Analysis on Cloning and Expression of Promoter of Flowering Time GmFT2a in Soybean
2022-04-06LiuTianmengLiXinWangQiFanYuhuanLiuZhengyaandZhaoLin
Liu Tian-meng, Li Xin, Wang Qi, Fan Yu-huan, Liu Zheng-ya, and Zhao Lin
Key Laboratory of Soybean Biology of Chinese Education Ministry, Northeast Agricultural University, Harbin 150030, China
Abstract: Soybean (Glycine max) is short-day (SD) plant. Flowering time is a key agronomic trait that determines the transition from vegetative to reproductive growth. The study on the expression and regulation mechanism of flowering time gene in soybean photoperiod control of flowering pathway is particularly theoretically significant for soybean genetic improvement. In this study, a dual-luciferase reporter gene system with the GmFT2a gene promoter as promoter sequence was constructed, and the method of Agrobacterium tumefaciens injection into tobacco leaves was selected to study the effects of long and short days on the activity of the GmFT2a gene promoter. The results of transient expression analysis showed that the GmFT2a promoter was strongly induced under the SD conditions in tobacco. Furthermore, analysis of the GmFT2a promoter sequence revealed several cis-acting elements, including G-Box, Box 4, GT1-motif and TCT-motif by PlantCARE search. It was speculated that these elements might promote the expression of GmFT2a gene in the SDs and played a role in promoting flowering. The results of this study provided a basis for a better understanding of the function of the GmFT2a gene and further exploration of the complex flowering mechanism of soybean.
Key words: soybean, promoter, GmFT2a, light-induced response element, photoperiod
Introduction
Soybean is a vital oil crop in China. As a facultative short-day (SD) plant, it will accelerate the transition from the vegetative stage to the reproductive stage under short-day conditions (SDs) by affecting flowering time. Flowering time is critical for adapting to the growth environment of broad ranges of latitudes and is regulated by the signals of endogenous hormones and environmental factors (Songet al., 2013). Photoperiod is the most crucial environmental factor in various environmental signals to help soybean adapt to the changes of day length (Bäurle and Dean, 2006). Moreover, photoperiod is a rhythmic change, and its photosensitivity contributes to plants to adjust flowering time by using the seasonal variation of day length. Rice (Song and Luan, 2012), corn (Zhang, 2001), soybean (Konget al., 2016), wheat (Shen and Guo, 2015), autumn chrysanthemum (Yanget al., 2007) and other plants have been confirmed that photoperiod has a related effect on their flowering period. It is worth noting that the flowering period is crucial for soybean. Therefore, the study on the expression and regulation mechanism of photoperiod response genes in the soybean photoperiod flowering pathway has important theoretically significance for soybean genetic improvement.
InArabidopsis, the flowering regulation system constructs a complex network system by integrating various signals of photoperiod and temperature pathways (Komeda, 2004). The FLOWERING LOCUS T(FT) is an integration factor in the complex flowering regulatory networks, the interaction between the FT and the bZIP transcription factor FD (FLOWERING LOCUS D) promotes flowering (Abeet al., 2005). To date, at least 10FT homologs have been identified in the soybean (Konget al., 2010), including flowering promoting genes and flowering inhibitory genes.It is worth nothing that early flowering in soybean relies on flowering promoting genes. The expression patterns of the FT homologous genesGmFT2aandGmFT5ain soybean closely change with the changes of photoperiod, and they are shown to be positive promoting flowering. Recent studies revealed thatE1 encodes a transcription factor that may down-regulateGmFT2aandGmFT5a(Xiaet al., 2012), and shows the most profound effect on flowering time (Upadhyayet al., 1994; Satoshiet al., 2004; Tsubokuraet al., 2014; Zhaoet al., 2016). By gene mapping, sequencing, and expression analysis demonstrated thatE9 is a homolog ofGmFT2agene inArabidopsis. TheE2 inhibits the expression of theGmFT2aunder the condition of natural day length (Watanabeet al., 2011). TheGmFT2aand theGmFT5aare up-regulated under the SDs and observably down-regulated under longday conditions (LDs). The plants were then transferred from the SDs to the LDs, the results showed that theGmFT2ais more sensitive to photoperiod changes than theGmFT5a(Konget al., 2010; Caiet al., 2018). The specificity of theGmFT2agene was demonstrated using the CRISPR/Cas9 system as a key gene for flowering in soybean. After directional mutation, the homozygousGmFT2amutant shows a lateflowering phenotype under the LDs and the SDs. These results suggested that the flowering promoting effect of theGmFT2ais stronger than that of theGmFT5aunder the SDs. It is speculated that there may be different in flowering regulation pathways between theGmFT2aand theGmFT5aunder the SDs and the LDs. Because of the remarkable flowering characteristics of theGmFT2ain the process of flowering in the SDs, this gene is chosen as the starting point to find its internal mechanism as a flowering promoting factor in the soybean early flowering regulation network system, which contributes to the expansion of geographical scope of soybean cultivation.
TheGmFT2ais a key gene for the improvement of photoperiod sensitivity in soybean; however, its specific expression and regulatory mechanisms are not clear. The promoter is one of the crucial determinants of gene expression. The upstream region of 5' URT in the full-length sequence of the gene is generally called the promoter region, which regulates the expression intensity of foreign genes in transgenic plants. Early research had shown that the TCT-motif (SNP-796G) in theGmGBP1 promoter results in higher expression levels of theGmGBP1 (Liet al., 2018). The mutation of the light-induced element W-box in the LFY promoter will decrease the expression level of the LFY and affect its normal function (Qiao and Yu, 2017). The upstream 1 500 bp sequence oftheCpFT1 gene ATG is rich in abscisic acid, gibberellin, salicylic acid and light response elements, and treatment with the above conditions can significantly induce the expression of theCpFT1, while gibberellin slightly induces the expression of theCpFT1 (Liuet al., 2016). The above results suggested that the study of gene promoter is not only helping to clarify the gene function and expression regulation pattern, but also can improve crop genetic mode from promoter regulation. TheGmFT2aplays a major part in promoting early flowering, but there are no specific studies and reports on promoter light-inducing elements and their special regulatory functions.
The double luciferase reporter gene has the advantages of high sensitivity in detecting the activity of transcription factor regulatory genes and has been widely used in the study of gene promoter activity. In the early stage of our laboratory, this method was successfully applied to analyze the daily length expression pattern of soybeanGmFT5a(Zhaoet al., 2020)andGmGBP1 (Liet al., 2018) gene promoters. Hence, in this study, the dual-luciferase reporter gene system of the soybeanGmFT2agene promoter was constructed, and the light response-related elements were screened by sequence analysis ofGmFT2apromoter region.Agrobacterium tumefacienswas injected into tobacco leaves to study the effects of long and short days on the activity of theGmFT2agene promoter.
Materials and Methods
Experiment materials
The soybean varieties Dongnong 42 and tobacoo (N. benthamiana) seeds were obtained from the Soybean Research Institute of Northeast Agricultural University. The pGreenⅡ0800 vectors were provided by Professor Fengning Xiang of Shandong University. In-Fusion®HD Cloing kit, PrimeSTAR®Max DNA Polymerase andSmaI restriction endonucleases were purchased from TaKaRa Company. Double luciferase reporter gene (LUC) detection kit was purchased from Promega Company. The PCR primer synthesis and the DNA sequencing were completed by Beijing Huada Genome Technology Co., Ltd.
Methods
Bioinformatic analysis
The promoter region of theGmFT2awas predicted by the promoter prediction website PlantCARE. Additionally, a sequence alignment program (DNAMAN) was used to determine thecis-acting elements related to the photoperiod response in the promoter.
Pro (GmFT2a)::LUC vector construction
The sequence of theGmFT2a(Glyma.16G150700) promoter was from phytozome, based on this, theGmFT2aP-F2(5-GAATTCCTGCAGCCCAGGGTGC TTTTGATTCAAGATA-3)/GmFT2aP-R(5-AGTAGT GGATCCCCCTACTTCCACTAGGCATGGGATA-3) primers adding theSmaI restriction endonuclease sites are listed in Table 1. Genomic DNA was extracted from the leaf tissue of soybean variety Dongnong 42 and used as a template to amplify the putative promoter of 1 496 bp. Then, the PCR products were purified and cloned into the pGreenII0800 vector. The vector above was digested bySmaI restriction endonucleases, which was ligated with aGmFT2apromoter fragment with the same restriction site by In-Fusion reagent. The reaction system and conditions were carried out, according to the In-Fusion®HD Cloing kit to form pro (GmFT2a)::LUC recombinant plasmid. After the reaction, 5 μL reaction solution was taken for gel electrophoresis detection.
Subsequently, the ligated product was transformed into Trans1-T1 competent cells by the heat shock method. According to the concentration of a bacterial solution, an appropriate amount of bacterial solution was smeared on LB solid medium containing ampicillin (50 μg • mL-1) for screening. Twelve hours later, monoclonal on the surface of the medium was placed on LB liquid medium, which contained the corresponding antibiotic (Kan). After shaking the culture at 37℃ for 12 h, the positive clones were tested by the PCR identification and sequencing, and the plasmids were extracted from the correct bacterial solution. The recombinant was transferred to the EHA105 competent state ofAgrobacterium tumefaciensby electroporation. The 40 μL of the bacterial solution was coated on YEP solid medium (50 mg • L-1Str, 25 mg • L-1Rif and 100 mg • L-1Spec) for screening. After upside-down culture at 28℃ for 1-2 days, a single spot was selected for the PCR identification. The positive clones were sequenced by Beijing Huada Genome Technology Co., Ltd. The identified positive recombinantAgrobacterium tumefacienswas preserved in 30% glycerol and the final concentration reached 15%. It was stored in an ultra-low temperature refrigerator (−80℃) for using in subsequent experiments.
Expression pattern of pro (GmFT2a)::LUC in tobacco
Tobaccoes were seeded in the surface of mixed soil with floral nutrient soil and vermiculite and placed in the growth chamber at 25℃ under the LDs. When the tobacco leaves were cultured for 25 days, they could be infected byAgrobacterium tumefaciens. Subsequently, the pro (GmFT2a):: LUC solution andAgrobacteriumtumefacienscontaining P19 stored in the cryogenic refrigerator were activated, and the heavy suspension containing MES-KOH, acetosyringone and MgCl2was selected. When the OD600value was 1, the activation was completed, and the activated P19 and pro (GmFT2a)::LUC bacteria solution was fully mixed, according to the proportion of 1 : 1, and then stored at room temperature for 4 h for injecting tobaccoes. The tobaccoes infected by bacterial liquid were cultured under the LDs and the SDs for 12 h, each treatment was repeated three times, and samples were collected from different treatments. Finally, with the sea kidney luciferase as the internal reference, the RLU values measured by firefly luciferase were divided by the sea kidney luciferase values. According to the ratio, the transcriptional activity of Pro (GmFT2a)::LUC was detected, and the differences of their expressions under long and short days were compared and analyzed.

Table 1 Cis-acting elements in soybean GmFT2a promoter
Results
Cis-acting element analysis of GmFT2a gene promoter
To investigate thecis-acting elements ofGmFT2agene promoter, theGmFT2apromoter sequence obtained from phytozome was screened. The promoter sequence of the gene was further analyzed by the PlantCARE website. The results showed that in addition to the main acting element TATA-box,GmFT2apromoter sequence also included various hormones and environmental factor response elements, such as gibberellin (GARE-motif), low temperature (LTR) and defense stress response (TC-rich repeats)cis-acting elements. Besides, there were four important light response promoter elements (Table 1), and the specific locations of which were G-Box, Box 4, GT1-motif and TCTmotifcis-acting elements in Fig. 1. It was proved that there were these four main light response promoters in the upstream promoter region of the cloned theGmFT2agene, which indicated that the expression of theGmFT2agene might be affected by photoperiod.
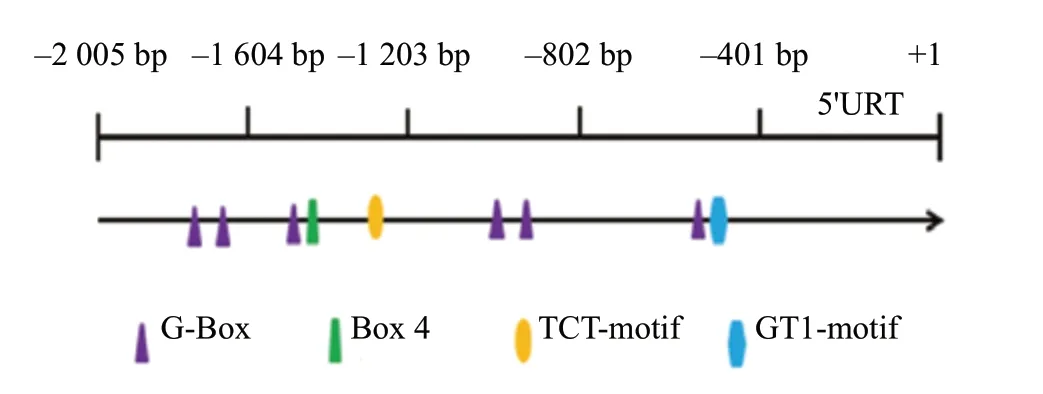
Fig. 1 Location of light-responsive cis-regulatory elements in GmFT2a promoter
Construction of pro (GmFT2a)::LUC fusion expression vector
To construct pro (GmFT2a)::LUC fusion expression vector, the target band of about 1 496 bp was amplified by primer PCR (Fig. 2A). The size of the amplifiedGmFT2apromoter was consistent with the expected design, indicating that theGmFT2apromoter had been successfully amplified. The PCR products of the correct size were recovered and then amplified again to obtain the target gene products with higher concentration, and the gel products were recovered.
Fig. 2B showed the plasmid construction process of theGmFT2a-promoter-Luc. TheSmaI restriction endonuclease was selected to digest and recover the cloning vector pGreen II 0800. The linear pGreen II 0800 vector was ligated to the promoter fragment (1 496 bp) ofGmFT2agene amplified with theSmaI cutting site by the In-Fusion cloning technique, which was named pro (GmFT2a)::LUC. After a successful connection, the clones were transferred into the Trans1-T1 competent cells, and the clones were selected and cultured in a liquid medium containing Knaresistant LB. The positive strains were verified by the PCR. The positive strains and pSoup plasmids were transferred to the EHA105viaelectroporation and the fragment size was 1 496 bp, thus the positive strains ofAgrobacterium tumefacienswere obtained (Fig. 2C). The above results revealed that the pro (GmFT2a)::LUC fusion expression vector was successfully constructed.
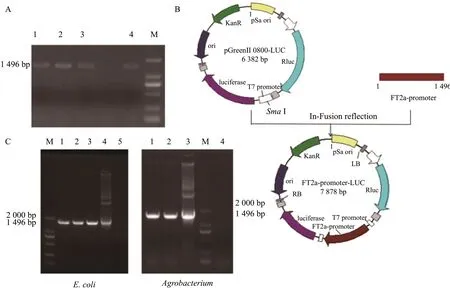
Fig. 2 Construction of pro (GmFT2a):: LUC vectorsA, GmFT2a gene promoter fragment; M, 2000 bp DNA Marker; B, Process of pro (GmFT2a)::LUC construction; C, PCR identification of recombinant. For E. coli: M, 2000 bp DNA Marker; 1-3, PCR identification of recombinant E. coli containing GmFT2a-pGreenII 0800; 4, Positive control; 5, Negative control. For Agrobacterium: M, 2000 bp DNA Marker; 1-2, PCR identification of Agrobacterium containing GmFT2a-pGreenII 0800; 3, Positive control; 4, Negative control.
Transient expression pattern of pro (GmFT2a)::LUC recombinant vector in tobacco
To detect the expression pattern ofGmFT2apromoter under the LDs and the SDs, the constructed pro (GmFT2a)::LUC fusion expression vector was injected into healthy 4-week-old tobacco leavesviasyringe, and the transient expression was traced. The results showed that the recombinant vector pro (GmFT2a)::LUC was expressed in both SD and long-day (LD) tobacco leaves after 12 h treatment under the LDs and the SDs, which proved that the constructedGmFT2apromoter had promoter activity. The SD activities of pro (GmFT2a)::LUC was two times as many as LD light (Fig. 3), indicating that theGmFT2apromoter could preferentially drive theGmFT2agene expression under the SDs. The results above supported the conclusion that the expression of theGmFT2ain the SDs was higher than that in the LDs (Konget al., 2010).
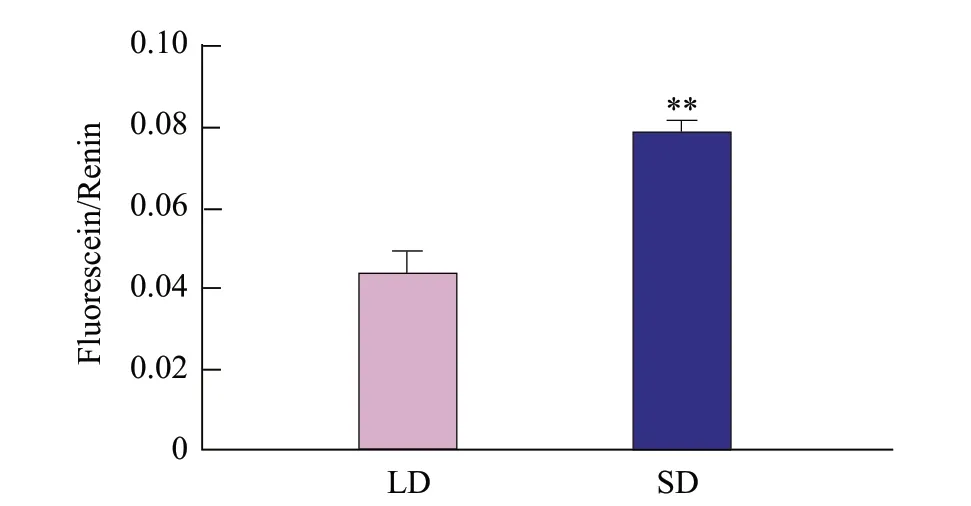
Fig. 3 Transcriptional activity of pro (GmFT2a)::LUC construct after 12 h under LDs andSDs
Discussion
Soybean is very sensitive to change in day length. The latitude of China is relatively broader, and there is a cultivation problem that a single variety adapts to a small planting range. Flowering time is directly related to soybean adaptability and yield, and photoperiod sensitivity has also become the main obstacle to the wide adaptability of soybean planting. The molecular techniques are benefiting to analyze the expression and regulation mechanism of photosensitive response genes in the soybean photoperiod flowering pathway to improve the soybean photoperiod regulation network system. Thus, it is an important theoretical significance for soybean genetic improvement. Previous studies had shown thatFTwas a key floral element, which was produced when plant leaves moved to the meristem at the tip of the stem to initiate flowering (Corbesieret al., 2007). The expression and regulation ofFTgene were extremely complex. Studying the function ofFTwas very vital to further understand the regulation mechanism of soybean flowering. The homology of theEhd1 gene in rice andFTinArabidopsiscould promote the development of floral organs with the same function asFT(Xuet al., 2014). The LDs inhibited the expression of theEhd1 pathway and delayed flowering, while the inhibition of theEhd1 gene in the SDs decreased, which led to the accumulation of anthocyanin in rice and shortened flowering time (Xueet al., 2008). Consistently, theGmFT2aand theGmFT5awere often mentioned in pairs in soybean, and they had been identified as flowering promoting factors (Konget al., 2010). However, there were still differences in flowering regulation pathways between theGmFT2aand theGmFT5aunder the SDs and the LDs. Based on the remarkable flowering characteristics of theGmFT2aunder the SDs, the special flowering regulation mechanism of this gene under the SDs was selected to find an improved molecular breeding method to reduce photoperiod sensitivity.
Transcription is a complex and strict process, the promoter plays a key role in the regulation of gene expression, and different activities of the promoter determines its function. The light-induced promoter is a kind of promoter classification, which usually contains a variety of elements that interact with light, such as G-Box, GA-motif and Box 4. These elements can regulate the strong expression of target genes through their specific properties. The existence of a light response promoter might provide opportunities in guiding light regulation genes and promoting the high expression of related genes (Wanget al., 2011). The double luciferase reporter gene had the advantages of high sensitivity in detecting the activity of transcription factor regulated genes. It was widely used in the study of gene promoter activity. In previous experiments, thein vivotransient expression system mediated byAgrobacterium tumefacienswas used to verify the diurnal response expression of theGmFT5agene (Zhaoet al., 2020). In this study, to analyze the diurnal length expression pattern of theGmFT2apromoter, a dualluciferase reporter gene system of the soybeanGmFT2apromoter was constructed. AfterAgrobacterium tumefaciensinfected tobacco leaves, the transient expression was discovered.LUCgene was driven by theGmFT2apromoter at basic level in both the SDs and the LDs. However, the promoter activity of pro (GmFT2a)::LUC under the SDs was about twice as high as that under the LDs, indicating that theGmFT2apromoter was strongly induced by the SDs. The light-inducedcis-response elements in theGmFT2apromoter region were analyzed by PlantCARE, and four-light response elements, G-Box, Box 4, GT1-motif and TCT-motif were screened. There was every prospect that G-Box, Box 4, GT1-motif and TCT-motif might play significant roles in the soybeanGmFT2apromoter as important light response elements. Therefore, the up-regulation of theGmFT2atranscript levels under the SDs might be a function of these elements, which in turn promote plant flowering.
Conclusions
The double luciferase reporter gene system of soybeanGmFT2apromoter was successfully constructed with a size of 1 496 bp, which proved that the SDs exposure could strongly induce the expression of theGmFT2apromoter. The PlantCARE analysis showed that there were four main light response elements of the soybeanGmFT2apromoter involving G-Box, Box 4, GT1-motif and TCT-motif. Therefore, it was speculated that these elements might significantly promote theGmFT2apromoter to up-regulate the expression of theGmFT2agene under the SDs. Thus it played a role in the early flowering of soybean. TheGmFT2apromoter had completed the screening of the light-induced elements of the gene. This study could facilitate the further study of how the elements in theGmFT2agene promoter combined to regulate gene expression under the SDs to provide clues and ideas for further exploration of complex transcriptional regulation in the photoperiod pathway.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effect of Allelic Variation of β-conglycinin α-subunit Locus cgy-2 on Soybean (Glycine max) Quality I. Evaluation of Free Amino Acid Content
- Improved Nucleic Acid Spot Hybridization Technique for Detection of Potato Spindle Tuber Viroid (PSTVd)
- Nutrient Accumulation and Distribution in Cotton Promoted by Removal of Mulch Film
- Effects of Sowing Periods on Growth and Development, Yield and Quality of Maize in Cold Area
- Cloning of GsTPS9 Gene from Glycine soja and Study on Its Responses to Stresses
- Comparative Analysis of Hydrolytic Amino Acids in Human and Cow Milk of Different Lactation Periods
