Characterization and Optimization of Amylase Production in Strains LZ-10 and LZ-11 Belonging to Bacillus subtilis
2022-04-06ZhaoShuqinYangXiaopuandGaoYuanFoundationExperimentTeachingCenterGansuAgriculturalUniversityLanzhou730070China
Zhao Shu-qin, Yang Xiao-pu, and Gao Yuan* Foundation Experiment Teaching Center, Gansu Agricultural University, Lanzhou 730070, China
2 College of Life Science and Technology, Gansu Agricultural University, Lanzhou 730070, China
Abstract: In order to obtain pure enzyme with high activity, two amylase producing strains were isolated from soil samples, and named as the strains LZ-10 and LZ-11. According to morphologic observation, physiology and biochemistry experiments, 16S rRNA and gyrB gene analysis, the strains LZ-10 and LZ-11 were identified as Bacillus subtilis. Adopted the method of ammonium sulfate, DEAE-52 anion purify enzyme, finally used polyacrylamide gel electrophoresis (SDS-PAGE) to detect molecular weight. The strain LZ-10 had an amylase activity of 123.3 U • mL-1, a purification factor of 6.8, a recovery rate of 69.5% and an optimal temperature of 50℃. The amylase activity of the strain LZ-11 was 59.91 U • mL-1, the purification factor was 4.5, the recovery rate was 60.5%, and the optimum temperature was 55℃. The commodity enzyme derived from Bacillus subtilis was 37.5 U • mL-1. The relative molecular weight of amylase activity from the two strains was 55 ku. Both thermal stability and pH stability were higher than those of commercialized amylase.
Key words: amylase, screening, enzyme purification, enzymatic property
Introduction
Starch is the highest level of nutrients in food crops. Deep processing of starch can produce glucose, fructose, oligosaccharides, etc (Wanget al., 2016). At present, amylase is produced by microbial fermentation in industrial production (Sennuret al., 2016). Alpha amylase hydrolyzes the alpha 1, 4-glycosidic bond inside the starch molecule, and the hydrolyzed products are often dextrin, maltose, glucose, etc (Nisha and Satyanarayana, 2017; Sennuret al., 2016). In recent years, dozens of excellent strains of alpha amylase bacteria have been discovered, including
Yarrowia,Aureobasidium,Pichia,Candida,Rhotolorulaand somebacillusspecies, such asBacillus subtilis,Bacillus licheniformis(Salemet al., 2016),Bacillus reconciliation(Niyonzima and More, 2014),Bacillus amyloliquifaciens,Bacillus cereus,Bacillus thermooleovorans,Anoxybacillus flavithermus,Anoxybacillus amylolyticus,Anoxybacillussp,Geobacillus stearothermophilus,Geobacillus thermooleovoransandGeobacillussp(Aceret al., 2016). Industrial microorganism alpha amylase is derived mostly fromBacillus subtilis(Caoet al., 2017).
Using enzymes to change the traditional technology can not only greatly reduce consumption, save food and improve efficiency, but also greatly promote the development of starch raw material processing industry (Bozicet al., 2014; Dhundaleet al., 2014). Therefore, in order to further improve the application of amylase, screening and developing a new type high catalytic activity and stability of microbial enzyme will be imperative.
Materials and Methods
Bacterial strain and medium
Samples: the strains used in this study were isolated from the soil of restaurants, sewers, flour mills, feed mills, etc. A total of 50 soil samples were collected, and numbered LZ1-LZ50. The 3, 5-dinitrosalicylic acid (DNS), commodity alpha amylase (purity 93%, fromBacillus subtilis) was purchased from Coolaber Company (Beijing, China). Bacterial genomic DNA extraction kit was purchased from TIANGEN Biochemical Technology Company (Beijing, China). Agarose was from Spain, packed by TanWei Biotechnology (Beijing, China), and all the chemicals were of analytical grade.
Screening and species identification
Preliminary screening of bacteria was done according to literature reports (Bekleret al., 2015; Syedet al., 2009). Picked out the strains which produced transparent starch hydrolysis circle as target strains for experiment (Aceret al., 2016). The bacteria were identified according to morphologic observation, physiology and biochemistry experiments, 16S rRNA sequence andgyrBgene analysis (Wanget al., 2007).
Enzyme activity assay
The 3,5-dinitrosalicylic acid method was used to determine enzyme activity (using maltose as standard curve), and a commercial enzyme which produced fromBacillus subtiliswas used as a control (Nishaet al., 2013). Unit of enzyme activity definition: under the condition of 40℃, pH 6.0, the amount of enzyme which released 1 mmol malt sugar from 1% soluble starch per minute was defined as a unit of enzyme activity (U) (Zhanget al., 2009).
Purification of alpha amylase
The supernatant shaking culture was precipitated with 65% ammonium sulfate after 72 h. The concentrated enzyme preparation was applied to Sephadex G-50 column (1.5 cm×20 cm), Sephadex G-100 column (1.5 cm×60 cm) and Cellulose DE-52 column (1.5 cm×80 cm). Protein content and enzyme activity were determined after each step. All the purification procedures were done at 4℃. Finally, enzyme purity was determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (Annamalaiet al., 2011).
Effects of temperature and thermostability, pH and pH stability of purified enzyme
Frist, the effects of temperature on alpha amylase activity were performed in the temperature ranges of 35℃-60℃ at pH 7 for 10 min (Dheeranet al., 2010). For the thermostability, the enzyme was incubated at pH 7 for 30, 60, 120 and 180 min at different temperatures, and the residual activity was determined under the enzyme assay conditions. The non-heated enzyme was considered as a control (100%).
The effects of pH on alpha amylase activity were performed in pH ranges of 4-9 at 40℃ for 10 min (Luet al., 2014). For the measurement of pH stability, the enzyme was incubated at 40℃ for 30, 60, 120 and 180 min in different buffers, and the residual activity was determined under the enzyme assay conditions (Debet al., 2013).
Effects of different metal ions and chemical reagents
Different metal ions and ethylenediaminetetraacetic acid (EDTA) were added into the reaction systems at a final concentration of 2.0 mmol • mL-1. The enzyme solution without metal ions was used as a control (Raiet al., 2014).
Results
Purpose strain screening and identification of strains
Two strains which had larger transparent circles were separated, and named as the strains LZ-10 and LZ-11, then measured their enzyme activity values after shake-flask culture 72 h. The strain LZ-10 was 41.6 U • mL-1, while the strain LZ-11 was 34 U • mL-1, the commodity enzyme was 37.5 U • mL-1, then used the two strains as preferred strains to the next experiment. Morphology observation: the strains LZ-10 and LZ-11 had similar colony size, but had more apparent differences in colors. The strain LZ-10 for white colonies had creases, folded shape like a small umbrella, by the central bulge spreading around, opaque and margin irregular; the strain LZ-11 with pink colonies had creases, folded like cleft leaves without rules, opaque, colony edge was in line with the central parts of the color and not neat (Fig. 1). After the gram staining, the microscopic examination found that the stratins LZ-10 (Fig. 2a) and LZ-11 (Fig. 2b) were negative for smallBacilluswith red capsule, had spores, both ends obtuse, diameter was about 0.5 and 2.0 µm long, dyeing uniformity. After using malachite green spore staining, the spores could clearly be seen, the form of the strains LZ-10 (Fig. 2c) and LZ-11(Fig. 2d) was very similar under microscope.
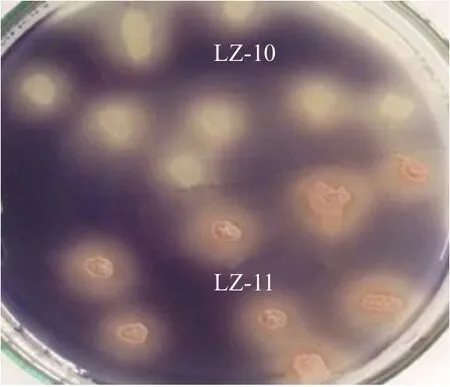
Fig. 1 Colony morphology of strains LZ-10 and LZ-11 after iodine staining
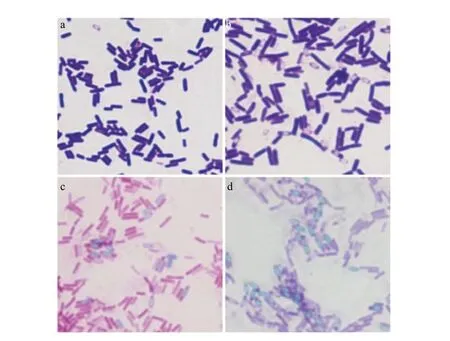
Fig. 2 Morphology of strainsa and b, Strains LZ-10 and LZ-11 after gram staining, respectively; c and d, Strains LZ-10 and LZ-11 after malachite green spore staining, respectively.
Physiological and biochemical experiment results (Table 1) showed that there were a lot of similarities between the strains LZ-10 and LZ-11. First, a variety of sugars could be fermented by the strains LZ-10 and LZ-11, just as fructose, xylose and so on. The gas couldn't be produced with them, and the gelatin could be liquefied. In addition, the results also showed positive of nitrate reduction test, urease test and hydrogen sulfide test. The difference was that the glucose and sucrose could be fermented by the strain LZ-10, but not the strain LZ-11 and the reaction was not the same in the catalase test and oxidase test. With morphology observation, it was preliminary concluded that the fungus belonged to the genusBacillus.

Table 1 Physiological and biochemical characteristics of strains LZ-10 and LZ-11
Molecular biology identification results showed that the length of 16s rRNA genes from the strains LZ-10 and LZ-11 about 1 600 bp (Fig. 3). Then, compared with the NCBI gene bank and found that the sequence of the strains LZ-10 (Fig. 4a) and LZ-11 (Fig. 4b) both had the highest similarity withBacillus subtilis,Bacillus tequilensis,Bacillus amyloliquefaciens,Bacillus licheniformis,Bacillus vallismortisandBacillus methylotrophicus. The similarity was 99%, therefore, the two bacteria could not be identified by 16s rRNA. A phylogenetic tree had been established to determine the affinity between the strains LZ-10 and LZ-11, and it was found that they had close genetic relationship.
From thegyrBgene sequencing analysis of the strains LZ-10 and LZ-11 (Fig. 5), the results showed that the similarity was 99% withBacillus subtilis. Therefore, the strains LZ-10 and LZ-11 were identified asBacillus subtilisthat coupled with morphological observation and physiological-biochemical characteristics.
Alpha amylase production and purification
The amylase production of both the strains LZ-10 and LZ-11 reached the maximum at 72 h, and the maximum values were 41.6 and 34 U • mL-1, respectively (Table 2).The steps used for the alpha amylase extraction from the strains LZ-10 and LZ-11 and purification are shown in Table 3. It could be seen that alpha amylase of the strain LZ-10 was purified up to 6.8-fold with a yield of 69.5% of the pure enzyme, and the enzyme activity was 123.3 U • mL-1. The strain LZ-11 was purified up to 4.5-fold with a yield of 60.5% of the pure enzyme, and the enzyme activity was 59.91 U • mL-1. The commodity enzyme derived fromBacillus subtiliswas 37.5 U • mL-1.
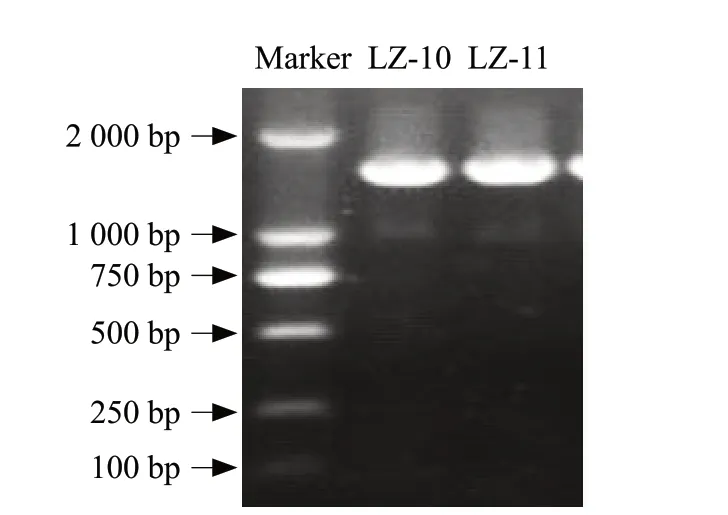
Fig. 3 Identification of 16s rRNA genes PCR product from the strains LZ-10 and LZ-11
SDS-PAGE showed that the molecular mass of the alpha amylase from the strains LZ-10 and LZ-11 was around 55 ku. The molecular masses of the alpha amylases from variousBacilliwere reported to be 55 ku (Wanget al., 2016).
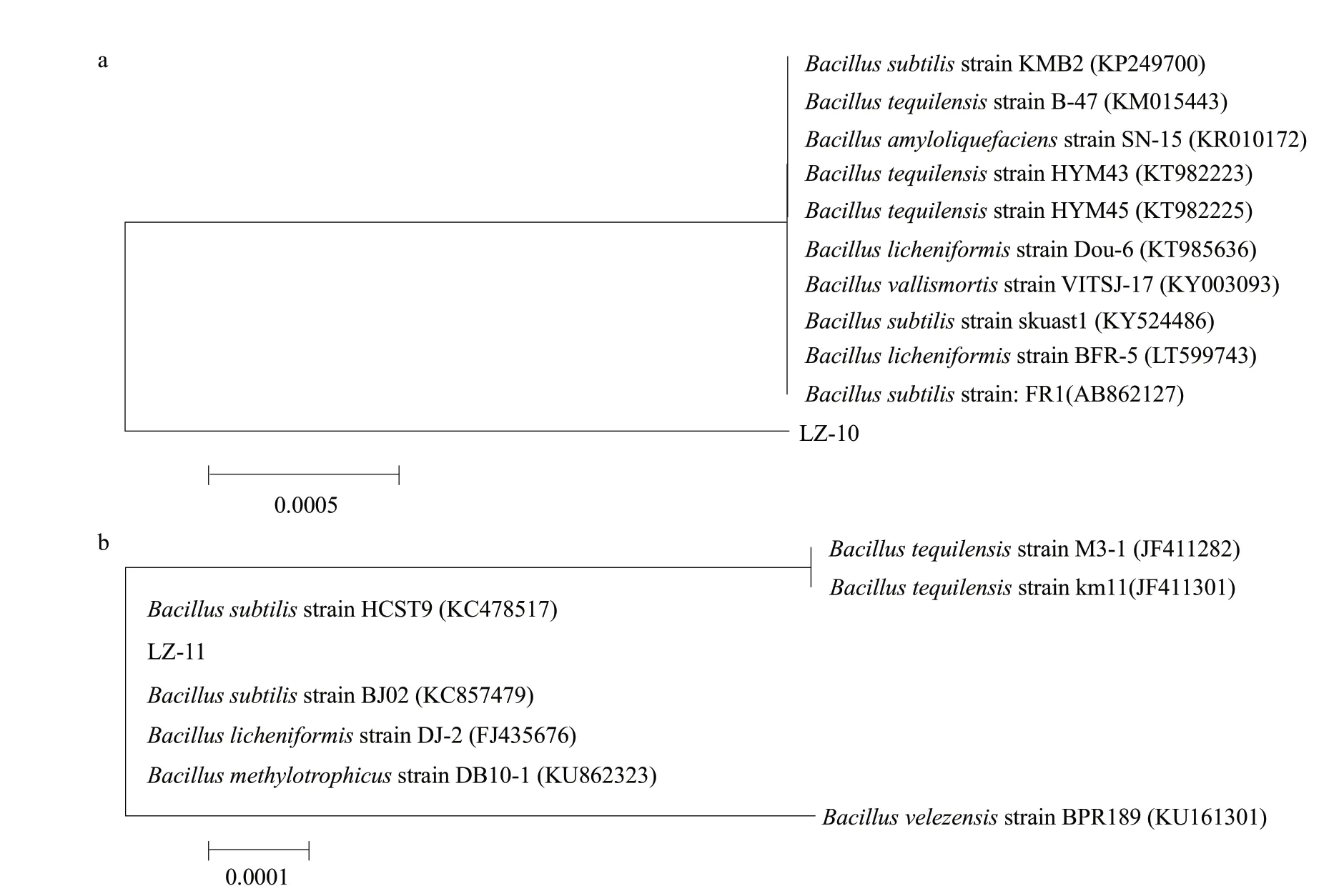
Fig. 4 16S rRNA sequence phylogenetic tree of strains a, Strain LZ-10; b, Strain LZ-11.
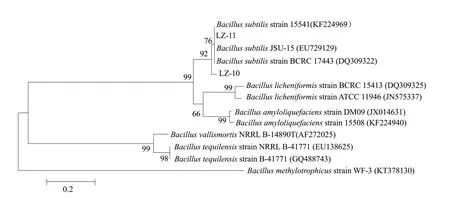
Fig. 5 Gene gyrB sequencing analysis of strains LZ-10 and LZ-11
Enzymology properties
The experiment results showed that the optimum temperature of the strain LZ-10 was 50℃, there was still a high catalytic activity at 40℃-55℃; the optimum temperature of the strain LZ-11 was 55℃ and there was higher catalytic activity at 40℃-65℃ (Fig. 6a); and the optimum temperature for commercial enzymes was 50℃ and there was a higher catalytic activity at 40℃-65℃. The optimum pH of the strain LZ-10 was 7, the strain LZ-11 was 6, commercial enzymes was 6.5, and there was still a higher catalytic activity at 5-8 (Fig. 6b). The thermal stability of alpha amylase results showed that the residual enzyme activity was close to 90% of the strains LZ-10 (Fig. 6c) and LZ-11 (Fig. 6d) which had processed 30 min at 50℃. There were small variations with extended time, and the enzyme activity could keep above 80% for 180 min, above 70% for 180 min at 60℃, and could maintain at 50% for 180 min at 70℃, 50% for 30 min at 80℃, 30% for 30 min at 90℃, the residual enzyme activity significantly reduced with extended time. By contrast, commodity enzyme was not very stable at 70℃, 80℃ and 90℃, it lost its activity very quickly in a short time, and the enzyme activity was almost completely lost in 10 min at 90℃. pH stability showed that the enzyme activity was almost no change at pH 7 for 180 min, over 70% at pH 6 for 180 min, around 60% at pH 5 for 180 min, and had relatively great variations at pH 4 and pH 8 with extended time, the enzyme activity could remain at about 40% for 180 min (Fig. 6e, f). By contrast, commodity enzyme activity dramatically decreased about 20% at pH 4 for 30 min, and about 40% at pH 8 for 30 min.
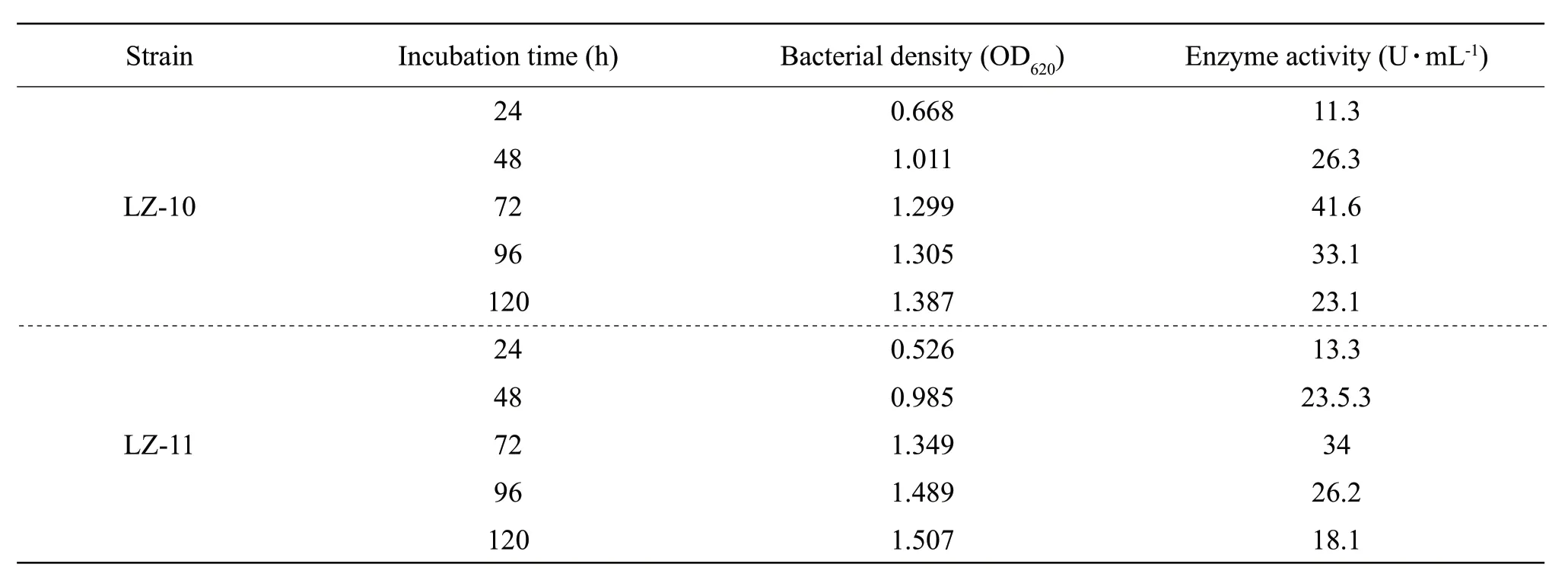
Table 2 Measurement of optimal enzyme culture time
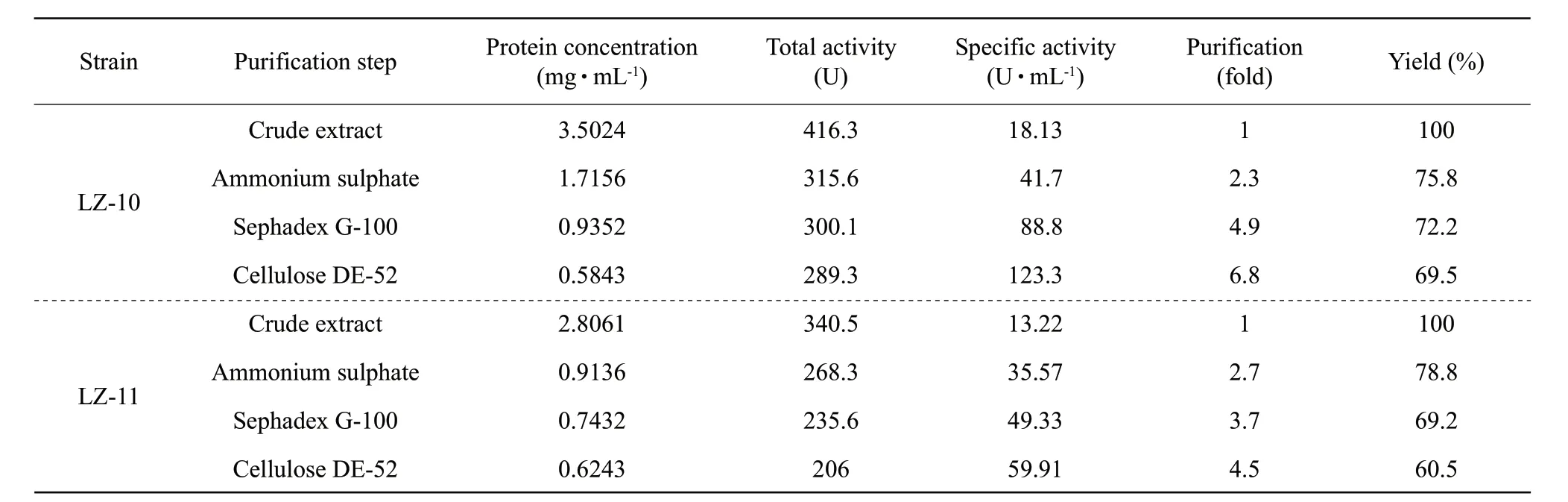
Table 3 Purification steps of alpha amylase
Effects of metal ions on alpha amylase activity
The results showed that Ca2+and Mg2+had significant activation and improvement effects on alpha amylase, while Na+and K+had weaker effects on alpha amylase. Fe2+and Zn2+had inhibitory effects on enzyme activity, and alpha amylase could be basically inactivated by EDTA (Fig. 6g).
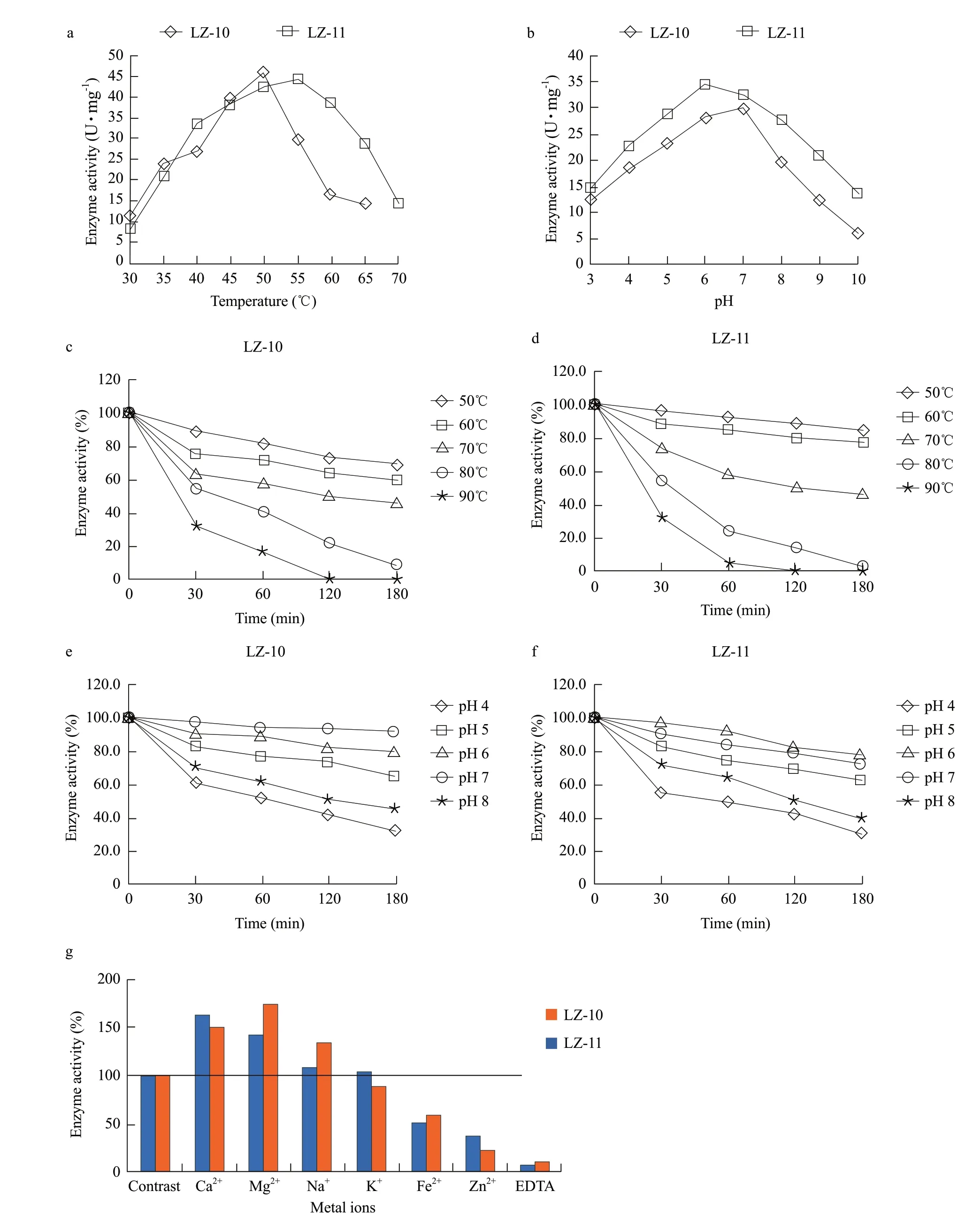
Fig. 6 Characterization of amylase assaya, Influence of temperature on enzyme activity; b, Influence of pH on enzyme activity; c and d, Effects of temperature and treatment time on enzyme activity, c is amylase from strain LZ-10, d is from strain LZ-11; e and f, Effects of pH and treatment time on enzyme activity, e is amylase from strain LZ-10, f is from strain LZ-11; g, Effects of metal ions on enzyme activity.
Discussion
Screening high alpha amylase yield strains
Amylases are among the most important enzymes and are of great significance in biotechnological processes. This experiment successfully isolated high alpha amylase yield strains from soil rich in starch, named as the strains LZ-10 and LZ-11, and measured enzyme activity values of 123.3 and 59.91 U • mL-1.
In general, most of the members fromBacillaceaefamily was reported to produce maximum enzyme production at activities ranging from 6.18 U • g-1to 15.84 U • g-1(Sennuret al., 2016). It was surpass alpha amylase activity thatBacillus subtilisproducts reported in literatures, also surpass with commodity enzyme derived fromBacillus subtilisthat the enzyme activity value was 37.5 U • mL-1. The optimal temperatures for alpha amylase activities were within the ranges (30℃-105℃) of the reported alpha amylases fromBacilli(Saxenaet al., 2007; Arikan, 2008; Asoodehet al., 2010).
In this experiment, the optimum temperature of the strain LZ-10 was 50℃, pH 7, and that of the strain LZ-11 was 55℃, pH 6. The thermal stability results showed that the residual enzyme activity was close to 90% of the strains LZ-10 and LZ-11 which had processed 30 min at 50℃, 50% for 30 min at 80℃ and 30% for 30 min at 90℃. These results were at agreement with the reports of Ezeji and Bahl (2006) and Teodoro and Martins (2000).
In those studies, the optimum temperature was diminished beyond of 55℃ and was inactivated at 95℃ for 10 min. pH stability showed that the enzyme activity was almost no change at pH 7 for 180 min, over 70% at pH 6 for 180 min, around 60% at pH 5 for 180 min, and remained about 40%for 180 min at pH 4 or 8. The commodity enzyme activity dramatically decreased about 20% at pH 4 for 30 min, and about 40% at pH 8 for 30 min.
When used two methods that one was the traditional iodine staining method and the other was the 3, 5-dinitrosalicylic acid method for determination of compared enzyme activity, the results showed that the 3, 5-dinitrosalicylic acid method was more accurate for determination of enzymatic activity, when the color indicator was iodine, the effect was not ideal. On the one hand, it was easy to decompose under the light; on the other hand, the most accurate absorbance values were from 0.3 to 0.8, when the starch in a test tube was hydrolyzed thoroughly by amylase, the color span was too big and beyond the limit of 0.3-0.8, the determination results was not accurate.
Species identification
The 16s rRNA sequence of the strains LZ-10 and LZ-11 both had the highest similarity withBacillus subtilis,Bacillus tequilensis,Bacillus amyloliquefaciens,Bacillus licheniformis,Bacillus vallismortisandBacillus methylotrophicus. All the similarities were 99%, and no one could reach 100%, therefore, the two bacteria could not be identified by 16s rRNA, but a lot of literatures only based on 16s rRNA could identify these bacteria (Zhanget al., 2009; Bozicet al., 2014). The strains LZ-10 and LZ-11 were very similar both with structure and size under the microscope.
They both produced biofilm, the difference was biofilm colors, the strain LZ-10 was white and the strain LZ-11 was red, they also had almost the same physiological and biochemical characteristics. They were fine selections as alpha amylase microbial sources considering they had very high enzyme activity.
In a follow-up experiment, the higher enzyme activity of mutant strain was screened out by trying to optimize the culture medium composition, mutagenesis screening, gene clone technology methods, and then with further researches and development to replace or compensate for the current commodity enzyme commonly used by the microorganisms of alpha amylase.
Conclusions
The strains LZ-10 and LZ-11 were successfully isolated from soil as they had high commercial values, the alpha amylase enzyme activity values were 123.3 and 59.91 U • mL-1after purification. It was surpass commodity enzyme derived fromBacillus subtilis(37.5 U • mL-1). The optimum temperature of the strain LZ-10 was 50℃, pH 7, and that of the strain LZ-11 was 55℃, pH 6.
The thermal stability showed that the residual enzyme activity was close to 90% which had processed 30 min at 50℃, 50% for 30 min at 80℃, 30% for 30 min at 90℃. pH stability showed that the enzyme activity was almost no change at pH 7 for 180 min, over 70% at pH 6 for 180 min, around 60% at pH 5 for 180 min, and remained about 40% for 180 min at pH 4 or 8.
The alpha amylase could be activated significantly by Ca2+and Mg2+, and had a weak effect by Na+, K+, Fe2+and Zn2+, and EDTA had inhibitory effects.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Analysis on Cloning and Expression of Promoter of Flowering Time GmFT2a in Soybean
- Effect of Allelic Variation of β-conglycinin α-subunit Locus cgy-2 on Soybean (Glycine max) Quality I. Evaluation of Free Amino Acid Content
- Improved Nucleic Acid Spot Hybridization Technique for Detection of Potato Spindle Tuber Viroid (PSTVd)
- Nutrient Accumulation and Distribution in Cotton Promoted by Removal of Mulch Film
- Effects of Sowing Periods on Growth and Development, Yield and Quality of Maize in Cold Area
- Cloning of GsTPS9 Gene from Glycine soja and Study on Its Responses to Stresses
