Electrospinning Nanofiber Membrane Reinforced PVA Composite Hydrogel with Preferable Mechanical Performance for Oil-Water Separation
2022-04-14GaoDeyuLiuZanChengZhilin
Gao Deyu; Liu Zan; Cheng Zhilin
(School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
Abstract: Nowadays hydrogels have been attracting the massive interest in oil-water separation due to their robust hydrophilicity and fantastic underwater oiliness features. However, the weak toughness and tensile strength shortcomings of hydrogels have thus inhibited their actual applicability. For this reason, we successfully fabricated the electrospun nanofiber membrane-reinforced PVA composite hydrogels. The PVA-PAN composite hydrogel has exhibited the excellent tensile strength and friction performance, separately enhancing 174.2% of the tensile strength, and reducing 20.7% of the friction coefficient and 58.7% of wear volume relative to the neat PVA hydrogel. Furthermore, the pull-out experiments indicated that the PAN nanofiber membrane exerted a stronger interface bonding effect with PVA hydrogel. The oil-water separation evaluation test showed that the separation efficiency reached up to 97.6% for treating the SA-100 lubricating oil/water system.
Key words: wear and tribology; nanoparticles; PVA hydrogel; electrospun fiber; oil-water separation
1 Introduction
With a growing concern for underwater activities in oil-contaminated aqueous environments, the interface materials with super hydrophilicity or hydrophobicity have attracted great attention on oil-water separation[1-2].Traditional oil removal methods, such as coagulation[3],air floatation[4], flocculation[5], and biological treatment[6],had the disadvantages of complex operation, low separation efficiency, and high operating cost. The field of oil-repellent materials has expanded from classic selfcleaning applications to more social problems[7].
In the past decade, a large number of materials have been successfully prepared to separate oil/water mixtures[8].Thereof, hydrogels are three-dimensional cross-linked polymers, which have been studied for oil-water separation due to their high hydrophilicity[9-10]. Among the well-defined hydrogels, polyvinyl alcohol (PVA)hydrogel has been widely used as an easily obtained soft material with high water content and hydrophilic polymer network structure for oil-water separation[11-12]. Fan, et al.[13]reported that the PVA-coated cellulose filter paper using glutaraldehyde (GA) as the crosslinker exhibited a strong ability to separate the oil/water mixtures even in strongly acidic, alkaline, and salty environments. Because of their poor mechanical property, this disadvantage has restricted the wide application of hydrogel materials in oil-water separation[14]. Liu, et al.[15]fabricated the crosslinked double-network PVA/P(AM-co-AA)/CS hydrogel(HEPC-Gel) with an excellent oil-water separation ability(having a separation efficiency of >99%). However, the preparation of these PVA composite hydrogels filled with macromolecules showed the complicated processing process conducted under harshly controlled conditions.
Owing to the unique 3D network structure, high porosity and excellent mechanical properties, the electrospun nanofiber membranes were also employed for oil-water separation[16-19]. Inspired by the advantages of electrospun nanofiber membranes, we have fabricated the sandwichstructured electrospun nanofiber membranes-filled PVA composite hydrogels in terms of the self-made PVA,PAN, and PMMA nanofiber membranes. The mechanical performance and oil-water separation efficiency of the asprepared composite hydrogels were tested.
2 Experimental
2.1 Materials
Polyacrylonitrile (PAN) and polymethyl methacrylate(PMMA) were provided by the Jiangsu SINVOCHEM Chemical Corp. Poly(vinyl alcohol) (PVA) was purchased from the Sinopharm Chemical Reagent Co., Ltd (China).SN-100 oil was purchased from the local supermarket.
2.2 Preparation of composite hydrogel
First, the preparation of the PVA, PAN, and PMMA electrospun nanofiber membranes was performed according to our previous report[20]. Then, two portions containing 40 mL of 10% PVA aqueous solution each were prepared. One portion of PVA solution thereof was poured into a self-made mold, which was subsequently frozen at -20 °C for 1 h and was afterwards thawed out at room temperature for 1 h. Next, the self-made 15 cm×15 cm PVA, PAN, and PMMA electrospun nanofiber membranes were put on the upper surface of the abovementioned PVA hydrogel, and the another portion of PVA solution was poured onto the membrane, which was successively frozen at -20 °C for 10 h and then was thawed out at room temperature for 2 h. The abovementioned operation was repeated at least 5 times,resulting in the PVA-electrospun nanofiber membranes composite hydrogels (denoted respectively as PVA-PVA,PVA-PAN, and PVA-PMMA), as shown in Scheme 1.
2.3 Characterizations and testing
The morphology of composite hydrogel was characterized using a scanning electron microscope (SEM, type Zeiss-Supra 55, Germany) operating at an accelerating voltage of 10.0 kV. The tribological behavior analysis of the composite hydrogel was conducted on a ball-on-slider reciprocating MFT-5000 type multi-function friction and wear tester (Rtec-Instruments, USA). The mechanical properties of hydrogels were tested on an electronic universal testing machine with a strain rate of 50 mm/min(Instron-3367, USA). The shape of the sample was tailed to form a dumbbell (46 mm×6 mm×2 mm). The formula of water content and swelling ratio of hydrogel are shown as follows:
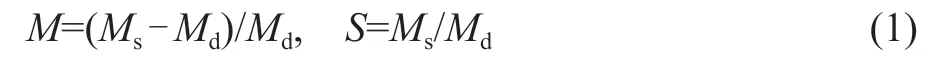
whereMsis the mass of wet hydrogel;Mdis the mass of dry hydrogel;Mis the water content;Sis the swelling ratio.
The separation efficiency (R) equation is presented below:

whereMarepresents the mass of water after separation andMbrepresents the mass of water before separation,respectively. At the same time, the time required for the whole separation process is ΔT(s). The effective area of the separation system isS(cm2), and the permeate flux (P)is defined as:

whereV(mL) is the volume of the water passing through the PVA-PAN hydrogel for the whole separation process.
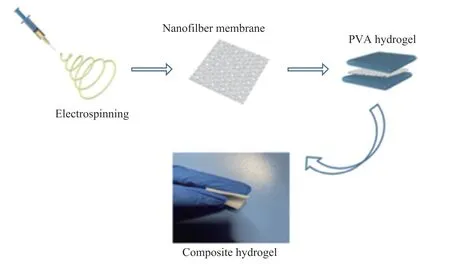
Scheme 1 The fabrication of the nanofiber membrane reinforced PVA composite hydrogels
3 Results and Discussion
Figure 1 shows the surface morphology and diameter distribution of the self-made PVA, PAN, and PMMA electrospun nanofiber membranes before and after immersion in water for 24 h. Obviously, three types of the nanofiber membranes have a typical nonwoven structure,which possesses high porosity and non-uniform pore size distribution[21]. Moreover, the overlapping nanofibers are randomly arranged, and the surfaces are smooth without humps. Before immersion in water, the average diameter of PVA, PAN, and PMMA nanofibers is approximately concentrated at 269.12 nm, 256.3 nm, and 357.8 nm,respectively. However, after immersion in water, the PVA nanofibers would suffer from disintegration owing to the hydrolysis of the substantial hydrophilic groups on the surface of PVA fiber[22], whereas the surface morphology of PAN and PMMA nanofibers could retain the intact structure.
Figure 2 displays the cross-sectional SEM images of the neat PVA hydrogel and three types of the composite hydrogels. The PVA-PAN shows a more compact interface structure than another two composite hydrogels.The PVA hydrogel is fully penetrated into the pores of nanofibers while being apt to yield the better interface contact, with which the nitrile group of PAN acts as a hydrogen bond acceptor to form hydrogen bonds,resulting in a close connection between PAN and PVA hydrogels[23]. However, the PVA fibers readily undergo hydrolysis reaction in PVA hydrogel, therefore leading to a poor enhanced ability (Figure 2 (a)-(b)). Meanwhile,thanks to the weak interfacial interaction of PMMA fibers with PVA hydrogels, the PVA-PMMA hydrogel displays the distinct interface and loose structure between PVA hydrogel and PMMA fibers.

Figure 1 The SEM images before immersion in water (a,d,g), diameter distribution (c,e,h) and SEM images after immersion in water (c,f,i) of PVA (a-c), PAN nanofiber (d-f) and PMMA nanofibers (g-i )
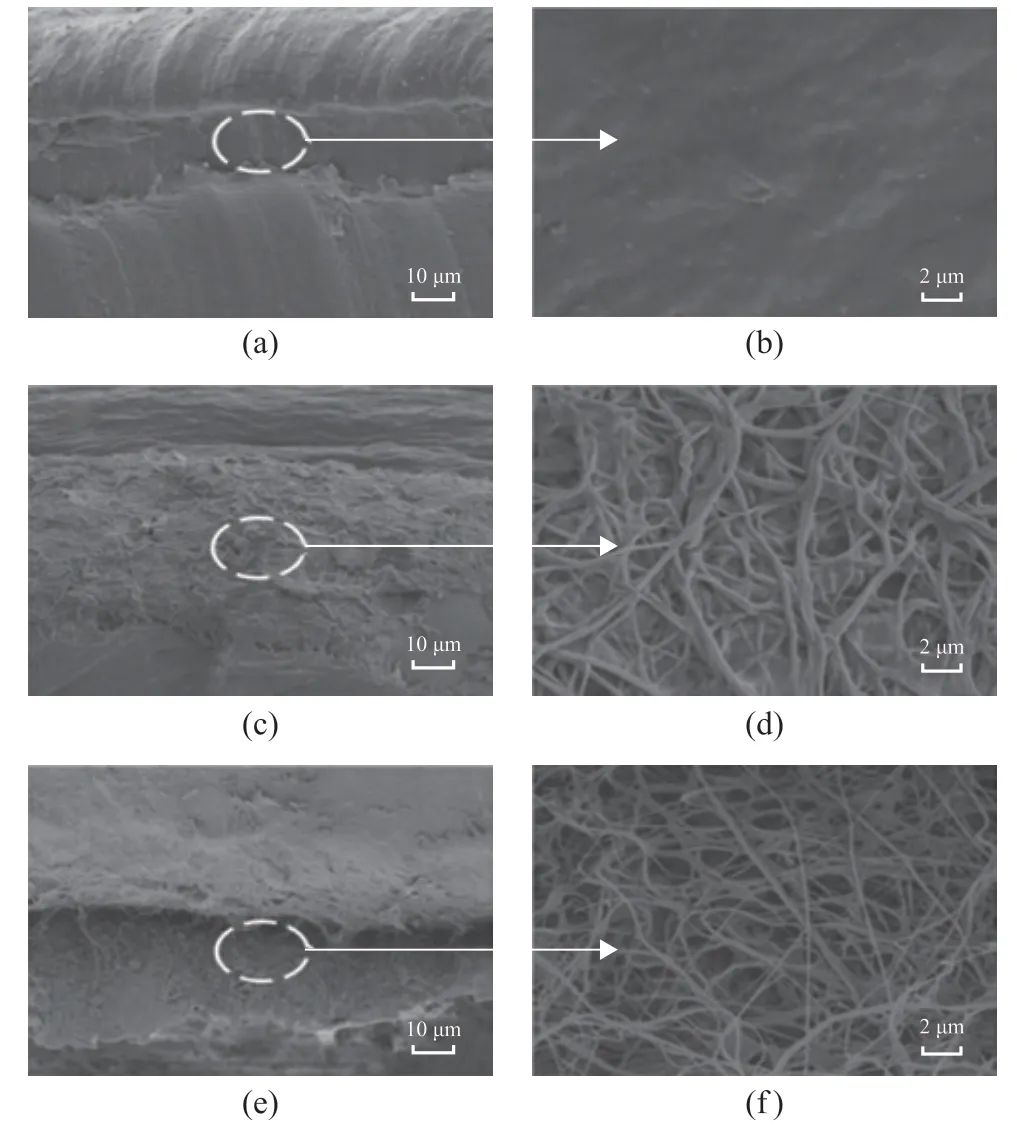
Figure 2 SEM images of (a, b) PVA-PVA, (c, d) PVA-PAN,and (e, f) PVA-PMMA
Figure 3 depicts the mechanical properties of the neat PVA hydrogel and three types of composite hydrogels. Among these hydrogels, the PVA-PAN hydrogel has the largest tensile strength reaching up to 1.79 MPa, while keeping an elongation at break of 158.8%, which is ascribed to the excellent mechanical properties and low elongation at break of PAN fibers[24-25]. Furthermore, the enhanced mechanical property of PVA-PAN is likely attributed to the mechanical percolation effect between PAN and PVA chains. The percolation effect, which can create firm interactions between PAN and PVA, has promoted the formation of a stiff interconnected network structure to resist the tensile process[26]. To further understand the bonding strength between nanofiber membrane and PVA hydrogel, we implemented the extraction testing of the nanofiber membrane from the hydrogel (Figure 3 (c), (d)).The results show that the PAN nanofiber membrane has the largest pull-out strength of the PVA-PAN hydrogel among three composite hydrogels, because a structure with more interface bonding effect exists between the PVA hydrogel and the PAN nanofiber membrane.
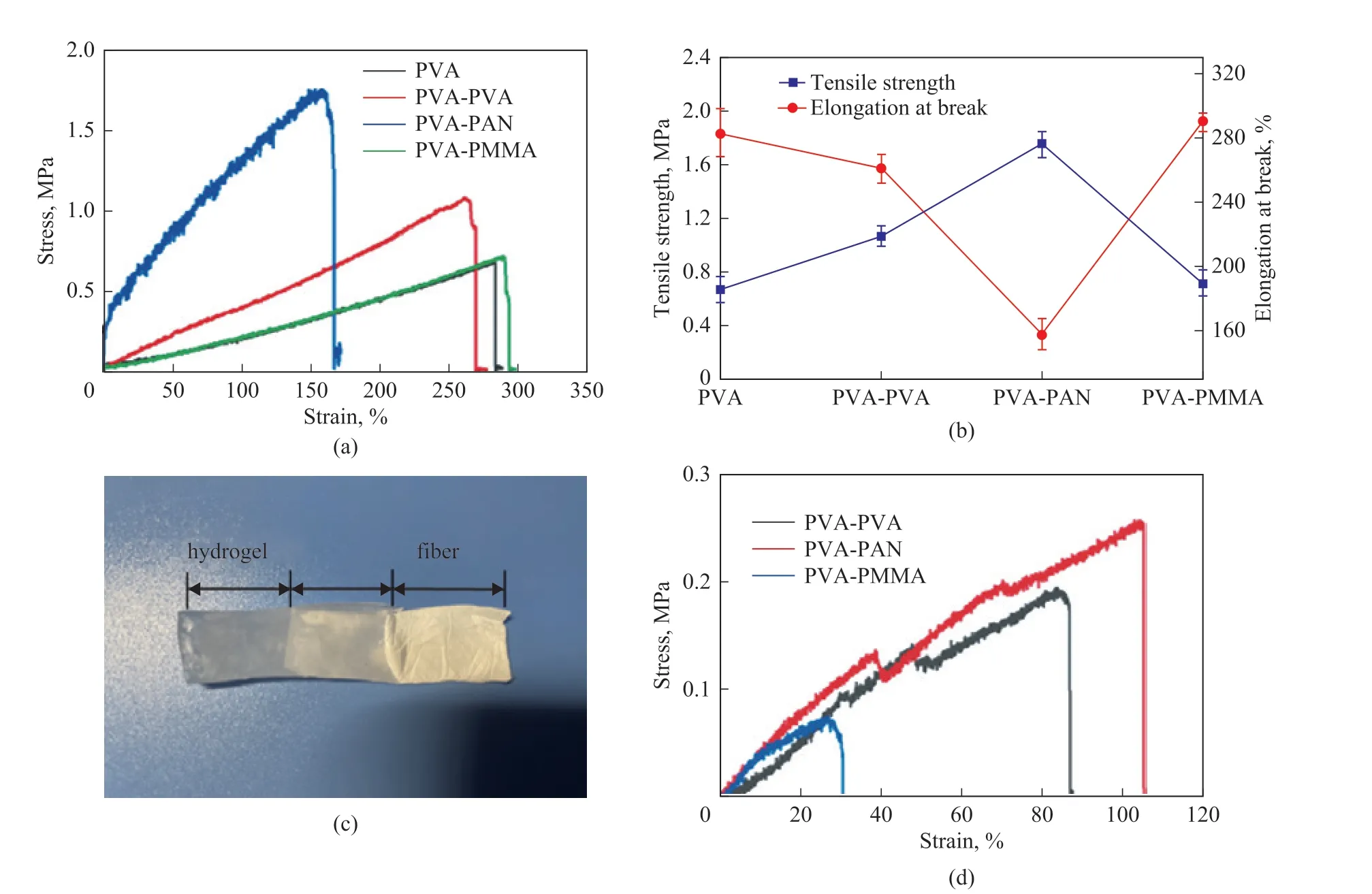
Figure 3 Mechanical performance of the neat PVA and composite hydrogels: (a) tensile strength; (b) elongation at break; (c)pull-out physical picture; (d) pull-out tensile strength
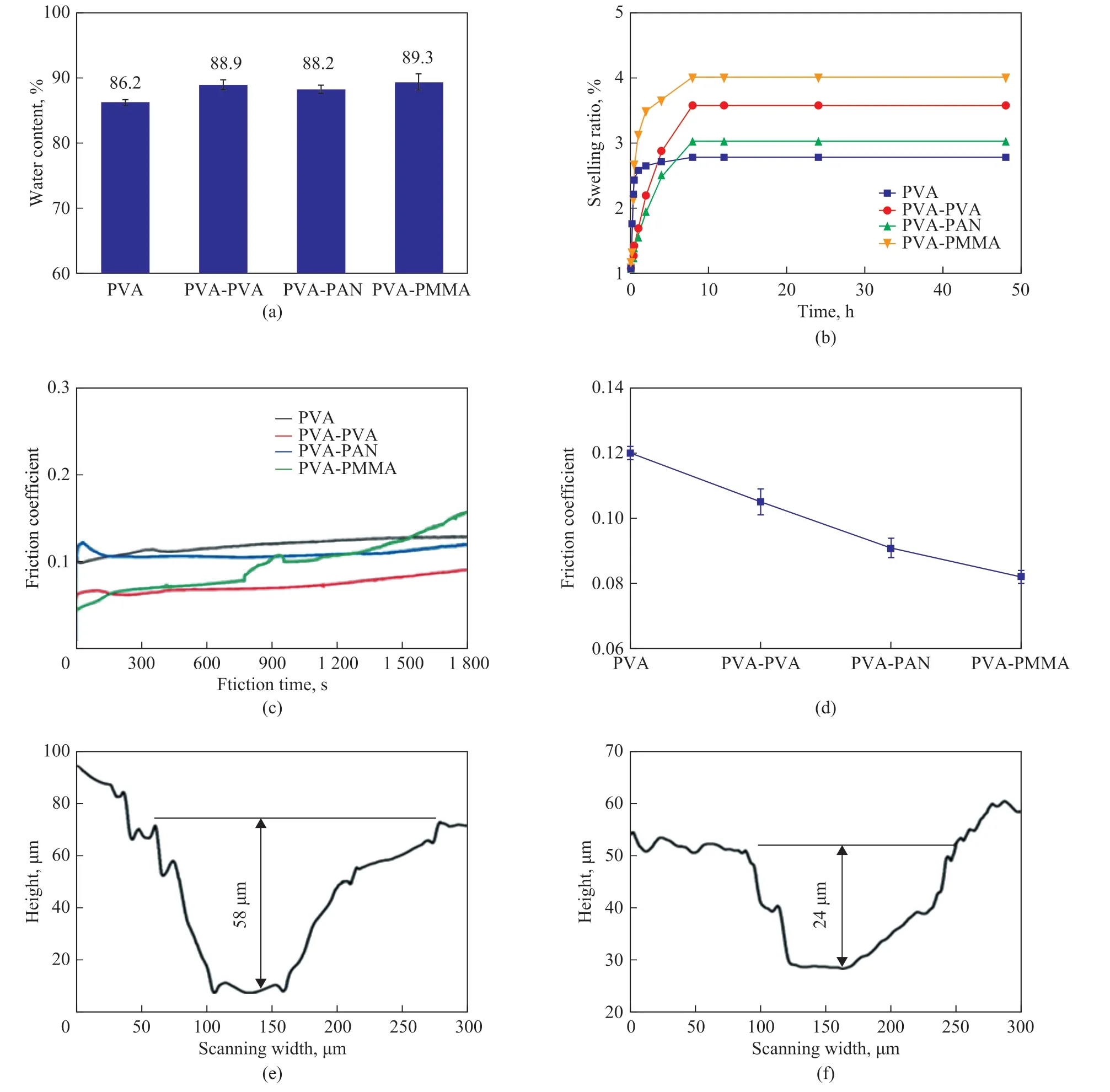
Figure 4 The water content (a), swelling ratio (b), the friction coefficients depending on time (c), and average friction coefficients (d) of PVA, PVA-PVA, PVA-PAN, and PVA-PMMA composite hydrogels; wear of PVA hydrogel (e); and wear of PVA-PAN (f)
As shown in Figure 4 (a), (b), the water content and swelling ratio of the neat PVA hydrogel are the smallest.More interestingly, the PVA-PAN composite hydrogel has the smallest water content and swelling ratio among three composite hydrogels, which is favorable to the oilwater separation[15]. Figures 4 (c), (d) illustrate the friction performance of the neat PVA hydrogel and three types of the composite hydrogels. Surprisingly, only the PVAPMMA cannot attain the steady state throughout the test duration. Other than PVA-PMMA composite hydrogel,the friction coefficients (COFs) of PVA, PVA-PVA, and PVA-PAN composite hydrogels can reach 0.12, 0.08, and 0.09 under a load of 5 N after attaining a steady state,respectively. The friction coefficient of the PVA-PAN is decreased by 20.7% compared to the neat PVA hydrogel,which should be ascribed to the formation of a more compact interface structure of PVA hydrogel with the PAN nanofiber membrane[27]. During the friction process,the indentation degree of the pressure head on the composite hydrogel is small, which leads to the decrease of the contact area, and would finally lead to the decrease of the friction coefficient[28]. The two-dimensional crosssectional profile perpendicular to the sliding direction of the neat PVA and PVA-PAN hydrogels is displayed in Figure 4 (e), (f). This result indicates that the wear volume of the PVA-PAN hydrogel is by 58.6% less than that of pure PVA hydrogel, which is attributed to the contact volume reduction between the friction counterpart and the composite hydrogel on account of the more compact interface structure[29].
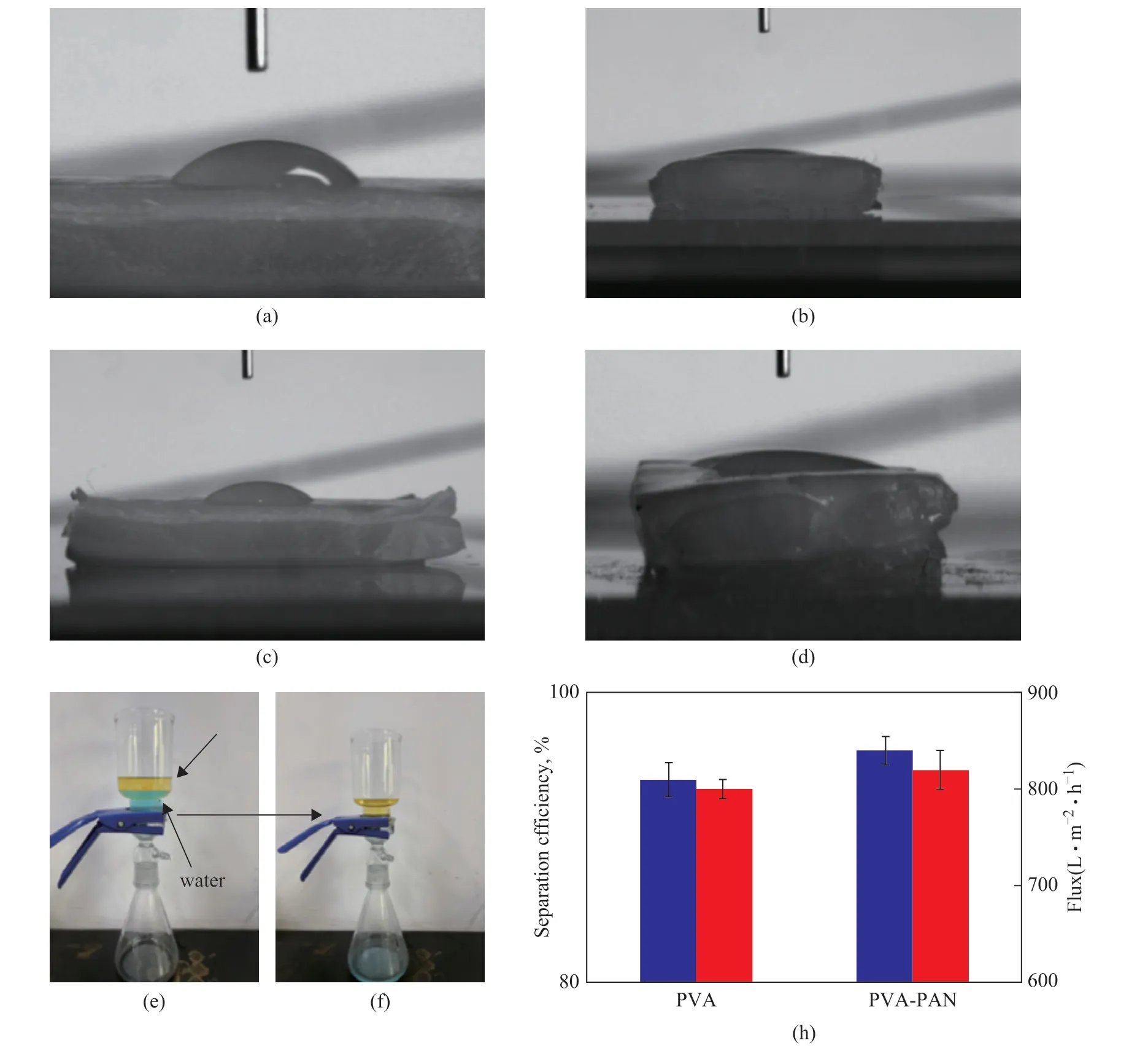
Figure 5 Contact angles of: (a) PVA, (b) PVA-PVA, (c) PVA-PAN, and (d) PVA-PMMA; (e, f) the initial and ending state of separation process; (h) separation efficiency and flux of neat PVA and PVA-PAN hydrogels
To our knowledge, the wettability of the composite hydrogel could exert an outstanding effect on the oilwater separation capacity, and the wettability was investigated first. According to the contact angle values of these hydrogels (Figure 5(a) - (d)), the wettability of the PAN-PVA composite hydrogel is optimum, suggesting that it is caused by the preferable hydrophilicity. The oilwater separation experiments are conducted by using a lubricant (SN-100) and water mixture (with an oil-water volume ratio of 1:1) (Figure 5(e), 5(f)). As shown in Figure 5h, the PVA-PAN hydrogel for oil-water separation has a separation efficiency of 97.6% and the permeation flux of PVA-PAN hydrogel reaches up to 820 L/(m2·h),which is obviously higher than the PVA hydrogel.
4 Conclusions
Above all, the electrospun nanofiber membranereinforced PVA composite hydrogels were successfully constructed in line with the design of sandwich structures.Among these composite hydrogels, the PVA-PAN composite hydrogel exhibited the preferable mechanical performance, in which the largest tensile strength was equal to 1.79 MPa, while achieving an elongation at break of 158.8%. At the same time, the PVA-PAN hydrogel for oil-water separation had a separation efficiency of 97.6%,and the permeation flux of PVA-PAN hydrogel reached up to 820 L/(m2·h).
Acknowledgment: This work was funded by the Jiangsu Yangzhou University Graduate Practice Innovation Program(XSJCX19-064) and the Jiangsu Provincial Colleges and Universities First-Class Project Program (PPZY2015B112). The data in this article were provided by the Test Center of Yangzhou University.
杂志排行
中国炼油与石油化工的其它文章
- Study on Viscosity Reducing and Oil Displacement Agent for Water-Flooding Heavy Oil Reservoir
- Preparation of Solid Waste-Based Activated Carbon and Its Adsorption Mechanism for Toluene
- Antibacterial and Corrosion Inhibition Properties of SA-ZnO@ODA-GO@PU Super-Hydrophobic Coating in Circulating Cooling Water System
- Investigation of Nitrite Production Pathway in Integrated Partial Denitrification/Anammox Process via Isotope Labelling Technique and the Relevant Microbial Communities
- Heteroatom-Doped Carbon Spheres from FCC Slurry Oil as Anode Material for Lithium-Ion Battery
- Study on Low-Temperature Properties of the Asphalt Modified by Carbon Nanotubes (CNTs) and Crumb Rubber (CR)
