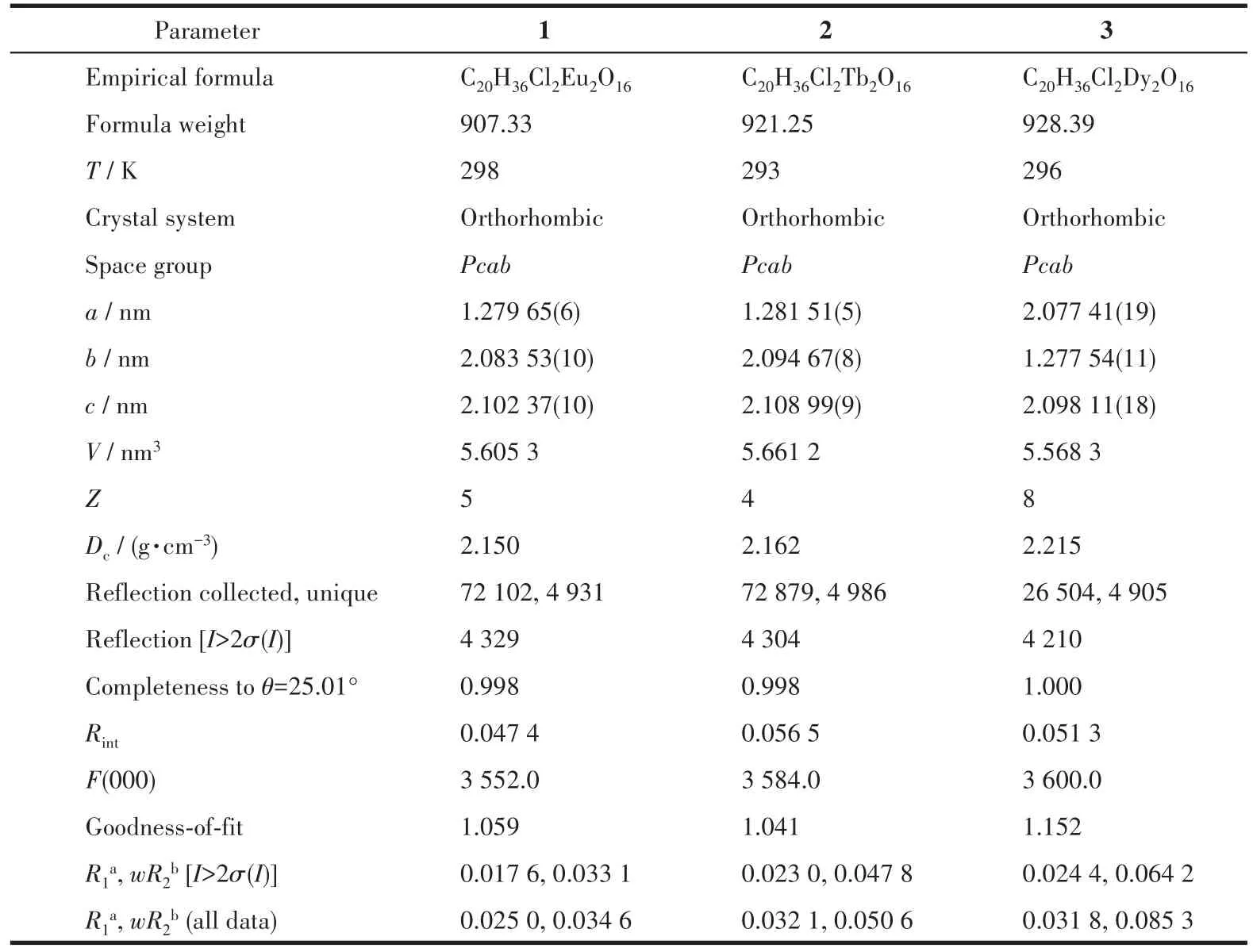
Table 1 Crystallographic data and structure refinement for complexes 1⁃3
CCDC:1940310,1;1940311,2;1940312,3.
2 Results and discussion
2.1 Description of crystal structures for 1⁃3
Complexes 1⁃3 are isostructural and crystallize in the orthorhombic system with the space group ofPcabrevealed by the single⁃crystal X⁃ray diffraction analy⁃ses,and here only 3 is selected as the representation to describe the structure detailedly.The asymmetrical unit of 3 consists of two crystallographic independent Dy(Ⅲ) ions,four dmpa−ligands,and two lattice Cl−ions.As shown in Fig.1a,Dy1 ion is nine coordinate by six O atoms of the carboxylates belonging to four dmpa−ligands and three O atoms of the hydroxyls from two dmpa−ligands,while Dy2 ion is eight coordinate finished by four O atoms of the carboxylates from four dmpa−ligands and four Ohydroxylatoms from three dmpa−ligands.The Dy—O bond lengths are in a range of 0.234 9⁃0.252 3 nm and 0.228 3⁃0.247 9 nm for Dy1 and Dy2,respectively.Notably,all hydroxyls serve as monodentate ligands.The continuous shape measure(CshM)method[30⁃31]realized by the SHAPE program is employed to estimate the coordination geometry sym⁃metry for Dy1 and Dy2 ions.And the results(Table S4)show the coordination polyhedron of Dy1 is mostly close to a muffin geometry ofCssymmetry with the devi⁃ation parameterS=1.406,while Dy2 is located in a bi⁃augmented trigonal prism geometry ofC2vsymmetry withS=3.738.Each Dy1 ion is connected with a near⁃est Dy2 ion by twoμ2⁃O atoms from two carboxylates,forming a dinuclear unit[Dy2],in which the distance of Dy1and Dy2 is 0.386 3 nm.Adjacent[Dy2]units are connected by twosyn,syn⁃μ⁃η1∶η1⁃COO−groups into parallelogram⁃shaped[Dy4]units(Fig.1b).The distance between neighboring[Dy2]units is 0.682 0 nm.Each[Dy4]unit links the other four neighboring[Dy4]units through four singleμ⁃1,3⁃carboxylate bridges affording the 2D layer structure of 3(Fig.1c).These 2D layers are further packed by Van Der Waals forces and inter⁃layer H⁃bonds between counter⁃anions Cl−ions and a hydroxyl group from dmpa−ligand to afford the final 3D supramolecular structure(Fig.S2).It is noted that all dmpa−ligands coordinate to two Dy(Ⅲ) ions and exhibit four different coordination modes which are plotted in Fig.2.
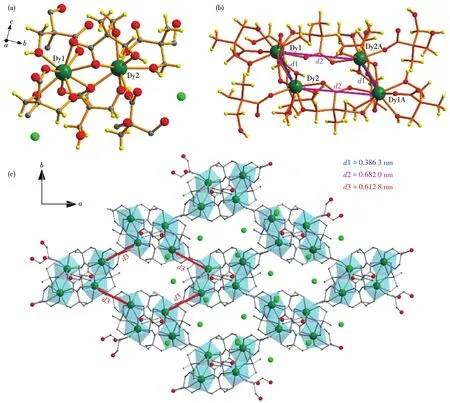
Fig.1 Structures of[Dy2]unit(a),[Dy4]unit(b)and 2D layer(c)in complex 3
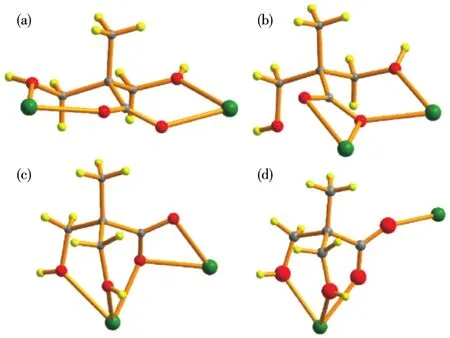
Fig.2 Four different coordination modes of dmpa−ligand in 3
2.2 PXRD and TG analyses
The PXRD data of 1⁃3 were collected at room temperature to confirm the phase purity.As shown in Fig.S3,experimental patterns of 1⁃3 were all in good agreement with the simulation pattern calculated from crystallographic data of 3,indicating that the bulk materials are all in pure phase.To identify the thermal stability of 1⁃3,TG analyses for 1⁃3 were performed with a heating rate of 10 ℃ ·min−1in a temperature range of 25⁃800 ℃ (Fig.S4).TG analyses indicate that the TG curves of 1⁃3 had similar trends due to the isostructural nature.Complexes 1⁃3 were stable up to about 250℃and then showed a dramatic weight loss,corresponding to the decomposition of the organic ligands.
2.3 Luminescent properties of complexes 1 and 2
The solid⁃state luminescent emission spectra of 1 and 2 at room temperature are shown in Fig.3.Upon excited at 309 nm,1 exhibited four characteristic emis⁃sion bands of Eu3+centered at 592,614,691,and 700 nm,respectively.The first two peaks can be ascribed to5D0→7F1and5D0→7F2transitions,respectively,while the last two peaks are both corresponding to5D0→7F4transition which decomposes into two parts due to the minor differences of the local coordination environments[32].The red color luminescence of 1 is dominated by the emission of5D0→7F2at 614 nm with the highest intensity.When excited at 300 nm,com⁃plex 2 showed green color luminescence with four main emission bands centered at 489,544,584,and 620 nm which can be assigned to the transitions of5D4→7FJ(J=6,5,4,and 3)of Tb3+ion,respectively[33⁃35].And the green color is dominated by the emission of5D4→7F5at 544 nm with the highest intensity.As 1 and 2 exhib⁃it 2D layer structure and most pores are eliminated by the staggered stacking of 2D layers,no tests on mole⁃cule and ion recognition for them were performed as they can only interact with other ions and molecules on the surfaces.
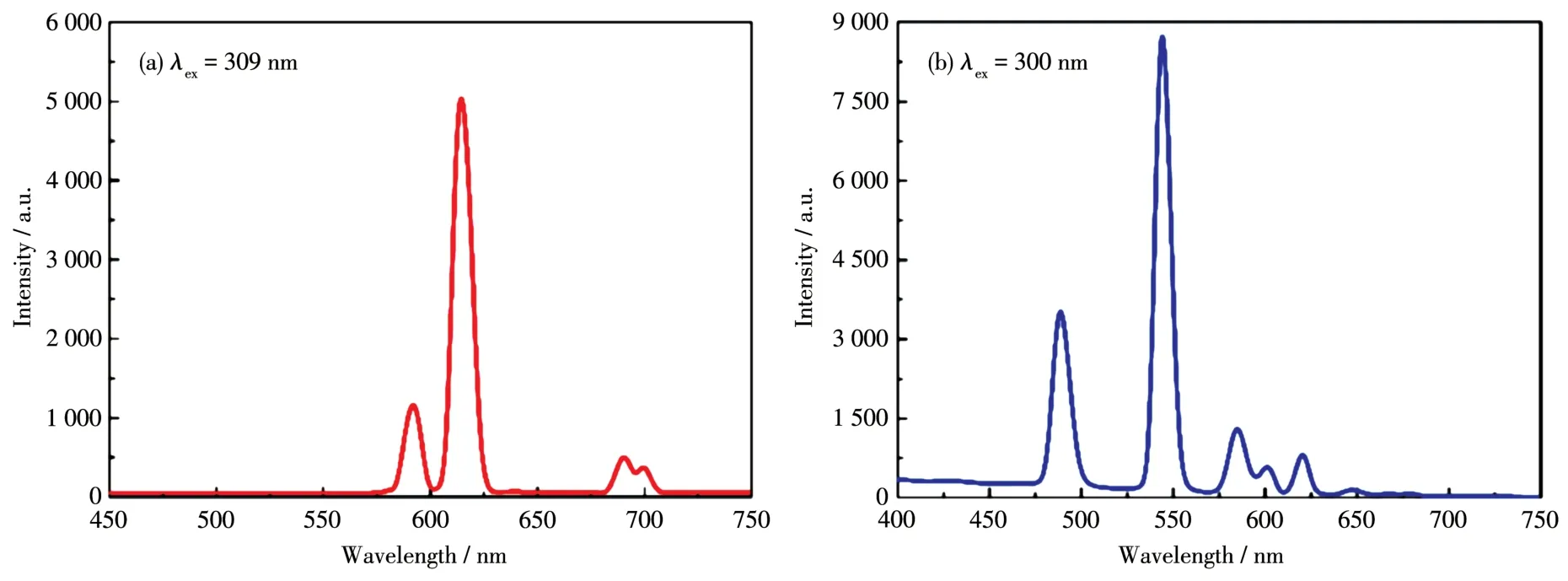
Fig.3 Solid⁃state photoluminescent spectra of complexes 1(a)and 2(b)
2.4 Magnetic property of complex 3
SRM behavior is an important research topic in lanthanide complexes for the understanding of the influences of LFs on electronic structures and their promising applications in high⁃density information stor⁃age.A double⁃degenerate ground state is a foundation for lanthanide complexes to show SRM behaviors which are guaranteed by Kramers′theorem for all Kramers ions in the absence of a magnetic field[36].However,for a non⁃Kramers ion,LF with strictly axial⁃symmetry geometry is a must for it to have a double⁃degenerate ground state[17⁃19].For complexes 2 and 3,as the LFs remarkably deviate from strictly axial symme⁃try demonstrated by the SHAPE results,only complex 3 based on the Kramers ion Dy(Ⅲ)is expected to show SRM behavior theoretically.And magnetic measure⁃ments were performed on it.
Variable⁃temperature magnetic susceptibility measurement was performed on the powder sample of 3 in a range of 300 to 2 K under 1 000 Oe applied field.As shown in Fig.4,the room temperature value ofχMTwas 27.68 cm3·mol−1·K and was slightly lower than the value of 28.34 cm3·mol−1·K for two free Dy(Ⅲ) ions with6H15/2ground state.Upon cooling,χMTdecreased gradu⁃ally first,reaching a minimum value of 22.72 cm3·mol−1·K at 6.0 K.And below 6.0 K,χMTincreased sud⁃denly and reached a value of 25.05 cm3·mol−1·K at 2 K.The plot ofχM−1vsTshowed well linear dependence and Curie⁃Weiss fitting gave the Curie constant(C)of 27.95 cm3·mol−1·K and a negative value of−2.61 K forθ.The decrease ofχMTabove 6 K and the negative val⁃ue ofθcan be attributed to the thermal depopulation of the LF split excited sublevels and/or the intralayer anti⁃ferromagnetic coupling.What is interesting was the observed increase ofχMTbelow 6 K,implying the pres⁃ence of interlayer ferromagnetic couplings or spin cant⁃ing behavior[37⁃38].
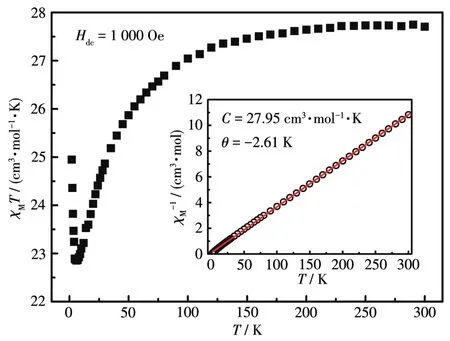
Fig.4 Temperature dependence of dc magnetic susceptibility for 3 under 1 000 Oe field
The field dependence of magnetization was mea⁃sured at 2 K up to 70 kOe for 3(Fig.5).The magnetiza⁃tion of 12.2Nβwas far less than the saturation value of 20Nβfor two free Dy(Ⅲ) ions.As the superexchange coupling among Dy(Ⅲ)ions is usually extremely weak,the large difference between experimental and satura⁃tion values suggests the presence of large single⁃ion magnetic anisotropy and/or thermal population on low excited sublevels.The dM/dHplot exhibited a maxi⁃mum atca.200 Oe.Considering the so small strength of the critical field,we attribute this step to the over⁃whelming of the antiferromagnetic interlayer interac⁃tions.As there was no other peak on the dM/dHplot,indicating the absence of a spin⁃flop transition[37⁃38],and the magnetization increased rapidly at low fields,we attribute the increase ofχMTbelow 6 K to the presence of weak ferromagnetic couplings rather than the spin canting behavior.And the ferromagnetic couplings are believed to be transmitted by the single⁃O(carboxylate)bridges within[Dy2]units,for other bridges separate Dy(Ⅲ)ions remarkably farther.
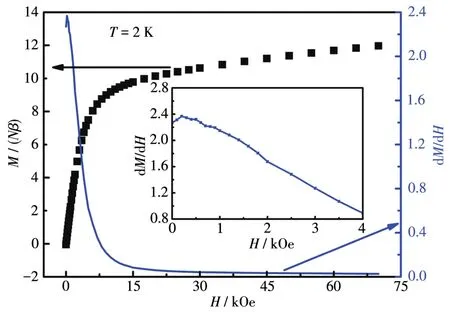
Fig.5 Field dependence of magnetization for 3 at 2 K
To study the dynamic magnetic behavior for 3,ac susceptibility was measured between 2 and 16 K with the frequency of 100 and 800 Hz under zero dc field.As shown in Fig.6,the imaginary part of ac data didn′t show any signals over the detected temperature and fre⁃quency range,suggesting the absence of SRM behav⁃ior.Considering the Kramers ion nature for Dy(Ⅲ)ion,we attribute the absence of SRM behavior to the presence of a fast relaxation process by quantum tun⁃neling mechanism which can occur between the double⁃degenerate ground states without absorbing or emitting a photon.As has been demonstrated,transverse anisot⁃ropy produced by non⁃axial LF parameters is the main perturbation factor that mixes the double⁃degenerate stark sublevels and causes a strong tunneling effect[39⁃40].To reduce the transverse LF parameters as far as possi⁃ble for Dy(Ⅲ)⁃based complexes,the LFs should be axi⁃al,meaning strong axial LF and weak non⁃axial LF,with a high symmetry likeD4d,D5h,Cn(n>7),etc[41].Such requirements are not satisfied in 3 according to the facts:(1)both Dy1 and Dy2 are located in LFs with low symmetry ofCsandC2v,respectively;(2)all coordi⁃nating O atoms are from either the neutral—OH groups or negative—COO−groups and both two kinds of ligands are weak ⁃field ligands which can′t build axial LFs when considering the high coordination num⁃bers of 9 and 8 for Dy1 and Dy2,respectively.And the tunneling effect should be strong and prevents the observation of SRM behaviors.As dc magnetic field was often employed to suppress the tunneling effect by introducing Zeeman splitting,we also tested the ac sus⁃ceptibility of 3 under dc field from 200 to 3 000 Oe(Fig.S5).However,no well⁃defined peaks inχ″can be observed indicating the external dc field can′t effec⁃tively suppress the strong tunneling effect alone.
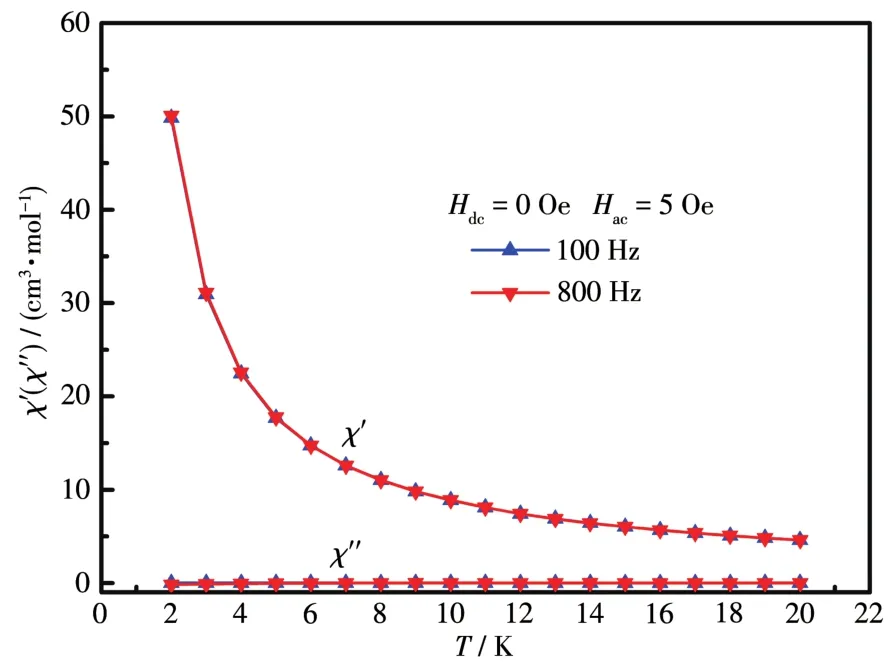
Fig.6 Temperature dependence of ac magnetic susceptibility for 3 under zero dc field
To the best of our knowledge,the three isostruc⁃tural complexes 1⁃3 are the unique kind of lanthanide complexes showing 2D layer structure based on dmpa−ligand.Usually,dmpa−was introduced to construct cluster complexes with high nuclearity because dmpa−was a significantly small organic molecule but with four O atoms which can serve as coordinating atoms simultaneously.And [Ni64Ln96][24], [Ln60][25], and[Ni36Ln102][26]complexes based on dmpa−have been syn⁃thesized which showed fascinating structures.To obtain these high⁃nuclearity structures,a certain kind of base was always introduced for the hydrolysis of lan⁃thanide ions,while no base was added in our synthesis procedure for 1⁃3 which may be the key reason that 2D layer structure complexes,not clusters,were obtained here.And our research demonstrates the high potential of Hdmpa ligand for constructing complexes with high dimensional structures beyond the clusters.In addi⁃tion,in the magnetic study on complex 3,ferromagnet⁃ic coupling was observed first in lanthanide complexes based on Hdmpa ligand.As high⁃spin ground state lan⁃thanide complexes based on ferromagnetic couplings are interesting in the fields of molecule⁃based magnets,magnetic cooling materials,and so on,our work also demonstrates the high potential of Hdmpa ligand in building such kinds of magnetic materials which will further promote the study on coordination chemistry based on Hdmpa ligand.
3 Conclusions
In summary,three new 2D lanthanide complexes 1⁃3 based on the ligand Hdmpa were prepared by sol⁃vothermal methods and fully characterized.These com⁃plexes are isostructural and show 2D layer structure.The studies of luminescent properties for 1 and 2 showed the typical red⁃light emission of Eu(Ⅲ) ion and green⁃light emission of Tb(Ⅲ) ion,respectively.Magnet⁃ic studies on 3 demonstrate the presence of weak ferro⁃magnetic couplings transmitted by single⁃O(carboxyl⁃ate)among the nearest Dy(Ⅲ)ions with a distance of 0.386 3 nm.However,due to the low symmetry of the coordination geometry of Dy(Ⅲ)ions,no slow relaxation of magnetization behavior was observed.
Acknowledgments:The authors thank the Modern Analy⁃sis and Testing Center of Xi′an Shiyou University for their support.
Supporting information is available at http://www.wjhxxb.cn
