Synthesis,Characterization and Antimicrobial Screening of Various Ferrocenyl Schiff Bases against Gram⁃positive and Gram⁃negative Bacteria
2022-04-18OladejiOlatundeIkhileMonisolaMamoMessaiNdunguPatrickNdintehDerek
Oladeji S.Olatunde Ikhile I.Monisola Mamo Messai Ndungu G.Patrick Ndinteh T.Derek
(Department of Chemical Sciences,Faculty of Science,University of Johannesburg,Johannesburg,South Africa)
Abstract:Ten ferrocenyl Schiff bases 1⁃10 were synthesized and characterized using UV⁃Vis spectroscopy,FT⁃IR,elemental analysis,1H and13C NMR spectroscopy.The synthesized compounds were evaluated against 11 bacteria strains(Bacillus subtilis ATCC19659,Klebsiella aerogenes ATCC13882,Enterococcus faecalis ATCC13047,Myco⁃bacterium smegmatis MC2155,Staphylococcus epidermidis ATCC14990,Staphylococcus aureus ATCC25923,Esche⁃richia coli ATCC25922,Enterobacter cloacae ATCC13047,Klebsiella oxytoca ATCC8724,Proteus mirabilis ATCC7002,Proteus vulgaris ATCC6380).The results obtained from antibacterial assay indicated that the synthe⁃sized Schiff bases 1⁃10 inhibited potential growth of Klebsiella aerogenes with the minimum inhibitory concentration(MIC)ranging from 15.6 to 31.25 μg·mL−1and Enterococcus faecalis with MIC the range between 31.25 and 125 μg·mL−1in comparison with the standard nalidixic acid and streptomycin sulfate.
Keywords:antibacterial;Schiff bases;nalidixic;synthesis;inhibition
0 Introduction
Cyclopentadienyl iron also known as ferrocene is a very important and precious compound that was discovered through a serendipitous process and its structure was determined over six decades ago[1⁃5].The discovery and determination of ferrocene structure have been positively influenced the growth of inorganic and bio⁃organometallic chemistry rapidly[6⁃7].Therefore,ferrocene⁃based organometallic compounds have attracted the attention of many researchers,especially in recent times based on their uncountable applica⁃tions.These numerous applications of ferrocene are attributed to its readily accessible reversible redox cou⁃ple,the stability of the ferrocene scaffold to air,temper⁃ature,and moisture[8].Also,the ability to easily func⁃tionalize the cyclopentadienyl rings in ferrocene skele⁃ton via electrophilic substitution which had led to the synthesis of monosubstituted,1,1′⁃disubstituted or 1,2⁃disubstituted ferrocenyl moieties is an advantage.In addition,its sufficient structural rigidity in a building block that gives an appropriate chiral environment[7,9]has made ferrocene a molecule of choice in organome⁃tallic synthesis.
It is important to note that ferrocene exhibited low cytotoxicity in biological systems as a result of its pos⁃session of aπ⁃conjugated system which resulted in its ability to transfer electrons easily[10].The cytotoxicity of its metabolites towards tumors[11]and its lipophilicity ability renders ferrocene derivatives to be a good mem⁃ber for the determination of biological activities[12].Fur⁃thermore,ferrocenyl compounds containing heteroatom like nitrogen,oxygen,sulfur have received much atten⁃tion in the last couple of decades as a result of their superior stability to function in various applications such as medicinal application[13⁃21],material science[22],catalysis[23⁃25],and electrochemistry[26⁃27].
Ferrocenyl Schiff bases containing imine function⁃al groups have been well studied due to their ability to reversibly bind oxygen[28⁃29],their redox system[30⁃31],and oxidative in DNA[32].Also,they were examined to exploit their redox properties in various applica⁃tions[33⁃34].Imine Schiff bases have been found to show good biological activities in the treatment of pathogen activities such as tuberculosis[35],antibacterial[36⁃38],anti⁃fungal[39],antitumor[40],antiviral[40],and herbicidal due to the presence of azomethine linkage(C=N—)pres⁃ent in living systems[41].Therefore,the incorporation of the imine functional group into ferrocenyl moieties will enhance their biological activities.
For this study,various ferrocenyl Schiff bases(1⁃10)were synthesized via refluxing with different aro⁃matic aldehydes.The reduction of nitro derivatives was achieved via Sn/HCl[42]and finally,the ferrocenyl Schiff bases were characterized and tested against elev⁃en strains of bacteria.
1 Experimental
1.1 Materials and methods
All chemicals used for the synthesis of the ferroce⁃nyl Schiff bases were purchased from Sigma Aldrich and Protea laboratory solutions(Pty)Ltd.,South Africa.Dry ethanol was used under nitrogen conditions.Other chemicals were used without further purification.Bruk⁃er topspin1H NMR(500 MHz)and13C NMR(125 MHz)were used to elucidate the compounds.FT⁃IR spectra were recorded on a PerkinElmer(spectrum 100)FT⁃IR Spectrometer while the UV⁃Vis spectrome⁃try analysis was determined using Agilent Technolo⁃gies Cary 60 UV⁃Vis.The melting points were carryout using Agilent 100 Melting point.The mass spectrome⁃try analysis of sample 1⁃10 was performed on a Bruker Compact Q⁃TOF high⁃resolution Compact mass spec⁃trophotometer.A 10 μL of the sample was injected into the UHPLC and run through a loop at 50% solvent A consisting of 0.1% formic acid in H2O(V/V)and 50% solvent B consisting of 0.1% formic acid in acetonitrile(V/V).The elemental analyses were recorded on 2400 series llCHNS/PerkinElmer analyzer.The antibacterial testing was carried out with 11 bacteria strains which consisted of Gram⁃positive and gram⁃negative bacteria(Bacillussubtilis(B.subtilis)ATCC19659,Klebsiella aerogenes(K.anogeneus)ATCC13882,Enterococcus faecalis(E.faecalis)ATCC13047,Mycobacteriumsmeg⁃matis(M.smegmatis)MC2155,Staphylococcusepider⁃midis(S.epidermidis)ATCC14990,Staphylococcusau⁃reus(S.aureus)ATCC25923,Escherichiacoli(E.coli)ATCC25922,Enterobactercloacae(E.cloacae)ATCC13047,Klebsiellaoxytoca(K.oxytoca)ATCC8724,Proteusmirabilis(P.mirabilis)ATCC7002,Proteusvulgaris(P.vulgaris)ATCC6380).They were purchased from the Centre of Excellence in Biomedical TB Research,University of the Witwa⁃tersrand,and were cultured overnight in Mueller⁃Hinton broth(Merck Chemicals,SA)at 25℃.The turbidity of the culture solutions was adjusted to match a 0.5 McFarland standard within 15 min before anti⁃bacterial testing.
1.2 Preparation of 3⁃nitrophenyferrocene
According to the procedure already published by Ikhile et al.[42],3⁃nitroaniline(13.4 g,96.8 mmol),30 mL of concentrated hydrochloric acid,and 30 mL of water were mixed in a two⁃necked round bottom flask and cooled to 0⁃5 ℃.Thereafter,a specific amount of sodium nitrite(9.0 g,130.44 mmol)solution was added dropwise with stirring.The mixture was stirred further for an additional 30 min and kept at a temperature below 5℃throughout this period.The mixed ferrocene(9.0 g,48.37 mmol)and hexadecyltrimethylammonium bromide were added to 100 mL of diethyl ether and cooled to 0⁃5 ℃.The diazonium salt was added drop⁃wise and the mixture was stirred for an additional 5⁃6 h at room temperature.Lastly,the reaction mixture was extracted with dichloromethane and dried with dehy⁃drated sodium sulfate and the crude extract was evapo⁃rated using a rotary evaporator.The product was dried in a vacuum,and an orange powder was given(13.2 g,90%).m.p.112.5⁃112.9 ℃.UV⁃Vis:λmax=447 nm.FT⁃IR(KBr,cm−1):3 087,2 937,2 864,1 591,1 519,1 339,1 102,1 001,890,802,741.1H NMR(500 MHz,CDCl3):δ8.23(d,1H,J=7.8 Hz,C6H4),8.01(d,1H,J=7.8Hz,C6H4),7.76(d,1H,J=7.8Hz,C6H4),7.53(t,1H,J=8.1 Hz,C6H4)4.70(s,2H,C5H4),4.39(s,2H,C5H4),4.04(s,5H,C5H5).13C NMR(125 MHz,CDCl3):δ148.57,148.16,142.17,135.36,134.72,131.73,130.44,129.32,123.82,123.06,121.69,120.45,120.30,82.44,69.92,66.82.Anal.Calcd.for C16H13FeNO2(%):H,4.27;C,62.57;N,4.56.Found(%):H,4.25;C,62.59;N,4.60.
1.3 Preparation of 3⁃ferrocenylaniline
3⁃Nitrophenylferrocene(4.02 g,13.09 mmol)was added to 30 mL of concentrated hydrochloric acid and 40 mL of ethanol containing granulated tin(8.82 g,74.29 mmol)according to the already published meth⁃od[42].The reaction mixture was heated to reflux at 110℃for 5 h.After cooling the reaction,150 mL of water was added together with NaOH to adjust the pH value to 14 and thereafter the mixture was filtered.The filtrate was extracted with water and dichloromethane(DCM)and the organic layer was dried with(Na2SO4).Rotary evaporation was used to remove the solvent.The concentrated crude was subjected to thin⁃layer chroma⁃tography(TLC)using hex/DCM(1∶1,V/V)and finally subjected to column chromatography as the eluent to give a pure compound.The product was dried in a vacuum,and an orange powder was given(2.1 g,91%).m.p.128⁃128.5 ℃.UV⁃Vis:λmax=447 nm.FT⁃IR(KBr,cm−1):3 441,3 357,3 103,2 919,2 720,1 598,1 513,1 465,1 307,1 232,1 169,1 100,999,810.1H NMR(500 MHz,CDCl3):δ7.13(d,1H,J=7.8 Hz,C6H4),6.96(dd,1H,J=5.0 Hz,C6H4),6.84(s,1H,C6H4),6.56(d,1H,J=7.8 Hz,C6H4),4.62(s,2H,C5H4),4.31(s,2H,C5H4),4.08(s,5H,C5H5),3.69(s,2H,NH2).13C NMR(125 MHz,CDCl3):δ146.26,140.29,129.18,117.06,113.06,112.89,85.65,69.57,68.69,66.52.Anal.Calcd.for C16H15FeN(%):H,5.39;C,69.12;N,5.08.Found(%):H,5.46;C,69.34;N,5.05.
1.4 General procedure for synthesis of ferrocenyl Schiff bases(1⁃10)
The synthesis of nitrophenylferrocene was carried out via arylation of ferrocene by diazonium salt under phase⁃transfer conditions and thereafter,the nitro derivatives were reduced using Sn/HCl to obtain ferro⁃cenylaniline(Scheme 1).Furthermore,aromatic alde⁃hydes were reacted with ferrocenylaniline to form imine(Scheme 2 and 3).The reactions were performed under nitrogen conditions.
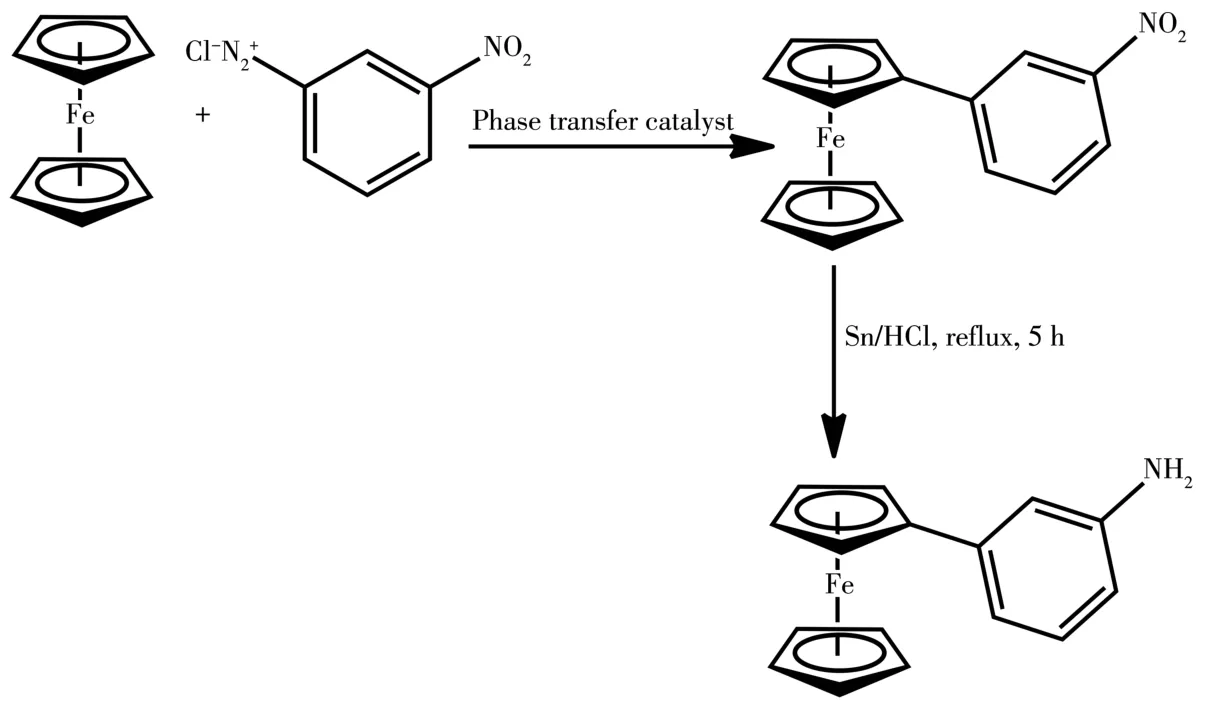
Scheme 1 Synthesis of 3⁃nitrophenylferrocene and 3⁃ferrocenylaniline
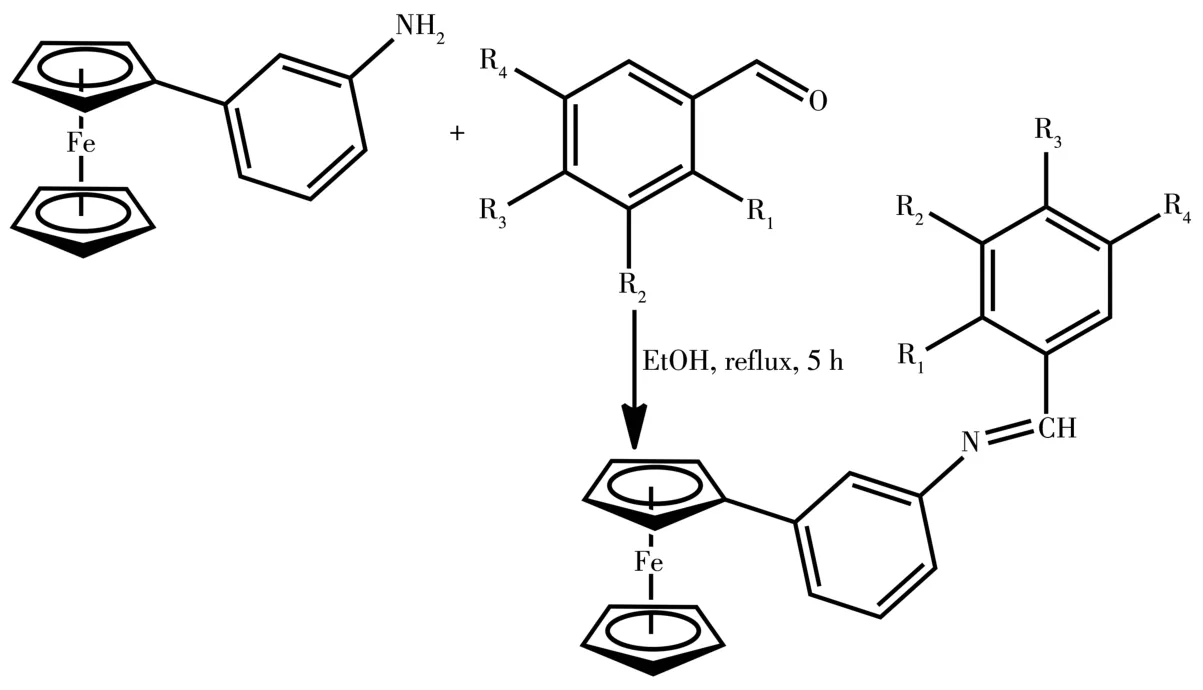
1:R1=OCH3,R2=OCH3,R3=R4=H;2:R1=OH,R3=OH,R2=R4=H;3:R3=Cl,R1=R2=R4=H;4:R3=NO2,R1=R2=R4=H;6:R1=NO2,R3=NO2,R2=R4=H;7:R3=NH(C8H9),R1=R2=R4=H;8:R2=Br,R1=R3=R4=H;9:R2=NO2,R1=R3=R4=HScheme 2 Synthesis of 3⁃ferrocenylphenylimine Schiff bases 1⁃4 and 6⁃9
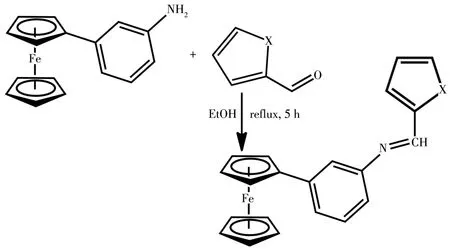
5:X=S;10:X=OScheme 3 Synthesis of 3⁃ferrocenylphenylimine Schiff bases 5 and 10
An equal volume of 3⁃ferrocenylaniline and aro⁃matic aldehyde were mixed and dissolved with 15 mL dried ethanol in a two⁃necked flask equipped with a magnetic stirrer.The reaction was performed under a reflux atmosphere and the mixture was heated for 5⁃6 h.Thereafter,the TLC was used to determine the for⁃mation of 1⁃10 after 5⁃6 h.A pressure vacuum was adopted to remove the solvent.
1.4.1 Synthesis ofN⁃(2,3⁃dimethoxy⁃benzylidene)⁃3⁃ferrocenylimine(1)
3⁃Ferrocenylanline(0.036 g,0.21 mmol)and 2,3⁃dimethoxybenzaldehydes(0.034 g,0.23 mmol)were used.The product was collected as brownish colour powder(0.051 3 g,63%).m.p.240.5⁃242.3 ℃.UV⁃Vis(nm):261,304,325,454.FT ⁃IR(KBr,cm−1):3 088(Ar—H),2942,2 873,2 830,2 753,1 680(CH=N),1 581(C=C),1 475,1 384,1 311,1 258(Cp),1 173(ν—C—O),1 068(ν—C—O),996,906,784,751(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ10.46(s,1H,N=CH),8.93(s,1H,C6H3),7.80(dd,1H,J=5.0 Hz,C6H3),7.38(d,1H,J=7.8 Hz,C6H3),7.34(t,2H,J=1.8 Hz,C6H3),7.17(t,1H,J=1.8 Hz,C6H3),7.07(dd,1H,J=5.0 Hz,C6H3),4.71(s,2H,C5H4),4.35(s,2H,C5H4),4.09(s,5H,C5H5),4.02(s,3H,OCH3),3.96(s,3H,OCH3).13C NMR(125 MHz,CDCl3):δ190.14,156.49,153.05,152.92,150.26,140.40,129.98,129.04,124.30,124.14,123.78,119.29,119.03,118.16,117.91,115.14,85.06,69.64,68.97,66.63,56.07,55.96.HRMS(ESI):m/zCalcd.for C25H23FeNO2:425.300 6;Found([M+H]+):426.110 2.Anal.Calcd.for C25H23FeNO2(%):H,5.45;C,70.60;N,3.29.Found(%):H,5.54;C,70.62;N,3.19.
1.4.2 Synthesis ofN⁃(2,4⁃dihydroxyl⁃benzylidene)⁃3⁃ferrocenylimine(2)
3⁃Ferrocenylanline(0.040 g,0.23 mmol)and 2,4⁃dihydroxylbenzaldehydes(0.048 g,0.25 mmol)were used.The product was collected as orange colour pow⁃der(0.073 3 g,81%).m.p.250.2⁃251.5 ℃.UV⁃Vis(nm):235,275,327,430.FT ⁃IR(KBr,cm−1):3 329(—OH),3 096(Ar—H),2 861,1 668(CH=N),1 585(C=C),1 495,1 439,1 326,1 223(Cp),1 159,1 120,973,800(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ11.24(s,1H,OH),9.73(s,1H,N=CH),8.55(s,1H,C6H3),7.46(d,2H,J=7.8 Hz,C6H3),7.35(s,1H,C6H5),7.12(d,1H,J=7.8 Hz,C6H4),6.42(d,1H,J=7.8 Hz,C6H4),6.52(m,2H,C6H4),4.70(s,2H,C5H4),4.37(s,2H,C5H4),4.09(s,5H,C5H5).13C NMR(125 MHz,CDCl3):δ194.34,164.45,163.47,160.73,141.10,136.05,134.34,129.39,124.40,118.66,117.69,115.55,108.69,107.71,103.70,103.18,84.48,69.67,69.18,66.61.HRMS(ESI):m/zCalcd.for C23H19FeNO2:397.247 3;Found([M+H]+):398.079 7.Anal.Calcd.for C23H19FeNO2(%):H,4.82;C,69.54;N,3.53.Found(%):H,4.81;C,69.27;N,3.50.
1.4.3 Synthesis ofN⁃(4⁃chlorobenzylidene)⁃3⁃ferroce⁃nylimine(3)
3⁃Ferrocenylanline(0.048 g,0.27 mmol)and 4⁃chlorobenzaldehydes(0.042 g,0.30 mmol)were used.The product was collected as yellow colour powder(0.094 1 g,87%).m.p.224.8⁃226.5 ℃.UV⁃Vis(nm):262,301,330,454.FT⁃IR(KBr,cm−1):3 099(Ar⁃H),2922,2864,1681,1657(CH=N),1578,1365,1285,1 166,1 084,1 008(Cp),813,710(Fc⁃Cp).1H NMR(500 MHz,CDCl3):δ10.01(s,1H,N=CH),8.50(s,1H,C6H4),7.91(d,2H,J=7.8 Hz,C6H4),7.50(d,2H,J=7.8 Hz,C6H4),7.36(dd,1H,J=5.0 Hz,C6H4),7.34(t,1H,J=1.8 Hz,C6H4),7.05(dd,1H,J=5.0 Hz,C6H4),4.71(s,2H,C5H4),4.36(s,2H,C5H4),4.09(s,5H,C5H5).13C NMR(125 MHz,CDCl3):δ190.76,158.79,151.84,140.58,137.42,134.77,130.89,129.47,129.14,129.11,124.02,118.93,117.73,117.06,113.06,112.90,84.84,69.65,69.06,66.58.HRMS(ESI):m/zCalcd.for C23H18FeNCl:399.693 7;Found([M+H]+):400.049 7.Anal.Calcd.for C23H18FeNCl(%):H,4.54;C,69.11;N,3.50.Found(%):H,4.48;C,69.17;N,3.57.
1.4.4 Synthesis ofN⁃(4⁃nitrobenzylidene)⁃3⁃ferroce⁃nylimine(4)
3⁃Ferrocenylanline(0.040 g,0.23 mmol)and 4⁃nitrobenzaldehydes(0.035 g,0.23 mmol).The product was collected as gold colour powder(0.081 0 g,86%).m.p.330.5⁃332.5 ℃.UV⁃Vis(nm):287,300,352,463.FT⁃IR(KBr,cm−1):3 097(Ar⁃H),2 934,2 853,1 702,1 660,1 511,1 338(C—N),1 192,1 101,1 003(Cp),840,678(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ10.19(s,1H,N=CH),8.63(s,1H,C6H4),8.40(dd,2H,J=5.0 Hz,C6H4),8.12(dd,2H,J=5.0 Hz,C6H4),7.45(d,1H,J=7.8 Hz,C6H4),7.37(t,1H,J=1.8 Hz,C6H4),7.10(d,1H,J=7.8 Hz,C6H4),4.72(s,2H,C5H4),4.37(s,2H,C5H4),4.09(s,5H,C5H5).13C NMR(125 MHz,CDCl3):δ190.14,157.80,151.12,147.14,141.63,141.14,140.88,130.44,129.42,124.80,124.30,119.04,84.57,69.66,69.15,66.60.HRMS(ESI):m/zCalcd.for C23H18FeN2O2:410.246 2;Found([M+H]+):411.051 1.Anal.Calcd.for C23H18FeN2O2(%):H,4.42;C,67.34;N,6.83.Found(%):H,4.68;C,67.41;N,6.72.
1.4.5 Synthesis ofN⁃(2⁃thiophenecarboxbenzylidene)⁃3⁃ferrocenylimine(5)
3⁃Ferrocenylanline(0.044 g,0.25 mmol)and 2⁃thiophenecarboxaldehyde(0.034 mL,170 mmol)were used.The product was collected as faint yellow colour powder(0.0701 g,76%).m.p.196.6⁃198.5 ℃.UV⁃Vis(nm):260,300,333,461.FT⁃IR(KBr,cm−1):3 099(Ar⁃H),2 934,2 873,2 753,1 680(N=CH),1 579(C=C),1 430,1 384,1 264,1 183,1 069(Cp),998,906,751(C—S),656(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ8.64(s,1H,N=CH),7.55(dd,2H,J=5.0 Hz,C4H5⁃S),7.37(dd,1H,J=5.0 Hz,C6H4),7.34(t,2H,J=1.8 Hz,C6H4),7.18(d,1H,J=7.8 Hz,C6H4),7.06(d,1H,J=7.8 Hz,C6H4),4.71(s,2H,C5H4),4.35(s,2H,C5H4),4.08(s,5H,C5H4).13C NMR(125 MHz,CDCl3):δ152.99,151.61,142.91,140.46,132.12,130.00,129.06,127.74,123,64,119.13,69.63,69.00,66.55.HRMS(ESI):m/zCalcd.for C21H17FeNS:371.276 4;Found([M+H]+):372.045 7.Anal.Calcd.for C21H17FeNS(%):H,4.62;C,67.93;N,3.77.Found(%):H,4.71;C,67.91;N,3.68.
1.4.6 Synthesis ofN⁃(2,4⁃dinitrobenzylidene)⁃3⁃ferro⁃cenylimine(6)
3⁃Ferrocenylanline(0.040 g,0.23 mmol)and 2,4⁃dinitrobenzylidene(0.045 g,0.23 mmol)were used.The product was collected as black powder(0.071 1 g,74%).m.p.235.8⁃236.5 ℃.UV⁃Vis(nm):261,296,360,465.FT⁃IR(KBr,cm−1):3 095(Ar⁃H),2 936,2 853,1 676(CH=N),1 531(C=C),1 338(C—N),1 194,1 101,1 003(Cp),845(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ10.19(s,1H,N=CH),8.65(s,1H,C6H4),8.40(dd,2H,J=5.0 Hz,C6H4),7.54(d,2H,J=7.8 Hz,C6H4),7.36(t,1H,J=1.8 Hz,C6H4),7.10(d,1H,J=7.8 Hz,C6H4),4.71(s,2H,C5H4),4.37(s,2H,C5H4),4.09(s,5H,C5H5).13CNMR(125MHz,CDCl3):δ151.20,149.03,148.40,147.46,140.43,136.16,131.23,127.35,126.85,121.89,120.35,84.11,69.74,69.46,66.60.HRMS(ESI):m/zCalcd.for C23H17FeN3O4:455.243 8;Found([M+H]+):456.057 1.Anal.Calcd.for C23H17FeN3O4(%):H,3.76;C,60.68;N,9.23.Found(%):H,3.62;C,60.67;N,9.37.
1.4.7 Synthesis ofN⁃(4⁃dimethyaminobenzylidene)⁃3⁃ferrocenylimine(7)
3⁃Ferrocenylanline(0.040 g,0.23 mmol)and 4⁃dimethyaminobenzaldehydes(0.038 g,0.25 mmol)were used.The product was collected as armor yellow colour powder(0.064 4 g,69%).m.p.210.6⁃212.5 ℃.UV⁃Vis(nm):236,266,343,442.FT⁃IR(KBr,cm−1):3 095(Ar⁃H),2 926,2 811,1 663(CH=N),1 585(C=C),1 443,1 437,1 361(C—N),1 231,1 159,1 003(Cp),973,808(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ9.77(s,1H,N=CH),8.39(s,1H,C6H4),7.83(d,1H,J=7.8 Hz,C6H4),7.77(d,1H,J=7.8 Hz,C6H4),7.33(t,1H,J=1.8 Hz,C6H4),6.79(dd,2H,J=5.0 Hz,C6H4),6.74(dd,2H,J=5.0 Hz,C6H4),4.71(s,2H,C5H4),4.33(s,2H,C5H4),4.08(s,5H,C5H5),3.11(s,3H,N—CH3),3.09(s,3H,N—CH3).13C NMR(125 MHz,CDCl3):δ190.04,152.90,140.42,131.98,130.50,128.96,119.06,117.96,111.64,111.04,85.06,69.63,68.88,66.57,40.18,40.06.HRMS(ESI):m/zCalcd.for C25H24FeN2:408.316 5;Found([M+H]+):409.133 2.Anal.Calcd.for C25H24FeN2(%):H,5.92;C,73.54;N,6.86.Found(%):H,5.60;C,73.58;N,6.88.
1.4.8 Synthesis ofN⁃(3⁃bromobenzylidene)⁃3⁃ferroce⁃nylimine(8)
3⁃Ferrocenylanline(0.094 g,0.53 mmol)and 3⁃bromobenzaldehyde(0.103 g,0.56 mmol)were used.The product was collected as orange colour powder(0.175 1 g,74%).m.p.210.4⁃212.8 ℃.UV⁃Vis(nm):262,299,321,458.FT⁃IR(KBr,cm−1):3 062(Ar⁃H),2 931,2 824,2 546,1 689(CH=N),1 565(C=C),1 467,1 420,1 286,1 186,1 062(—Br),933,746(Fe⁃Cp).1H NMR(500 MHz,CDCl3):δ9.52(s,1H,N=CH),8.50(s,1H,C6H4),7.91(d,2H,J=7.8 Hz,C6H4),7.50(d,2H,J=7.8 Hz,C6H4),7.36(dd,1H,J=5.0 Hz,C6H4),7.35(t,1H,J=1.8 Hz,C6H4),7.05(dd,1H,J=5.0 Hz,C6H4),4.71(s,2H,C5H4),4.36(s,2H,C5H4),4.09(s,5H,C5H5).13C NMR(125 MHz,CDCl3):δ190.73,158.61,151.54,140.58,137.31,134.23,130.89,129.47,129.14,129.11,127.64,124.20,119.00,84.76,69.65,69.06,66.58.HRMS(ESI):m/zCalcd.for C23H18FeBrN:444.144 7;Found([M+H]+):445.000 6.Anal.Calcd.for C23H18FeBrN(%):H,4.08;C,62.20;N,3.15.Found(%):H,4.13;C,62.27;N,3.17.
1.4.9 Synthesis ofN⁃(3⁃nitrobenzylidene)⁃3⁃ferroce⁃nylimine(9)
3⁃Ferrocenylanline(0.040 g,0.23 mmol)and 3⁃nitrobenzaldehydes(0.035 g,0.23 mmol)were used.The product was collected as gold colour powder(0.731 g,78%).m.p.335.5⁃337.5 ℃.UV⁃Vis(nm):259,296,328,441.FT⁃IR(KBr,cm−1):3 085(Ar⁃H),2 936,2 853,1 701,1 665(CH=N),1 531,1 346(NO2),1 192,1 101,1 002(Cp),841,731(Fc⁃Cp).1H NMR(500 MHz,CDCl3):δ10.19(s,1H,N=CH),8.64(s,1H,C6H4),8.40(dd,2H,J=5.0 Hz,C6H4),8.12(dd,2H,J=5.0 Hz,C6H4),7.55(d,1H,J=8.7 Hz,C6H4),7.36(t,1H,J=1.8 Hz,C6H4),7.10(d,1H,J=7.8 Hz,C6H4),4.72(s,2H,C5H4),4.37(s,2H,C5H4),4.09(s,5H,C5H5).13CNMR(125 MHz,CDCl3):δ189.65,157.18,151.03,148.77,140.89,137.43,134.57,134.09,130.37,129.82,124.66,124.30,119.08,84.61,69.66,69.15,66.60.HRMS(ESI):m/zCalcd.for C23H18FeN2O2:410.246 2;Found([M+H]+):411.074 3.Anal.Calcd.for C23H18FeN2O2(%):H,4.42;C,67.34;N,6.83.Found(%):H,4.48;C,67.41;N,6.72.
1.4.10 Synthesis ofN⁃(2⁃furabenzylidene)⁃3⁃ferroce⁃nylimine(10)
3⁃Ferrocenylanline(0.096 g,0.55 mmol)and 2⁃furaldehyde(0.092 g,0.96 mmol)were used.The prod⁃uct was collected as black colour powder(0.140 7 g,76%).m.p.193.5⁃195.2 ℃;UV⁃Vis(nm):259,298,329,466.FT⁃IR(KBr,cm−1):3 357(C—O),3 092(Ar⁃H),2 865,1 676(CH=N),1 594,1 464,1 389,1 338,1 277,1 230,1 155,1 010(Cp),928,882,812,749(Fc⁃Cp).1H NMR(500 MHz,CDCl3):δ9.70(s,1H,N=CH),8.37(s,1H,C6H4),7.69(d,1H,J=7.8 Hz,—OC4H3),7.43(t,1H,J=1.8 Hz,C6H4),7.34(t,1H,J=1.5 Hz,—OC4H3),7.09(dd,1H,J=5.0 Hz,—OC4H3),6.61(dd,1H,J=5.0 Hz,C6H4),6.40(d,1H,J=7.8 Hz,C6H4),4.70(s,2H,C5H4),4.35(s,2H,C5H4),4.08(s,5H,C5H5).13C NMR(125 MHz,CDCl3):δ177.86,153.07,148.01,147.65,145.69,140.55,129.11,124.00,119.62,117.49,116.27,112.55,112.19,69.64,69.03,66.55.HRMS(ESI):m/zCalcd.for C21H17FeNO:355.210 8;Found([M+H]+):356.067 9.Anal.Calcd.for C21H17FeNO(%):H,4.82;C,71.01;N,3.94.Found(%):H,4.82;C,70.80;N,3.21.
All the spectra of the compounds can be found in Supporting information.
1.5 Antibacterial testing
Antibacterial studies of the ferrocenyl Schiff bas⁃es were carried out by determining the minimum inhibi⁃tory concentration(MIC)of all the strains through broth microdilution assay described by Andrew[43].The test compounds were accurately weighed and dissolved in DMSO to yield 1 mg·mL−1.The dissolved compounds were then serially diluted in Mueller⁃Hinton broth till the lowest volume of 100 μL.All dilutions were tested five⁃fold against each bacterial strain.100 μL of the bacterial suspension was mixed with 100 μL of the pre⁃diluted test compound in a 96 microwell plate and left to incubate overnight at 37℃.A 0.02% resazurin dye(10 μL)as the solution was added to each well and the plates were re⁃incubated for 2 h.Visual change of the solution from blue to pink indicated that the bacteria were still alive.MIC was determined as the minimum concentration of a compound where no color change could be observed.The MICs of all strains tested were compared to two reference antibiotics(nalidixic acid and streptomycin sulfate).This was because whilst streptomycin is a broad⁃based antibiotic,nalidixic acid is exclusively active against Gram⁃negative bacteria[44].
2 Results and discussion
2.1 FT⁃IR and NMR analysis
The synthesized compounds were characterized using FT⁃IR spectroscopy and the absorption bands at 3 441 and 3 357 cm−1correspond to the N—H stretch⁃ing band of aniline on aromatic compounds while the bands at 3 103 and 2 919 cm−1are attributed to the C—H band of symmetric and asymmetric stretching of aromatic compounds[45].The formations of the pure products were monitored using TLC in conjunction with FT⁃IR.
All ferrocenyl Schiff bases revealed the absence of N—H and disappearance of C=O stretching peak at 1 720⁃1 712 cm−1on aldehyde compound,and the appearance of absorption at 1 681⁃1 669 cm−1indicate the formation of C=N imine functional group[42,45].The vibrational stretching of the C—N band for all ferroce⁃nyl Schiff bases appeared at 1 361 ⁃1 330 cm−1while the presence of 3 329 cm−1indicates the vibrational stretching of the—OH group on aromatic moieties[45].
The1H NMR and13C NMR spectra of the synthe⁃sized Schiff bases were recorded in CDCl3relative to the reference TMS.In1H NMR spectra of all azome⁃thine compounds,the CH=N proton happens to be the most de⁃shielded and it appeared as a singletδranging from 8.45 to 10.46 while aromatic protons showed their signals in theδrange of 7.45⁃8.42.Four distinct pro⁃tons are directly attached to benzene indicating a dou⁃blet and doublet of double.Substituted cyclopentadi⁃ene attached to an aromatic compound contains two types of protons,which appeared as two singlets atδ=4.3 and 4.7,respectively.Furthermore,the unsubstitut⁃ed five protons of cyclopentadiene that are attached directly to ferrocene were chemically equivalent and showed one singlet atδ=4.0.13C NMR spectra revealed azomethine carbon to be the most de⁃shielded,which appeared in aδrange of 152 ⁃190.Aromatic carbon atoms showed their signals atδ=124,129,134,and 158,respectively.Carbon atoms of the ferrocenyl moi⁃ety appeared atδ=66.6,69.0,69.6,respectively[42,45].
2.2 UV⁃Vis spectroscopy
The UV⁃Vis spectroscopy analysis shown in Fig.1 and Table 1 revealed three major absorptions in the ranges of 200⁃300 nm,300⁃400 nm,and 400⁃425 nm.The absorption peaks in 200⁃300 nm are assigned toπ⁃π* transition of metal⁃to⁃ligand charge transfer(MLCT),which permits the charge transferred from cyclopentadienyl to be delocalized while the absorption in the range between 300 and 400 nm corresponds ton⁃π*transition of the aromatic compound of Schiff bases.Furthermore,the absorption peaks in 400⁃425 nm are ascribed ton⁃π*intramolecular charge transfer(ICT)of lone pairs of the electron from imine nitrogen atom(N=CH)to antibondingπorbital in conjunction with the Schiff base derivative[6].
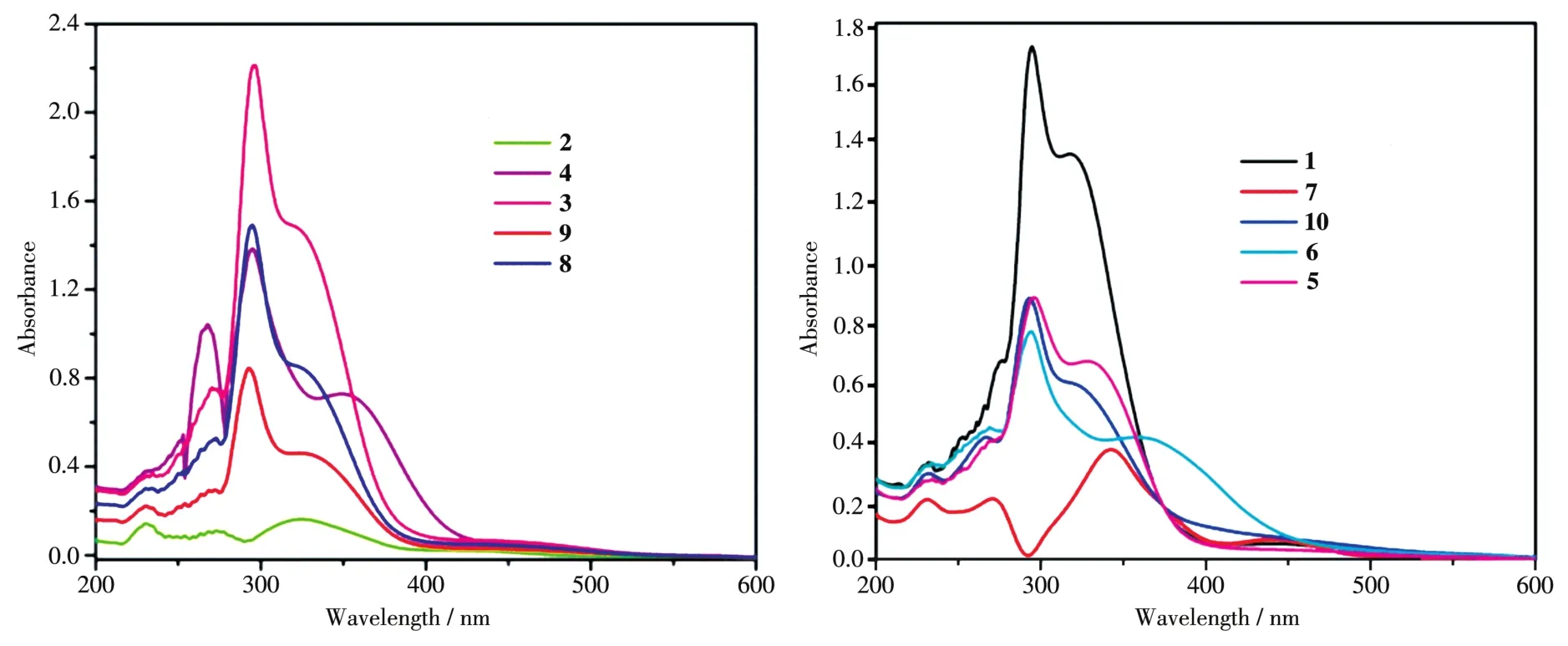
Fig.1 UV⁃Vis absorption for Schiff bases 1⁃10
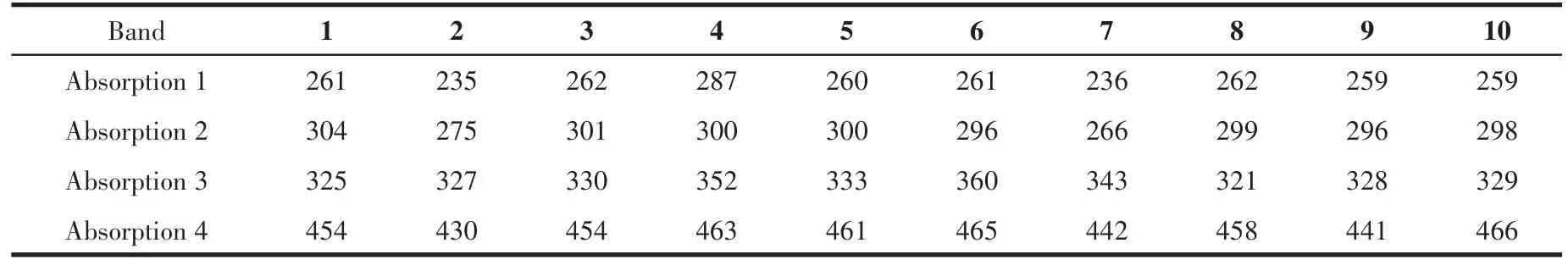
Table 1 UV⁃Vis spectroscopy bands of 3⁃ferrocenylphenylimine Schiff bases 1⁃10 nm
2.3 Antibacterial analysis
The potent activities of the synthesized com⁃pounds 1⁃10 were investigated against eleven microbes of bacteria strains.The antibacterial result is shown in Table 2.Compounds 1 and 2 show significant activities againstK.anogeneusandE.faecalisthan the reported standard streptomycin sulfate with the MIC of 31.25 μg·mL−1and nalidixic acid with the MIC range of 15.6⁃125 μg·mL−1.The presence of electron⁃donating groups of their substituents is attributed to it which shows that the biological activities of the ferrocene⁃containing compounds could be very active against microbes when more electron donor substituents are attached with ferrocene molecules[33].This will also interest you to know that all the synthesized compounds 1⁃10 show more significant active activities than the reported stan⁃dards againstK.anogeneuswith the MICs range of 15.6⁃31.25 μg·mL−1andE.faecaliswith the MIC range of 31.25⁃125 μg·mL−1except for 4.Their good activities could be attributed to the good lipophilic nature of dif⁃ferent substituents attached to imine Schiff bases,which allows easy permeation of the compounds through the lipoid layer of the microbial membrane.This is surprising as it is against the studies carried out by Muhammad et al.[45],where all the compounds show no good activities against six tested microbes[45].Theelectron⁃withdrawing group on aromatic compound 4 mitigates the potent of 4 against theK.anogeneusby disallowing 4 penetration into the lipoid layer of the microorganism membrane.Furthermore,the activities of 6 and 7 compounds againstB.subtilismicroorganism revealed good potent than the standards with the MIC of 15.62 μg·mL−1as against the streptomycin and nali⁃dixic acid having MIC value of 16 μg·mL−1,which is attributed to the strong electron⁃withdrawing group of the nitro couple with ferrocene molecule which facili⁃tates easy penetration into the lipoid layer of microbe while others show a relatively high value than the reported standards.
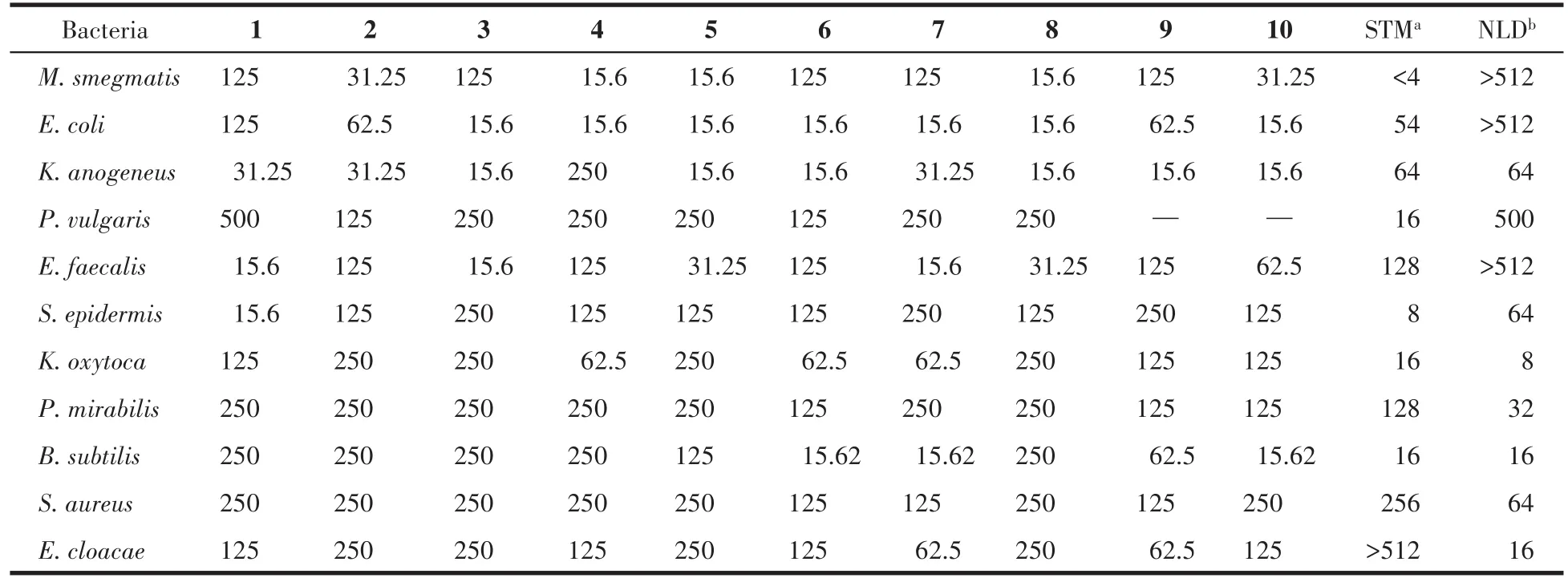
Table 2 Potential inhibitory activities of the synthesized compounds against eleven strains of bacteria μg·mL−1
A similar trend was also observed withS.aureusandE.cloacaemicroorganisms against all tested com⁃pounds 1⁃10.They showed superior activities than stan⁃dard streptomycin sulfate with the MIC range of 125⁃250 μg·mL−1forS.aureusand the MIC range of 62.5⁃250 μg·mL−1forE.cloacae.Although,we observed relatively low activities for compounds 1⁃10 againstS.aureusandE.cloacaethan the reported standard nalidixic acid(MIC of 64 and 16 μg·mL−1).However,most of the compounds display good activities againstE.coli,while compounds 1,2,and 4 exhibited weak activities when compared with the standards(MIC of 54 and>512 μg·mL−1),which could be attributed to the strong electron⁃donating effect of their attached substituents.Also,againstM.smegmatisthe tested compounds show high activities than streptomycin stan⁃dard and show weak activities than nalidixic acid with MIC of<4 and>512 μg·mL−1.It could be observed that all compounds displayed different activities and the antibacterial activity of compounds 1⁃10 againstE.coliandS.aureusmicrobes are similar to the report⁃ed activities of the compounds H1⁃H12and L1,L6,L11by Liu et al[46].Generally,most of the compounds displayed weak to mild activities against certain micro⁃bial organisms at the prepared concentration of 500 μg·mL−1.These activities could be attributed to the redox behavior of the central ferrocenyl moiety which inhibits their penetration ability through the lipoid layer of the microorganism membrane[45].
3 Conclusions
Ten compounds were successfully synthesized,characterized by various techniques like FT⁃IR,UV⁃Vis spectroscopy,NMR,and tested against eleven strains of bacteria under a sterilized condition.The results displayed a good activity of compounds 1⁃10 againstK.aerogeneswith MIC ranging from 15.6 to 31.25 μg·mL−1and againstE.faecaliswith the MIC ranges between 31.25 and 125 μg·mL−1except for compound 4.6 and 7 also displayed good activities againstB.subtiliswith the MIC of 15.62 μg·mL−1.It was also observed that most compounds experienced low to mild activities against some microorganisms.Therefore,to understand the full biological potential activity of 1⁃10 compounds,there is a need to perform other activities such as antifungal,cytotoxicity,and minimum bactericidal concentration(MBC)to ascer⁃tain their level of performance against various microor⁃ganisms.
Acknowledgments:All the authors are very grateful to the Department of Applied Chemistry,the University of Johan⁃nesburg for providing an enabling condition and funding to carry out this project.
Supporting information is available at http://www.wjhxxb.cn
