Preparation of Solid Waste-Based Activated Carbon and Its Adsorption Mechanism for Toluene
2022-04-14LiZhiLiYunpengLiuJunSiWenzheZhangYongfaLiJunhua
Li Zhi; Li Yunpeng; Liu Jun; Si Wenzhe; Zhang Yongfa; Li Junhua
(1. Taiyuan University of Technology, College of Chemistry and Chemical Engineering,State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan 030024;2. Tsinghua University, College of Environment, National Engineering Research Center for Coordinated Control of Air Pollutants and Greenhouse Gases, Beijing 100084;3. Zibo Pengda Environmental Protection Technology Co., Ltd., Zibo 255420)
Abstract: Regenerated activated carbon (RAC) samples were prepared by carbon activation using waste activated carbon from solid waste resources as the carbon source precursor coupled with adding alkaline additives, and then were further modified by potassium ferrate to finally prepare high-performance carbon for VOCs adsorption. At the same time, the samples before and after modification were systematically studied through characterization techniques such as SEM,Raman spectrometry, FT-IR, XPS, and dynamic/static adsorption. The results showed that the specific surface area and pore volume of the RAC after modification by the strong oxidant potassium ferrate increased by 1.4 times; the degree of defects was enhanced and the content of oxygen-containing functional groups on the surface increased significantly. Among them,the sample modified with potassium ferrate for 24 h had the best dynamic toluene adsorption performance (375.5 mg/g),and the dynamic adsorption capacity was twice that of the original sample (192.8 mg/g). The static adsorption test found that the maximum adsorption capacity of RAC-6%K2FeO4+H2SO4-24h was 796 mg/g, which indicated that the potassium ferrate modification treatment could significantly increase the VOCs adsorption performance of RAC. In addition, through consecutive toluene adsorption-desorption cycle tests, it was found that the RAC-6%K2FeO4+H2SO4-24h sample still retained 91% of adsorption activity after the fifth regeneration cycle. This indicates that RAC-6%K2FeO4+H2SO4-24h has good cycle stability and great application value for the efficient purification of industrial waste VOCs gas.
Key words: waste activated carbon; toluene adsorption; potassium ferrate modification; oxygen-containing functional groups; regeneration ability
1 Introduction
Volatile organic compounds (VOCs) are organic compounds that participate in atmospheric photochemical reactions, including complex and diverse harmful substances, and are important precursors for the formation of ozone (O3) and fine particulate matter(PM2.5) pollution[1]. The current concentration of volatile organic compounds in the atmosphere and their increasing trends have been threatening the human health and the environment. Volatile organic compounds(VOCs) can damage the human blood system, causing respiratory problems and even cancer[2]. Therefore, the removal of VOCs has attracted great attention. At present,various technologies such as adsorption, condensation,distillation, catalytic oxidation, incineration, and biological treatment have been developed and used for VOCs emission reduction[3]. Among the VOCs removal technologies, the adsorption process is recognized as a mature and efficient technology due to its flexibility, low cost and low energy consumption[4].
Porous carbon materials have been attracting more attention from the scientific community in different research fields due to their simplicity, environmental friendliness, and large-scale availability. Among the numerous carbonaceous adsorbents, activated carbon(AC) is considered to be the most promising adsorption technology due to its large surface area, abundant micropores and excellent adsorption capacity[5]. On the other hand, there have been many successful cases of preparing activated carbon from different wastes and biomass, which not only can reduce the production cost of porous carbon, but also can control the accumulation of wastes in the environment. Tehrani, et al.[6]used waste asphalt from the petroleum industry to synthesize a series of porous carbon materials with high nitrogen (N) and sulfur (S) contents. Zhang, et al[7]obtained biomassderived activated carbon with high surface area and good adsorption capacity through an inexpensive preparation process. At the same time, many studies have focused on agricultural and industrial waste (such as cork flour[8],corncob[9], wood chips[10], rice husks and waste tires[11]).However, there are few reports on the regeneration and utilization of waste activated carbon, so it is of great significance to provide a practical process technology.
Generally speaking, activated carbon with larger surface area has higher physical adsorption capacity for various VOCs. However, the adsorption properties of a material are not only determined by its porous structure but also strongly influenced by its surface chemistry. As a nonpolar adsorbent, AC itself has lower chemisorption and VOCs selectivity due to its fewer surface adsorption active sites[12]. Many studies are under way to significantly improve the surface activity of AC by using different chemical methods. For example, the introduction of functional groups[13](such as carboxyl, carbonyl,hydroxyl, phenols, etc.) or active substances[14](such as metal oxides, organic compounds, etc.) on the AC surface will improve the affinity between AC surface and VOCs. However, most studies would change the chemical properties of AC surface, while inevitably causing damage to the physical structure, which would greatly hinder the efficient removal of VOCs.
In this study, the waste activated carbon (AC) was utilized as a carbon source to synthesize a series of porous carbon materials, which were then modified using potassium ferrate. Toluene, a typical VOCs molecule, was used as the adsorbate to take part in the dynamic and static adsorption tests. Meanwhile, a series of characterization techniques such as BET, Raman spectroscopy and XPS were used to explore the main influencing factors of improving adsorption performance. This study not only can provide a sustainable and effective solution for solid waste resource utilization, but also can open up a new way to prepare high-performance VOCs adsorbent, which is of great significance for industrial VOCs governance.
2 Experimental
2.1 Synthesis of RAC
According to our previously independently developed AC preparation technologies[15], we had synthesized a series of high-performance VOCs adsorption carbon materials from industrial waste carbon through mesoporous-macroporous reconstruction and catalytic graphitization technologies.Specifically, after a certain proportion of waste activated carbon, ferric chloride, and petroleum coke were fully mixed, they were transferred to a ball mill and ground to a grain size of 400 mesh to form the mixture precursor.Subsequently, a non-asphalt-based binder was added to the mixed precursor for mixing and kneading, and then the hydraulic granulator was used for granulation. The granulated samples were dried at 105 °C for 24 h and then were subjected to pyrolysis in a carbonization furnace under N2flow at a temperature increase rate of 10 °C/min to be treated at 800 °C for 2 h. After cooling to room temperature, the carbonized samples were transferred to an activation furnace and heated in the presence of water vapor at 900 °C for 4 h. After being cooled down to room temperature again, the finally obtained sample was named as the regenerated activated carbon (RAC).
2.2 RAC modification
In this experiment, the typical magnetic agitationhigh temperature calcination method was adopted, and sulfuric acid was used as the solvent, while potassium ferrate, a strong oxidant, was used as the solute to modify activated carbon. The specific methods are presented as follows: 5 grams of the prepared RAC sample were slowly placed into a round-bottom flask containing 80 mL of concentrated sulfuric acid (30%),and ultrasonic vibration was carried out for 30 min to make the mixture of sulfuric acid and RAC uniform.After the ultrasonic treatment, potassium ferrate with a mass ratio of 6% was weighed and slowly added into the flask, then the flask was transferred to a constant temperature oil bath at 60 °C under magnetic stirring,and the effect on the adsorption of toluene by the recycled carbon was investigated through different treatment times (5 h, 12 h, 24 h). After the modification of the recycled carbon by potassium ferrate, the sample was left standing at room temperature, and the activated carbon was filtered and washed until the discharged water showed a neutral reaction. Then the sample was placed in a vacuum drying oven to be dried at 60 °C for 12 h. Then, the dried samples were transferred to a tubular furnace, were heated to 800 °C at a temperature increase rate of 5 °C/min under nitrogen atmosphere,and then were kept at 800 °C for 2 h for activation. The final samples were obtained after being cooled down to room temperature. The samples modified by potassium ferrate over different times were named as RAC-6%K2FeO4+H2SO4-5h, RAC-6%K2FeO4+H2SO4-12h,and RAC-6%K2FeO4+H2SO4-24h.
In order to compare the effect of potassium ferrate on activated carbon modification, blank samples were introduced for comparison. After ultrasonic vibration with sulfuric acid, the samples were modified without adding potassium ferrate. The samples were immersed in sulfuric acid under magnetic stirring for 24 h, and then the samples were washed until the discharged water showed a neutral reaction. Finally, the washed samples were transferred to a tube furnace for activation at 800 °C under nitrogen atmosphere for 2 h, and the final sample was named RAC-H2SO4-24h.
2.3 Dynamic toluene adsorption
Typically, toluene was used as adsorbate to test the adsorption performance of RACs samples. The experimental device is shown in Figure 1. The specific experimental procedure is as follows: Before the breakthrough test of toluene, all samples were subjected to heat treatment at 373 K to remove adsorbed water and impurities. Then 0.2 g of the sample was transferred to an 100-mm diameter glass tube reactor. The VOCs vapor is produced by the nitrogen bubble method, which involves the flow of N2gas controlled by a mass flow controller (MFC, D08-3F), resulting in a stable concentration of VOCs vapor. By adjusting the flow rate of the two N2streams, the concentration of VOCs vapor required for the experiment was generated. In this experiment, the gas mixture was introduced into the reaction bed at a flow rate of 50 mL/min. The concentration of vent VOCs was detected by a gas chromatograph (GC, SP-6890).
2.4 Characterization
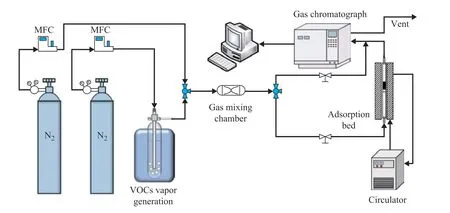
Figure 1 The schematic of toluene adsorption system
Nitrogen adsorption-desorption isotherms were measured by a surface area and porosity analyzer (ASAP2460,USA) at 77 K to analyze the specific surface area and pore size structure of the sample. The specific surface area and pore size distribution was calculated using the Brunauer-Emmett-Teller (BET) method. The surface morphology of RAC and other samples was measured by a scanning electron microscope (SEM, JSM-7500F) operating at 15 kV. The Raman spectra in the range of 800-2000 cm-1were measured by a Takram and Teksan microscope with a 532 nm laser source. The Fourier transform infrared spectrometer WQF-510 was used to determine the functional group composition of RAC samples. The surface chemical composition of porous carbon was analyzed by X-ray photoelectron spectroscopy (XPS)using an Escalab 250Xi instrument. The binding energy was calibrated by C1s (BE = 284.8eV) to compensate for surface changes.
3 Results and Discussion
3.1 Dynamic adsorption performance
The RAC samples were tested for toluene adsorption performance using the detection device shown in Figure 1.The experiment was carried out at 25 °C, the toluene inlet concentration was 1300 μg/g, the flow rate was 50 mL/min, and the mass of adsorbent was 0.2 g.
It can be seen from Figure 2 that the five samples have a similar adsorption trend, but their breakthrough times and equilibration times are quite different. After modification with potassium ferrate, the penetration time was extended from 99 min for the original RAC sample to 260 min for RAC-6% K2FeO4+H2SO4-24h. In addition, the adsorption capacity of VOCs was calculated according to the numerical integration of the breakout curve region. Figure 2(b) shows the dynamic toluene adsorption saturation capacity of five RAC samples. With the extension of potassium ferrate modification time, the toluene adsorption capacity of samples also gradually increased, reaching 375.55 mg/g (RAC-6%K2FeO4+H2SO4-24h), which was twice as high as that of the original RAC sample (192 mg/g). Compared with the blank sample RAC-H2SO4-24h, it was found that the toluene adsorption performance of the sample treated with sulfuric acid for 24 h (295 mg/g) was almost the same as that of RAC-6%K2FeO4+H2SO4-5h treated with potassium ferrate for 5 h (283.6 mg/g). This result also proves that the sample modified by potassium ferrate can improve the adsorption of toluene.
3.2 SEM analysis
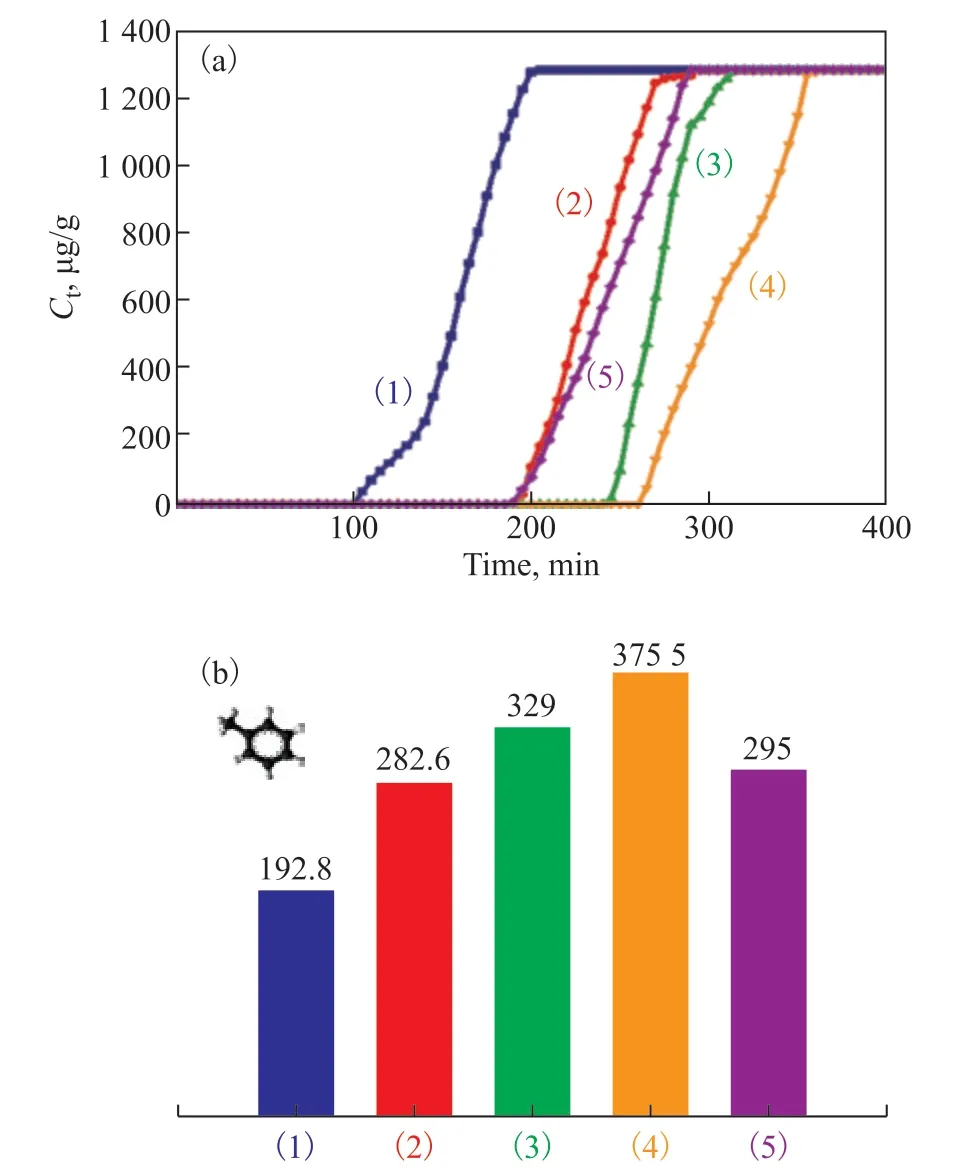
Figure 2 (a) Toluene adsorption breakthrough curve of RAC samples; (b) Toluene dynamic adsorption saturation capacity of RAC samples
A scanning electron microscope (SEM) was used to observe the microscopic morphology and structure of RAC, RAC-H2SO4-24h, and RAC-6% K2FeO4+H2SO4-24h samples. It can be seen from Figure 3(a) that the RAC has a loose surface morphology after being regenerated. Figure 3(b) shows that more irregular block structures are formed on the surface of RAC-H2SO4-24h after only 24 hours of sulfuric acid modification treatment, resulting in a more“gully” structure, which increases the defects of activated carbon. The reason is that acid etching makes the activated carbon react with sulfuric acid (C + 2H2SO4→ CO2+ 2SO2+ 2H2O), and the sulfuric acid etching removes impurities in the activated carbon and forms a larger specific surface area. Figure 3(c) shows the surface structure of the sample RAC-6%K2FeO4+H2SO4-24h. Due to the presence of strong oxidant, the pore size of weak strength in the structure of activated carbon collapses, leading to oxidation and reduction of functional groups on the surface of activated carbon. Therefore, compared with the sample RAC-H2SO4-24h, the defect structure on its surface is more disorderly and the number of defects increases.of nitrogen partial pressures (p/p0> 0.45), the nitrogen adsorption and desorption curves show an obvious IVtype hysteresis loop, which indicates that the sample has multi-layer adsorption. In addition, it can be seen from Figure 4 (b) on pore size distribution of samples that RAC is mainly dominated by mesopores distributed at 11 nm and supplemented by micropores distributed at 1.8 nm, forming unique micro-small mesoporous carbon materials. According to relevant literature, when the pore size of the adsorbent is 1 - 3 times the target molecule, it is beneficial to promoting the adsorption capacity of the target molecule. Since the kinetic diameter of toluene molecule is about 0.7 nm, so the micropores at 1.8 nm in the sample after potassium ferrate modification become the active points of adsorption. The small mesopores at 11 nm can provide good transport channels for toluene molecules to accelerate the mass transfer efficiency.
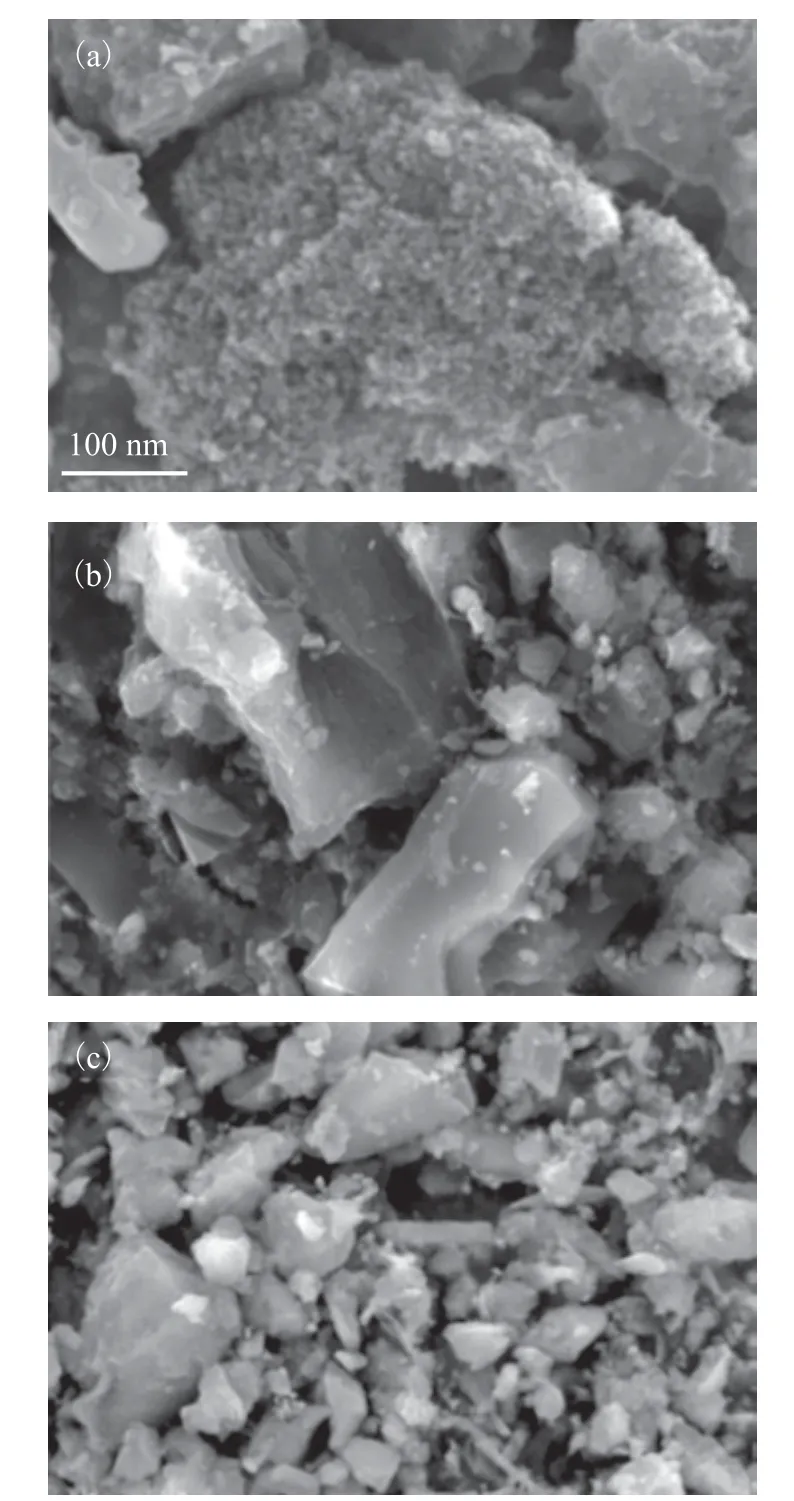
Figure 3 SEM images of different RAC samples: (a)RAC;(b)RAC-H2SO4-24h; (c)RAC-6%K2FeO4+H2SO4-24h
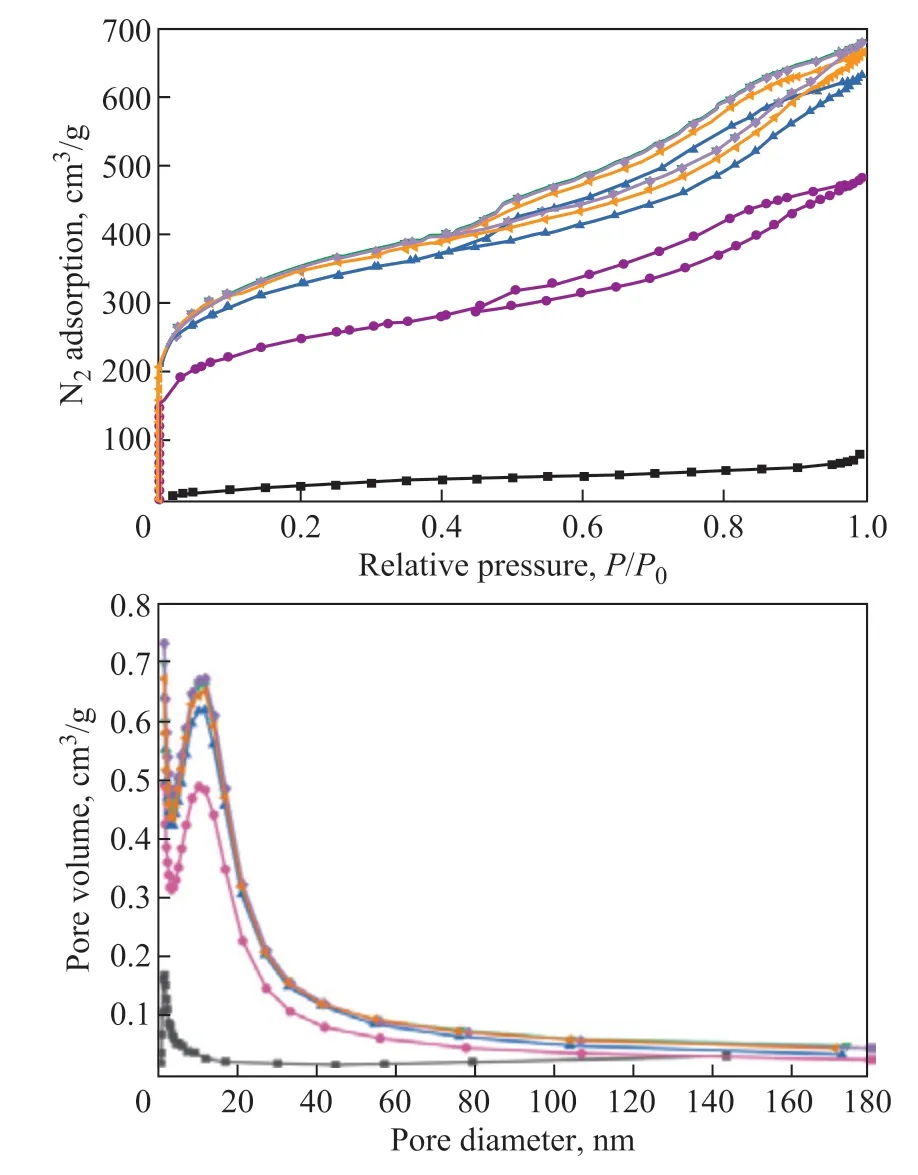
Figure 4 (a) N2 adsorption and desorption isotherms of different RAC samples; (b) Pore size distribution of RAC samples
3.3 Pore structure analysis
In order to further study the influence of pore size structure of regenerated and potassium ferrate modified RAC on toluene adsorption capacity, nitrogen adsorption and desorption experiments were conducted on six samples, and the nitrogen adsorption and desorption isotherms were obtained, as shown in Figure 4(a). The basic pore size parameters of six activated carbons were obtained from isotherms (Table 1).
By comparing the nitrogen adsorption curve, it can be found that the waste activated carbon has a weak nitrogen adsorption capacity, while the adsorption capacity of the sample modified by RAC and potassium ferrate increases greatly. At a very low partial pressure of nitrogen (p/p0< 0.1), the nitrogen adsorption capacity increases rapidly almost along a straight line,indicating a monolayer adsorption. With the increase
Table 1 shows the pore size parameters of six RAC samples. The specific surface area and pore volume of RAC modified by potassium ferrate increased significantly, which was about 1.41 times higher than that of activated carbon after regeneration, providing a favorable physical structure for toluene adsorption.It is worth noting that the physical parameters of the four samples modified by potassium ferrate and sulfuric acid are very similar, and the specific surface area and pore structure are about 1200 m2/g and 1.06 cm3/g,respectively, while the adsorption capacity for toluene is different. This indicates that the optimization of physical structure is one of the reasons for improving toluene adsorption capacity, and the essential influencing factor is that the addition of potassium ferrate changes the surface functional group content and the surface chemical properties of activated carbon, resulting in the difference of toluene adsorption capacity.
3.4 Raman and FT-IR spectroscopy
In this study, different RAC samples were characterized by Raman spectrometry, and the results were analyzed as shown in Figure 5(a). All samples showed a peak D at 1350 cm-1and a peak G at 1580 cm-1[16]. In general,the occurrence of peak D is attributed to the presence of amorphous or disordered carbon (related to the sp3defect pattern)[17], while the peak G corresponds to the in-plane vibration of the C-C double bond, which is related to the sp2form of the carbon atom[18]. The intensity ratio of peak D to peak G (ID/IG) reflects the degree of disordered carbon and ordered graphitized carbon in carbonaceous materials[19]. The higher theID/IGratio, the more defect sites and disordered carbon in the sample. The results of Raman spectroscopic analysis showed that theID/IGratio of RAC, RAC-H2SO4-24h, RAC-6%K2FeO4-5h, RAC-6%K2FeO4-12h and RAC-6%K2FeO4-24h was 0.94, 1.02,1.05, 1.10 and 1.18, respectively. This outcome shows that the modification of strong oxidant leads to a stronger disorder degree in RAC, and the disorder degree is more obvious with the prolongation of treatment time.
As shown in Figure 5(b), the functional group composition of RAC samples was analyzed by infrared transmission characterization. The band at 3221 cm-1is attributed to O-H stretching vibration (related to phenolic hydroxyl group)[20], and the transmission peak at near 1450 cm-1corresponds to the stretching vibration of COO- group[21]. The strong peak at 981 cm-1is related to C-O stretching vibration of lactone, phenol and ether group[22]. Similarly, the characteristic peak at 500 cm-1-800 cm-1is attributed to the vibration of C-H bond in aromatic group[23]. It can be seen from Figure 5(b) that the strength of C-O peak gradually increases with an increasing time for modifying RAC by strong oxidant potassium ferrate, indicating that the oxidation process can effectively increase the oxygen-containing group of activated carbon. In addition, the intensity of C-H peak also increased gradually, indicating that the addition of potassium ferrate inhibited the decomposition of some straight chain molecules.
3.5 XPS spectroscopy
In order to explore the relationship between enhanced toluene adsorption performance and surface chemical properties after RAC modification, the surface chemical composition and content changes of activated carbon were further analyzed by XPS characterization. The relative content of each element on the surface of RAC and modified samples was calculated according to XPS full scanning spectrum (Table 2). By comparing the molar ratio of O/C, it can be seen that the O/C ratio of samples modified by potassium ferrate decreases gradually, indicating that RAC-6%K2FeO4+ H2SO4-24h has better aromatic structure and non-polar surface, which is one of the factors for improving the adsorption performance of weakly polar toluene molecules. However, by comparingthe surface nitrogen content of the four samples, it can be found that there is a good linear relationship between toluene adsorption performance and nitrogen content. As shown in Figure 6(a), the fitting degree reaches 99%, indicating that the amount of nitrogen content on the sample surface can affect the adsorption performance of toluene.
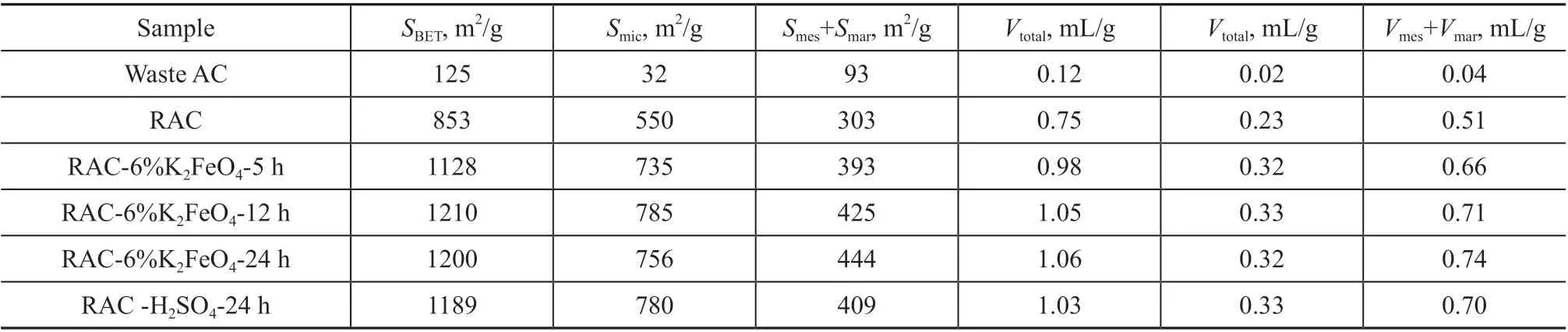
Table 1 List of pore size structure parameters of RAC samples
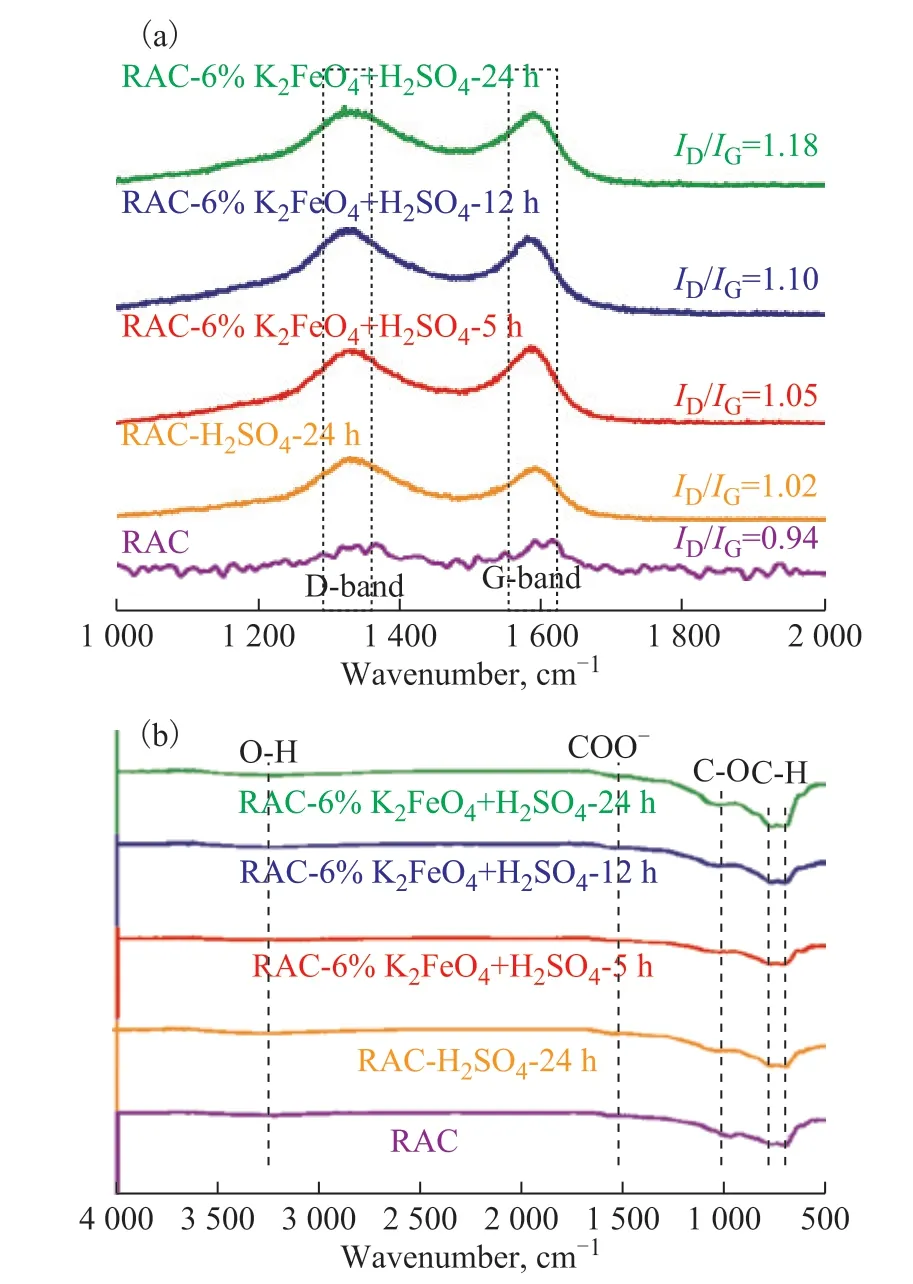
Figure 5 (a) Raman spectra of different RAC samples; (b)FT-IR spectra of different RAC samples
The nitrogen spectrum peak analysis of RAC-6%K2FeO4+H2SO4-24h with a highest nitrogen content shows that there are four different surface nitrogencontaining functional groups (Figure 6(b)), viz.: pyridinelike nitrogen (N-6, 398.1-398.8 eV), pyridine-like nitrogen (N-5, 400.0-400.4 eV), pyridine-like nitrogen(N-Q, 401.0-401.2 eV), and protonated pyridine-like nitrogen (N-x, 402.5-402.8 eV), respectively. Pyridine nitrogen (N-6) exists as a six-membered ring at the edge of the graphite layer of biochar and has a pair of unbonded lone pair electrons. Therefore, it has strong electron-donating ability and can effectively increase the alkalinity of the material. The graphitized nitrogen (N-Q)is located in the middle of the graphite layer of biomass carbon, which is formed by replacing a carbon atom in the original six-membered ring by nitrogen atoms.The graphitized nitrogen atoms form delocalized large π bond with the six-membered ring in the form of sp2hybridization, which can increase the conductivity of the material. These nitrogen-containing functional groups can enhance the toluene adsorption performance.
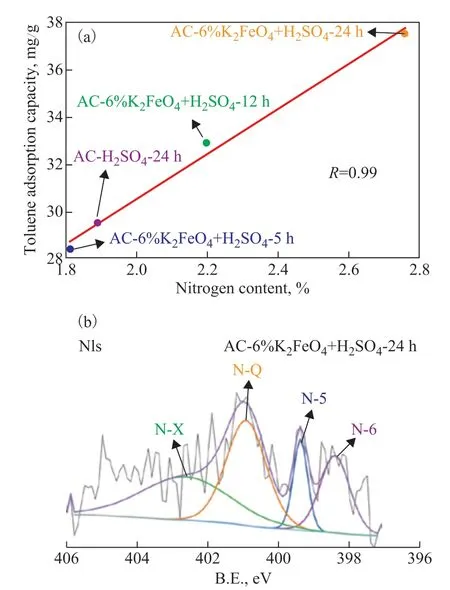
Figure 6 (a) The relationship between nitrogen content and toluene adsorption performance of RAC samples; (b) N1s high-resolution image of RAC-6%K2FeO4+H2SO4-24h
Separate C1s and P2p spectra were further de-integrated,and the relative contents of functional groups in the prepared biochar were recorded (Table 3). The measured spectra are mainly shown as the deconvolution spectra of C1s and O1s, as shown in Figure 7. The C1s spectra of RAC samples (Figure 7(a)) show five visible peaks[24]: (i) C-C peaks derived from sp2-C and sp3-C(284.0 eV-285.1 eV); (ii) C-O peaks of phenols and ethers (285.3 eV-286.3 eV); (iii) C=O peak of carbonyl or quinone (286.8 eV-288.1 eV); (iv) O=C-O peaks corresponding to ester, carboxyl, and anhydride groups(288.5 eV-290.0 eV), and (v) π-π*transition peaks(290.2 eV-292.1 eV). According to the integral results shown in Table 3, the content of π-π* functional groups increased with the increase of potassium ferrate treatment time. However, the dispersion force of π-π* functional group can increase the intimacy between the non-polar adsorbent and the weakly polar molecule of toluene,and attract more toluene molecules, thus enhancing the toluene adsorption performance of RAC samples.

Table 2 List of elemental compositions of different RAC samples
In the O1s spectrum, five different groups of oxygen can be identified (Figure 7(b)): (i) the C=O group assigned to quinones (531.0-531.3 eV), (ii) the C=O group attributed to esters and anhydrides (532.4 eV-532.7 eV); (iii) the non-carbonyl oxygen C-O group contained in anhydrides,esters, and phenols (533.2 eV-533.5 eV), (iv) the oxygen atoms in acidic COOH groups (534.5 eV-535.3 eV); and(v) the oxygen atoms in adsorbed water/oxygen (535.6 eV-537 eV)[25]. It can be seen from Table 3 that due to the addition of potassium ferrate, the inert carbonyl C=O group breaks bonds and is transformed into ester and anhydride C=O group and non-carbonyl oxygen in C-O group. According to previous reports, the basic functional groups on the surface of activated carbon can help to improve the adsorption capacity of activated carbon for non-polar or weakly polar molecules. The XPS results showed that the increase of oxygen-containing functional groups was consistent with the results of FT-IR analysis,and the increase of basic oxygen-containing functional groups improved the toluene adsorption capacity of RAC.
3.6 Static adsorption performance
In this study, three typical adsorbents (RAC, RAC-H2SO4-24h and RAC-6%K2FeO4+H2SO4-24h) were studied in static adsorption tests. As shown in Figure 8(a), with the increase of toluene vapor partial pressure, the toluene adsorption capacity of the three sorbents also increased gradually, indicating the occurrence of multilayer adsorption.
As shown in Figure 8 (b), the maximum adsorption capacity at a partial pressure of 0.95 was drawn as a histogram. It was found that the maximum adsorption capacity of RAC-6%K2FeO4+H2SO4-24h was 796 mg/g,indicating that the activated carbon prepared in this study had a strong toluene adsorption capacity. In addition,it is found that the maximum adsorption capacity of RAC-H2SO4-24h and RAC-6%K2FeO4+H2SO4-24h is not significantly different, which may be due to the same physical structure (specific surface area and pore size structure) of the two adsorbents. Therefore, it is speculated that the static adsorption is mainly affectedby the physical structure of the adsorbent, while the adsorption process (dynamic adsorption) in the actual environment is affected by both the physical structure and the surface chemical properties.
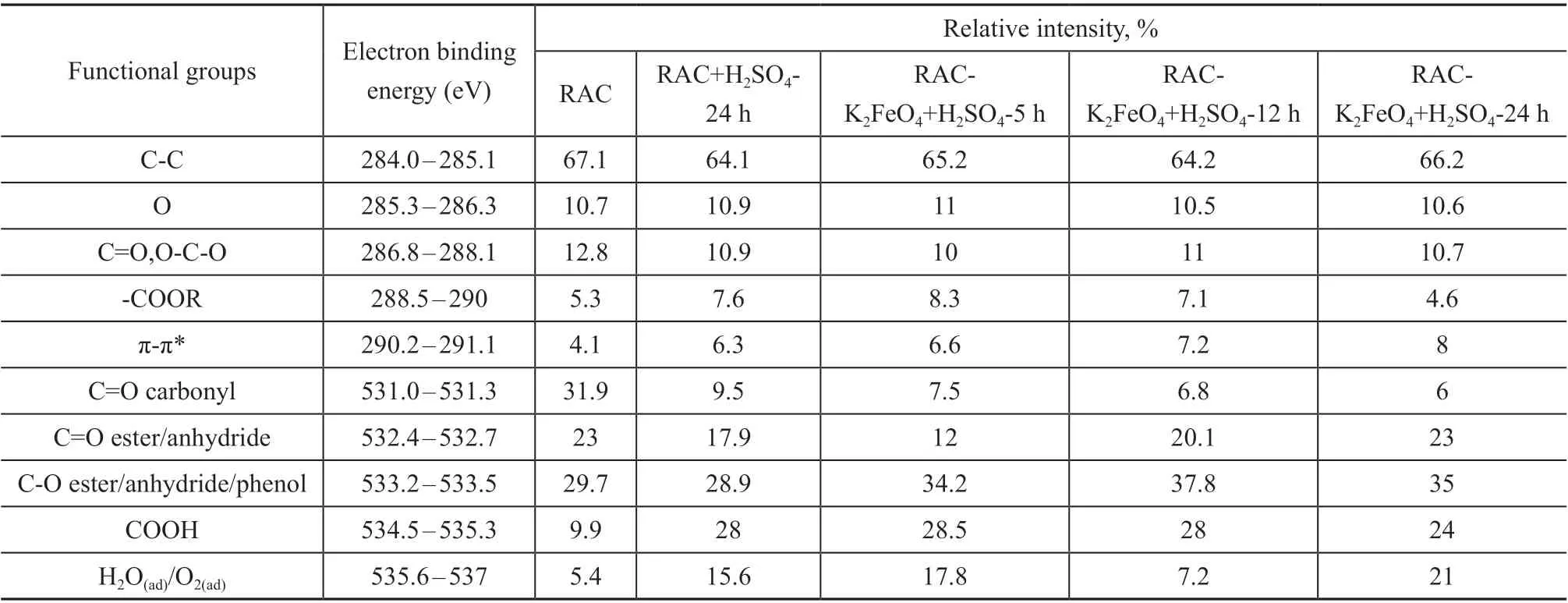
Table 3 List of relative contents of C1s and O1s functional groups in RAC samples
3.7 Recyclability of samples
Further, the toluene adsorption and desorption cycling experiments were carried out for three typical adsorbents(RAC, RAC-H2SO4-24h, RAC-6%K2FeO4+H2SO4-24h) in this study. Each saturated activated carbon was degassed in nitrogen atmosphere at 150 °C for 6 h, and then toluene adsorption experiment was repeated under initial conditions (1300 μg/g, 25 °C, 50 mL/min), and the data of final adsorption saturation were summarized. As shown in Figure 9, the sample RAC-6%K2FeO4+H2SO4-24h still retained 91% of the adsorption performance after the fifth regeneration cycle, while the adsorption performance of RAC and RAC-H2SO4-24h dropped to 86% and 80%, respectively. The degradation in the adsorption performance of nanoporous carbon can be mainly attributed to the following two reasons: a) the destruction of the pore structure or the functional groups at such a high regeneration temperature, and b) the incomplete degasification of VOCs molecules during the regeneration process[26]. According to the experimental results, the RAC-6%K2FeO4+ H2SO4-24h not only has strong toluene adsorption capacity, but also has high toluene cyclic adsorption capacity. Therefore, RAC-6%K2FeO4+ H2SO4-24h is a promising VOCs adsorption material with good cyclic energy and long lifetime industrial practicability.
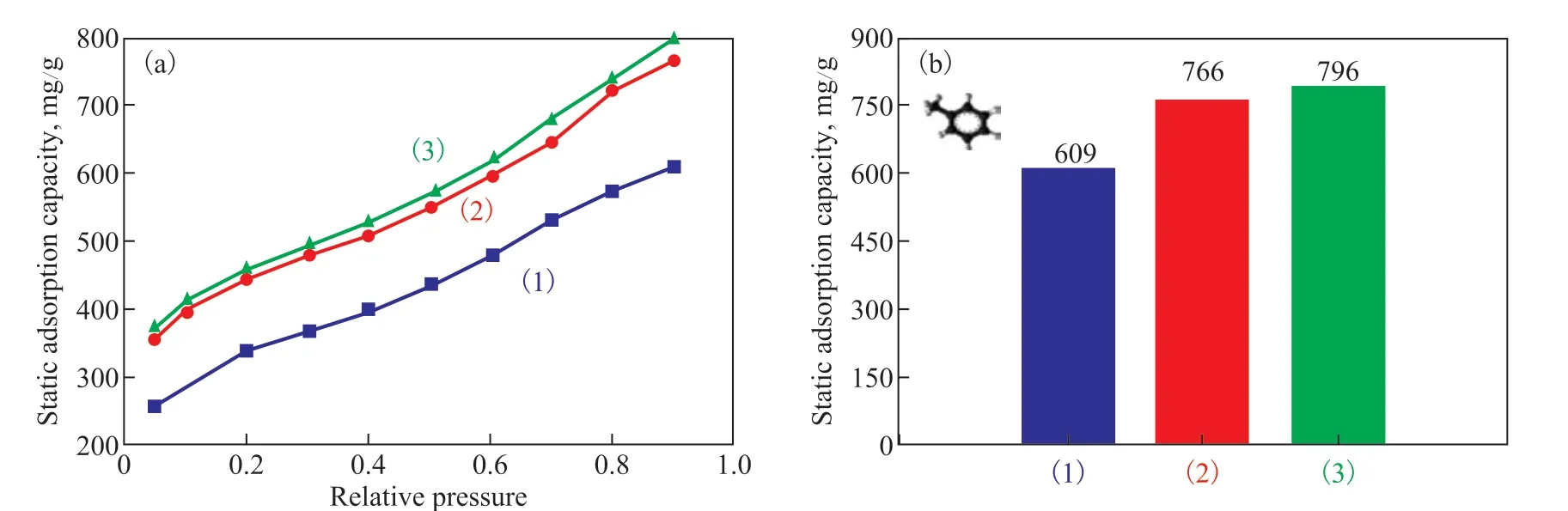
Figure 8 (a) Static adsorption isotherm of RAC sample; (b) Static adsorption saturation capacity of RAC samples
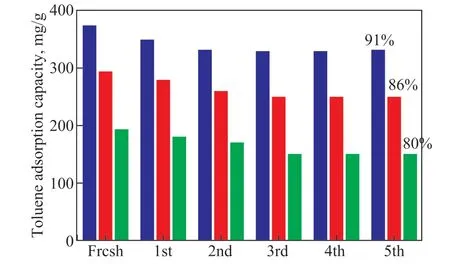
Figure 9 Toluene adsorption cycle performance test of RACs
4 Conclusions
The goal of this study is to efficiently remove toluene waste gas by synthesizing low-cost adsorbents with excellent adsorption capacity. Using waste activated carbon from solid waste resources as carbon source,the high-performance VOCs adsorption carbons were prepared by virtue of self-developed mesoporous/macroporous reconstruction, catalytic graphitization and other preparation technologies, and were further modified by potassium ferrate. Through the analysis of experimental characterization results,it was found that the specific surface area (853 m2/g) and pore size structure of the RAC-6%K2FeO4+H2SO4-24h sample modified by potassium ferrate improved 1.4 times as compared with the original RAC sample. With the increase of potassium ferrate modification time, the surface π-π* dispersion force, the content of basic functional groups C=O (anhydride, ester and phenolic non-carbonyl oxygen group) and nitrogenous functional groups in RAC and other samples would also increase.These factors all lead to a significant improvement in the toluene adsorption capacity of RAC-6%K2FeO4+H2SO4-24h. The dynamic adsorption saturation capacity of RAC-6%K2FeO4+H2SO4-24h reaches 375.5 mg/g, which is twice as high as that of the original RAC (192.8 mg/g), and its static adsorption saturation capacity reaches up to 796 mg/g. Moreover, multilayer adsorption occurs in the sample during the adsorption process. After the fifth regeneration, RAC-6%K2FeO4+H2SO4-24h sample still retains 91% of adsorption activity, indicating that the adsorbent has good cycling stability and has great application value for the efficient purification of industrial VOCs waste gas.
Acknowledgments:This work was financialy supported by the National Natural Science Foundation of China(No.21936005,52070114, 21876093), and the Postdoctoral Science Program of China (No. 2019M660061).
杂志排行
中国炼油与石油化工的其它文章
- Study on Viscosity Reducing and Oil Displacement Agent for Water-Flooding Heavy Oil Reservoir
- Electrospinning Nanofiber Membrane Reinforced PVA Composite Hydrogel with Preferable Mechanical Performance for Oil-Water Separation
- Antibacterial and Corrosion Inhibition Properties of SA-ZnO@ODA-GO@PU Super-Hydrophobic Coating in Circulating Cooling Water System
- Investigation of Nitrite Production Pathway in Integrated Partial Denitrification/Anammox Process via Isotope Labelling Technique and the Relevant Microbial Communities
- Heteroatom-Doped Carbon Spheres from FCC Slurry Oil as Anode Material for Lithium-Ion Battery
- Study on Low-Temperature Properties of the Asphalt Modified by Carbon Nanotubes (CNTs) and Crumb Rubber (CR)
