Therapeutic strategies for gastroenteropancreatic neuroendocrine neoplasms:State-of-the-art and future perspectives
2022-04-01ElettraMerolaAndreaMichielanUmbertoRozzanigoMarcoEriniSandroSferrazzaStefanoMarcucciChiaraSartoriChiaraTrentinGiovannidePretisFrancaChierichetti
Elettra Merola,Andrea Michielan,Umberto Rozzanigo,Marco Erini,Sandro Sferrazza,Stefano Marcucci,Chiara Sartori,Chiara Trentin,Giovanni de Pretis,Franca Chierichetti
Elettra Merola,Andrea Michielan,Sandro Sferrazza,Giovanni de Pretis,Department of Gastroenterology,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Trento 38122,Italy
Umberto Rozzanigo,Department of Radiology,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Trento 38122,Italy
Marco Erini,Franca Chierichetti,Department of Nuclear Medicine,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Trento 38122,Italy
Stefano Marcucci,Department of Surgery,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Trento 38122,Italy
Chiara Sartori,Department of Pathology,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Trento 38122,Italy
Chiara Trentin,Department of Medical Oncology,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Trento 38122,Italy
Abstract Although gastroenteropancreatic neuroendocrine neoplasms(GEP-NENs)have always been considered rare tumors,their incidence has risen over the past few decades.They represent a highly heterogeneous group of neoplasms with several prognostic factors,including disease stage,proliferative index(Ki67),and tumor differentiation.Most of these neoplasms express somatostatin receptors on the cell surface,a feature that has important implications in terms of prognosis,diagnosis,and therapy.Although International Guidelines propose algorithms aimed at guiding therapeutic strategies,GEP-NEN patients are still very different from one another,and the need for personalized treatment continues to increase.Radical surgery is always the best option when feasible;however,up to 80% of cases are metastatic upon diagnosis.Regarding medical treatments,as GEP-NENs are characterized by relatively long overall survival,multiple therapy lines are adopted during the lifetime of these patients,but the optimum sequence to be followed has never been clearly defined.Furthermore,although new molecular markers aimed at predicting the response to therapy,as well as prognostic scores,are currently being studied,their application is still far from being part of daily clinical practice.As they represent a complex disease,with therapeutic protocols that are not completely standardized,GEP-NENs require a multidisciplinary approach.This review will provide an overview of the available therapeutic options for GEP-NENs and attempts to clarify the possible approaches for the management of these patients and to discuss future perspectives in this field.
Key Words:Gastroenteropancreatic neuroendocrine neoplasms;Therapeutic strategies;Radical surgery;Medical treatments;Overview;Future perspectives
BlOGRAPHY
Elettra Merola,MD,PhD:She is a gastroenterologist and researcher currently working at the Department of Gastroenterology of Santa Chiara Hospital(APSS),Trento(Italy).She received her MD degree with honors in 2005 from Campus Bio Medico University,Rome(Italy),and during medical school she was visiting student and researcher at the University of Illinois in Chicago(United States)and at Temple University in Philadelphia(United States).In 2009,she completed her residency in Gastroenterology and in 2013 her PhD in Digestive Oncology at Sant’Andrea Hospital,Sapienza University,Rome(Italy),where she developed a particular interest in neuroendocrine neoplasms(NENs).Her experience in this field grew,joining as a visiting researcher the European Neuroendocrine Tumor Society(ENETS)center of excellence of Charité University,Berlin(Germany)(2015-2017),and then working as an NEN specialist at the NEN center of FAU Erlangen University,Erlangen(Germany)(2017-2018).Dr.Merola is internationally recognized for her expertise in NENs and she has led several cooperative studies aimed at improving the clinical management of these patients.In March 2017 she was awarded the ENETS Center of Excellence Academy Fellowship Grant for an international,cooperative research project regarding curative surgery in neuroendocrine tumors.She moved to Santa Chiara Hospital(APSS)in Trento(Italy)in 2018,where she promoted the management of NEN patients in a multidisciplinary setting,coordinating a dedicated NEN tumor board.She is also responsible for the outpatient clinic of neuroendocrine neoplasms in the Gastroenterology Department(Figure 1).

Figure 1 Elettra Merola,MD,PhD,Department of Gastroenterology,Santa Chiara Hospital,Azienda Provinciale per i Servizi Sanitari(APSS),Largo Medaglie D’Oro 9,Trento 38122,Italy
lNTRODUCTlON
Although gastroenteropancreatic neuroendocrine neoplasms(GEP-NENs)have always been considered rare tumors,their incidence has risen in recent decades,up to 3-5 casesper100000 personsperyear[1,2].They represent a highly heterogeneous group of neoplasms with varying biological behavior.Several prognostic factors have an impact on GEP-NEN survival,including the proliferative index(Ki67)[3],disease stage according to the European Neuroendocrine Tumor Society(ENETS)tumor-nodemetastasis(TNM)staging system[4,5],and the World Health Organization(WHO)classification[6].
In particular,if the definition of NENs is adopted for all neoplasms with a neuroendocrine differentiation in general,based on immunolabeling for chromogranin A and synaptophysin,the novel WHO 2019 classification[6]distinguishes two different subgroups in terms of morphology,genetics,response to therapy,and prognosis:NETs and neuroendocrine carcinomas(NECs).NETs are well-differentiated neuroendocrine neoplasms,characterized by a population of cells with uniform nuclear features,“salt and pepper” chromatin,organoid architecture and sometimes minimal necrosis.NETs are classified according to proliferation fraction in G1(mitotic count < 2per2 mm2and/or < 3% Ki-67 index),G2(mitotic count 2-20per2 mm2and/or 3%-20% Ki-67 index),and G3(mitotic count > 20per2 mm2and/or> 20% Ki-67 index).Instead,NECs are highly aggressive poorly differentiated neoplasms that grow in sheets,usually with abundant necrosis.They are further classified into small cell NECs or large cell NECs,based on the cell morphology.NECs are high grade by definition;grading for these neoplasms is not assigned to avoid confusion regarding the NET G3 category.
The expression of somatostatin receptors(SSTRs)also has an important role in therapy selection and characterizes nearly 90% of NENs.This feature is mainly identified by functional imaging tests,which are pivotal in diagnosis,disease staging,and the therapeutic management of NENs.They include octreotide scintigraphy with radiolabeled somatostatin analogs(SSAs)(Octreoscan®),limited by the low accuracy in detecting small lesions(< 1 cm in diameter)and by a difficult semiquantitative analysis[7].The subsequent development of different radiolabeled DOTA-conjugated peptides(DOTANOC,DOTATOC,DOTATATE)for positron emission tomography/computed tomography(PET/CT)has changed the landscape of nuclear medicine.Following the first published paper introducing68Ga-DOTATOC-PET/CT,a series of further papers showed that this test could detect no less than 30% more neuroendocrine lesions than Octreoscan®and conventional CT[8].
Although International Guidelines propose algorithms aimed at guiding therapeutic strategies[9-13],NEN patients are still very different from one another and the need for personalized treatments continues to increase.Although radical surgery is always the best option when feasible,up to 80% of cases are metastatic upon diagnosis and data on adjuvant treatments are still insufficient for this disease.Regarding medical treatments,as NENs are characterized by a relatively long overall survival(OS),multiple therapy lines are adopted for these patients during their lifetime,but the best sequence to be followed has never been clearly defined.Furthermore,new molecular markers aimed at predicting therapy response and prognostic scores[14,15]are currently being studied,but their application is still far from being part of daily clinical practice.A recent network meta-analysis including only phase-III randomized controlled trials(RCTs)has attempted to identify the best therapeutic strategy for controlling tumor growth,proposing the combination of peptide receptor radionuclide therapy(PRRT)and SSAs as the option with the best progression-free survival(PFS).However,this analysis seems very speculative and hard to apply to real-life settings.
This review will explore the available antiproliferative therapeutic options for GEP-NENs,based on evidence reported in the literature and on many years of experience in the field.It also includes the contribution of the specialists working in the multidisciplinary setting dedicated to NEN patients at Santa Chiara Hospital(APSS)in Trento(Italy).A separate session will be dedicated to new frontiers in the therapy landscape.Genetic syndromes and management of clinical syndrome(i.e.carcinoid syndrome)will not be discussed in this manuscript.
RESECTABLE DlSEASE
Endoscopic treatment
The incidence of GEP-NENs has increased in the last two decades also due to the extensive use of endoscopy,particularly following the worldwide implementation of bowel cancer screening programs.Endoscopic resection is reserved to small,localized NETs,mainly located in the rectum,stomach and duodenum.The endoscopist must have extensive knowledge of the macroscopic appearance of these lesions and perform endoscopic ultrasound(EUS)for staging when an invasive NET is suspected,and perform a biopsy when lesions arise from the deep mucosal layer and then extend into the submucosa[16].A thorough evaluation of tumor location,size,and depth of invasion are mandatory and a multidisciplinary consultation is recommended prior to resection even in case of small and low-grade lesions[17,18].
In this session,the endoscopic approach for gastrointestinal NETs will be discussed according to site,and our proposal for endoscopic management is reported in Table 1.

Table 1 Proposed endoscopic management for gastrointestinal neuroendocrine tumors
Colorectal NETs:Colonic NETs are located in the right colon in 70% of cases,can reach a very large size without obstructive symptoms,and are usually aggressive[19].Given their advanced stage at the time of diagnosis,endoscopic treatment has only been reported in case series,with a significant burden of complications and incomplete resections[17].
Rectal NETs(r-NETs)appear as small,sessile lesions,located within 5-10 cm of the anal verge,with overlying normal or yellowish mucosa.Larger lesions may also be semi-pedunculated or have central depression or ulceration[19].
Staging with EUS is not required for lesions < 10 mm in size due to the negligible risk of invasion[16,20].The endoscopist may be tempted to perform a standard snare resection but must bear in mind that the complete removal rate for polypectomy is approximately 30%,and for conventional endoscopic mucosal resection(EMR)it is highly variable(17%-90%)due to the submucosal nature of these nodules[19,21,22].
Modified EMR techniques have been employed to obtain a deeper resection.Cap-assisted EMR(EMR-C)uses a dedicated cap with a circumferential rim that can lodge a crescent snare.After saline injection of the submucosa,the lesion is suctioned within the cap and cut.Band-ligation EMR(EMR-L)also requires saline injection.Once the lesion has been adequately captured by the deployment of an elastic band(usually employed for variceal ligation),a snare resection is performed below the band.
The rate of histologically complete resection by modified EMR is high,particularly for EMR-L(93%-100%vs71%-100% for EMR-C)and comparative studies and a meta-analysis confirmed a higher complete resection rate than conventional EMR[20,23,24].Resection by EMR-C and EMR-L are both used for r-NETs,and the only comparative retrospective study available to date demonstrated similar effectiveness[23].The higheren blocresection rate for EMR-L was explained by the authors by the larger quantity of submucosa captured by the thickness of the elastic band.
Another technique for advanced endoscopic resection is endoscopic submucosal dissection(ESD).This technique is superior in terms of radical histologic resection in r-NETs ≥ 10 mm[17],but has similar outcomes to EMR-C and EMR-L for small r-NETs(< 10 mm)despite a longer procedure time[20,25].
Gastric NETs:Gastric NENs(g-NENs)usually arise from enterochromaffin-like(ECL)cells and are divided into three types.More specifically,Type I arises in the setting of a chronic atrophic gastritis,Type II is associated with gastrinomas,and Type III is sporadic and independent from gastrin levels.Two additional categories of g-NENs have been recently described and are currently being investigated:Type IV lesions arise from non-ECL endocrine cells,whereas another subtype of g-NETs might be determined by the chronic use of proton pump inhibitors[19,26,27].
Type I and II g-NETs have a highly variable endoscopic aspect(red or yellow,depending on the vascular supply)and are sometimes characterized by a central depression.They usually appear as smooth and rounded multiple polypoid lesions,with size < 20 mm and located in the gastric body and fundus[19,28,29].As Type I g-NETs are mainly characterized by indolent behavior,conservative management with endoscopic surveillance +/- resection is safe and effective also in the case of recurrent lesions[17,30,31].
Disease staging by EUS prior to resection is not required for small Type I g-NETs(< 10 mm)but it is mandatory when lesions are ≥ 10 mm,when Ki67 is > 3% or in the case of Type II g-NETs[17].The data regarding ESD show complete resection achieved in 75%-100% of cases,with a lower rate of positive vertical margins at histology compared to standard EMR[32-34].Modified EMR techniques(EMR-L or EMR-C)are currently being used for Type I g-NETs,and should be considered for small lesions(≤ 10 mm)that can be completely suctioned within the cap in order to obtain theen blocresection(Figure 2).

Figure 2 Endoscopic management of gastric neuroendocrine tumors presentation of a clinical case referred to our hospital.A 78-year-old female patient was referred to our Endoscopy Unit for resection of a lesion of the gastric fundus.Staging by endoscopic ultrasound showed hypoechoic lesion of 19 mm × 12 mm,with well-defined margins,originating from the third hyperechoic layer.Fine-needle cytology diagnosed a NET G1(Ki67 < 2%).The lesion was then resected by endoscopic submucosa dissection(ESD).Histological evaluation described a gastric NET(g-NET)G1,associated with autoimmune gastritis(Type I).During follow-up,another minor lesion(< 10 mm)suspected for NET was reported along the greater curvature,and resected by Band-ligation endoscopic mucosal resection(EMR-L).Histological report confirmed a Type I g-NET.A:Cardial area reflexed view;B:Resection base after ESD;C:Oriented and pinned specimen;D:Hematoxylin-eosin stain showing monomorphic cells in a nested architecture without necrosis;E:Corresponding Chromogranin A immunostain(20 × magnification);F:Corresponding Ki67 immunostain(20 × magnification);G:Endoscopic appearance of the lesion detected during follow-up;H:EMR-L:Rubber band release;I:Resection base after EMR-L.
Type II lesions are extremely rare,and in the absence of high-quality level data,their management is generally similar to Type I[27].However,considering their size upon presentation(≥ 10 mm)they usually require ESD for completeen blocresection that is better than EMR.
Type III g-NENs are larger,solitary lesions located anywhere in the stomach,sometimes with a broad fixed base and ulceration indicating deeper invasion[17,28,29].They require a complete disease staging,including EUS.As lymph node involvement is present in more than 50% of cases upon diagnosis andliver metastases is in 22%-75%,an endoscopic approach is not frequent in these cases[18,27].A recent systematic review included 121 patients from eight studies with small localized Type III g-NETs who underwent endoscopic resection.The complete resection rate varied from 72% to 87%,but details about the endoscopic technique were often not reported,preventing comparisons of the EMR and ESD outcomes[18].
Type IV g-NENs are described as aggressive lesions,with a size of > 40 mm upon diagnosis,and in the case of localized disease,surgical resection is preferable[19].
Small bowel NETs:Jejunal and ileal NETs are usually > 20 mm,multifocal in 40% of cases,and with lymphatic involvement upon diagnosis in 70% of cases[19].Due to these features,and as they are often beyond the reach of a device-assisted enteroscopy,a surgical approach is recommended for localized disease.Endoscopy may instead be helpful for diagnosis,in the case of bleeding,or for tattooing of the lesion[19].
Duodenal NETs(d-NETs)are usually small,sessile and solitary lesions,mainly located in the duodenal bulb or second part[28].As even sub centimetric tumors present lymphatic spread in 40%-60%upon diagnosis,EUS is mandatory,and resection by EMR or ESD is reserved to submucosal lesions < 10 mm with no lymphatic involvement[17,19].The management of intermediate(10-20 mm)lesions is controversial and based on local expertise[28].Considering the thin duodenal wall,some authors prefer to use standard EMR rather than modified EMR[29].Standard EMR has indeed shown outcomes that are comparable to EMR-C and EMR-L,although higher rates of complete histological removal(70%-92%)has been reported for EMR-L in a small case series[35-38].Some authors even suggest the autoamputation of small d-NETs using band ligation without snare resection[39].
The rate of radical resection by ESD in the duodenum is variable(67%-100%),due to the technical challenge of scope maneuvering in this anatomical district and the scarce submucosal lifting[36,40].Moreover,the complication rate may be higher than in other gastrointestinal districts,especially perforation(13%-67% in small case series)[36,41,42].Based on these considerations,ESD may be offered depending on local expertise and preferentially reserved to poor surgically-suited candidates.
Endoscopic full-thickness resection(EFTR)is usually reserved for subepithelial tumors originating from the muscularis propria.It has only been described for NETs in small case series and ideally should not provide a clear advantage compared to ESD as most NETs remain submucosal[17,43].The ability of EFTR to secure the intestinal wall with an over-the-scope clip under the cutting plane may overcome the risks of endoscopic resection in the duodenum[40].However,this advantage may be hampered by the technical drawbacks of operating this unwieldy device in the already difficult duodenal anatomy.
Duodenal NETs originate from the periampullary region in 20% of patients.In these cases,current guidelines recommend surgical resection because they have a more aggressive biology and their metastatic potential is independent of tumor size[18,28,30,35].Nevertheless,a growing body of evidence favors a prior attempt with endoscopic papillectomy[21,44].Prospective data are needed to evaluate the efficacy of this approach.
Esophageal NETs:Esophageal NETs(e-NETs)account for only 0.2% of total gastrointestinal NETs.Their appearance is similar to other gastrointestinal NETs,but they tend to have a central ulceration and may sometimes be multiple[29].Endoscopic resection can be considered in low-risk cases:Lesions ≤ 10 mm,without ulceration and confined to the submucosa according to EUS evaluation.Bothen blocEMR and ESD have been effectively used for complete removal.However,the exceptionally rare incidence of e-NETs does not allow high level comparative studies for these techniques[17].Regarding EMR,EMR-C and EMR-L are advocated to obtain a deeper submucosal resection than standard EMR.
Future perspectives and open questions:The available data regarding the use of SSAs in the management of Type I g-NETs derive from small,retrospective cohorts,resulting in controversial conclusions[45].Prospective trials exploring this approach would be useful in understanding the indications and the potential benefit of this alternative option which is currently considered only experimental.A prospective study describing the endoscopic appearance of gastrointestinal NETs and proposing an endoscopic classification would help recognize these lesions and select the suitable technique for endoscopic resection.
Surgery with radical intent
Surgery with radical intent is the preferred option in the management of all GEP-NENs,when feasible.Preoperative work should include complete disease staging with both morphological and functional imaging tests.We will discuss the surgical approach of these patients according to the tumor primary site and focusing on the main critical issues regarding this therapeutic option.
NETs of the appendix:Appendiceal NETs are usually incidental found during surgery for acute appendicitis.For this reason,radicality of the intervention and the indications to right hemicolectomy with lymphadenectomy still represent critical issues in the management of these patients.The European Guidelines for NETs have established,based on the literature,certain criteria aimed at guiding this decision according to the features of the tumor[46].More specifically,appendicectomy is considered sufficient when the tumor is < 1 cm and resection is R0.Right hemicolectomy is instead recommended when the tumor is > 2 cm.Regarding the “grey zone” of intermediate tumor size(1-2 cm),additional risk factors indicating a surgical re-intervention are represented by a G2 histology,signs of histological vascular or lymphatic invasion(V1 and/or L1)or a mesoappendiceal infiltration > 3 mm.
Small bowel NETs:Pre-operative tests to be performed in the case of small bowel NETs(Sb-NETs)should also include echocardiography(to evaluate carcinoid heart disease)and colonoscopy.The surgical procedures for resection should include the intraoperative exploration of all abdominal cavities and extensive lymphadenectomy,as one-third of the cases(regardless of primary tumor size)have lymph node metastases upon diagnosis.As these lesions are in almost 80% of cases small,multiple nodules,undetectable by conventional imaging tests,palpation of the entire jejunum and ileum is mandatory to achieve radical resection.These tumors are often characterized by mesenteric fibrosis,and in 5% of cases by small peritoneal implants.For this reason,Sb-NETs are sometimes diagnosed for acute intestinal obstruction.Resection of mesenteric metastases is usually feasible,unless in cases of complete vascular encasement or retroperitoneal involvement[12,47].
Pan-NETs:Regarding pre-operative evaluation for Pan-NETs,vascular involvement(superior mesenteric vein,superior mesenteric artery,coeliac axis and common hepatic artery)must be accurately assessed in order to discuss the feasibility of a curative resection.When patients are candidate to enucleation,EUS or magnetic resonance cholangiopancreatography help evaluate the relationship of the tumor with the pancreatic duct[12].There is an open debate about the management of non-functioning Pan-NETs < 2 cm and with no involvement of the main pancreatic duct.The two possible proposals are surgical resectionvsfollow-up.As long-term data concerning safety of the conservative management are insufficient,surgery can be considered in young,healthy patients.Parenchyma-sparing pancreatic resections(enucleation or central pancreatectomy)can be performed in these cases;however,complete surgery with these techniques is uncertain because lymphadenectomy is crucial to reach the radicality.In fact,recent data report that 12% of resected small Pan-NETs have lymph nodal metastases at surgery,with poorer recurrence-free survival(RFS)rates in the case of tumors of 15-20 mm[12,48].The decision to operate or just observe these patients also needs to be based on the general conditions of patients,as the benefit of surgery can be counterbalanced by significant morbidity and mortality rates compared to conservative management[49].
Locally advanced or metastatic disease:Regarding advanced Sb-NETs,surgery can be considered when patients suffer from symptoms due to mesenteric involvement but must be performed in specialized centers.In fact,radical resection or debulking surgery can significantly improve the quality of life of these patients[12].Encouraging results of curative resection are also available for GEP-NEN patients with TNM stage IV disease,but after ruling out the presence of extra-abdominal disease.When radical resection is feasible,survival rates are indeed better than debulking or medical treatments.For Pan-NETs,median OS for these three options accounts for 97,89,and 36 mo,respectively[50].However,careful patient selection is mandatory in order to reduce the risk of complications.The data regarding the use of neoadjuvant treatment associated with radical surgery are scarce.The RMPanNET trial will compare the survival outcomes of metastatic Pan-NETs treated with resection on the primary tumor and metastases after neoadjuvant systemic treatment(SSAs,targeted therapy or chemotherapy)vscontinuing only systemic treatment(Supplementary Table 1).The NEONEC trial will instead investigate the role of neoadjuvant treatment in terms of RFS in patients with localized NECs,adopting a cisplatin(or carboplatin)/etoposide regimen(Supplementary Table 1).
Role of adjuvant treatments:Unlike other cancers,the data regarding adjuvant treatments in GEPNENs after curative surgery are scarce,and this approach is not routinely applied in clinical practice.This limitation is probably due to the relatively long survival rates after radical resection without any other treatments(especially for GEP-NETs G1-G2)and to the lack of validated risk scores aimed at identifying patients at high risk of disease recurrence.
A recent retrospective,multicenter study from the United States has reported survival outcomes of 91 GEP-NETs treated with adjuvant treatments(chemotherapy or SSAs)after curative-intended surgery,compared to patients receiving surgery only[51].The results showed that adjuvant therapy had negative impact on RFS rates,with no benefit in terms of OS.Another piece of analysis from the Surveillance,Epidemiology,and End Results-Medicare(SEER)database included 318 colorectal NENs treated with radical surgery.Focusing on stage I-III TNM disease,no benefit in terms of OS or RFS was observed when adopting adjuvant chemotherapy compared to surgery only[52].These data discourage the use of adjuvant treatments,but might be read with caution due to the inevitable selection bias of retrospective studies.In fact,patients receiving post-surgical treatment,in a retrospective analysis,are characterized by more aggressive tumor features,and should not be compared to patients with theoretically less aggressive tumors.
Focusing on NENs G3,the available data concerning the use of adjuvant treatments after curative surgery are derived from retrospective cohorts and provide controversial results.In a series of 73 digestive NECs,with the majority having a colorectal primary tumor site,43 received chemotherapy,either neoadjuvant and/or adjuvant.The median OS and RFS for patients receiving chemotherapy were 62 and 13 mo,respectively,showing the potential prognostic impact of chemotherapy on survival outcomes[53].Another study compared the survival rates of 394 radically resected non-metastatic colorectal NECs receiving adjuvant chemotherapyvs412 undergoing radical surgery only.The median OS was significantly longer for patients treated with adjuvant therapy[57.4vs38.2 mo for patients treated with surgery only;hazard ratio(HR):0.73,P< 0.01],especially in the subgroup of patients with left-sided NECs[54].Discouraging results were reported by Linet al[55],who analyzed the data of 804 gastric NECs or MiNENs treated with radical surgery +/- adjuvant therapy.The study showed no statistically significant different OS between the two groups.In another retrospective series of 60 GEPNENs G3 with TNM stage I-III disease receiving radical surgery,the 2-year OS of the total population was 64.5% and the median RFS was 14 mo.Adjuvant therapy,adopted in 20 patients,did not improve either the OS or RFS rates[56].
Future perspectives and open questions:The ASPEN study is prospectively assessing clinical outcomes of patients with Pan-NENs < 2 cm managed by radical surgeryvsfollow-up[57](Supplementary Table 1).The validation of risk scores in prospective cohorts might help stratify resected GEPNENs according to the risk of disease recurrence.Patients at high risk might be enrolled in RCT evaluating the potential benefit of adjuvant therapies compared to curative surgery only.Studies evaluating response to adjuvant treatments should also include NETs,as data showing a potential benefit of this therapeutic option so far available were mainly obtained in the setting of NEC patients.
ADVANCED OR METASTATlC DlSEASE
Surgical resection of the primary tumor
Beyond the need for debulking in uncontrolled functioning syndrome,resection of the primary tumor is another possible surgical indication in metastatic disease.Some series have recently proved that,in addition to symptomatic relief(for example,for obstruction due to the mesenteric involvement in Sb-NETs),this approach has also a prognostic impact.In fact,in a retrospective series of 14510 GEP-NETs,a benefit in terms of survival has been observed for G1 and G2 patients[58].A very recent publication from the SEER Registry,including 2219 GEP-NETs,confirms these results for all sites excluding the rectum,with an overall HR of 0.65.In addition,the study highlights the importance of a careful patient selection in a multidisciplinary setting[59].These conclusions may however be limited by a selection bias,as in retrospective analysis the surgical approach might be reserved to patients with a better performance status or more localized disease[60].
Future perspectives and open questions:Prospective studies comparing the survival outcomes of patients with metastatic GEP-NENs treated with primary tumor resectionvspatients not undergoing this option would assess the potential prognostic impact of this surgical approach.
Locoregional treatments
Indications,efficacy,and safety:Up to 80% of GEP-NETs present liver metastases at the time of initial diagnosis.Current guidelines recommend vascular and ablative locoregional treatments only for NETs G1-G2 in the case of metastases involving only or predominantly the liver with stable extrahepatic disease.The goals are the relief of symptoms caused by hormone secretion or mass effect in order to improve quality of life,and survival prolongation by slowing the growth of liver lesions.In very select cases,locoregional treatments can be bridging therapies to liver transplantation[61,62].These treatments should be offered after discussion in a multidisciplinary team consultation,in the case of hepatic disease progression(DP),and might be also considered in conjunction with other systemic therapies or combined with surgery.The choice is based on liver tumor burden,patient symptoms,general clinical condition,but also on the local expertise and availability of the various procedures.
Liver-directed therapies for metastatic GEP-NETs include thermal ablation,transarterial embolization(TAE)or transarterial chemoembolization(TACE)and transarterial radioembolization(TARE),also known as selective internal radiation therapy(SIRT).The data regarding their anti-tumor efficacy primarily comes from retrospective studies using heterogeneous protocols,and consequently it is currently unclear which technique is preferable.
Ablation techniques(radiofrequency ablation,microwave ablation,and cryotherapy)require imaging guidance,and are only applied in the case of limited liver disease:Less than three lesions ≤ 3 cm,or a single lesion < 5 cm,or even in association with liver surgery[61].When feasible,thermal ablation shows lower complication rates than surgery(3.9%vs20%,respectively)and a good clinical response(with relief of symptoms in up to 92%)[63].Unfortunately,the benefits of ablation alone in terms of survival rates are difficult to demonstrate,due to the influence of subsequent lines of therapy in the calculation.
Vascular treatments are based on the rationale that neuroendocrine liver metastases are hypervascular,deriving all their blood supply from the hepatic artery,whereas the normal hepatic parenchyma is mainly supplied by the portal vein(75%).Arterial embolization makes it possible to deliver a tumoricidal dose of chemotherapy(TACE)or β-radiation(SIRT)in association with the ischemic effect on the lesions,thereby reducing systemic adverse effects(AEs)and limiting toxicity for the normal liver parenchyma through the use of a selective technique.The feared carcinoid crisis due to massive release of serotonin or vasoactive peptides in the case of secreting GEP-NETs is prevented by octreotide premedication and by scheduling the presence of the anesthesiologist during the procedure[11].A multicenter retrospective study showed better results for catheter-based therapies in terms of OS and hepatic PFS for lower grade NETs and for liver tumor burden ≤ 50%,regardless of the primary tumor site(Pan-NETs or Sb-NETs)[64].Previous studies have instead reported a higher morphological response rate(RR)and/or better OS for non-pancreatic cases[65].
The TACE uses a mixture of chemotherapy drugs and a temporary embolic agent(degradable starch microspheres of 50 μm,with a half-life of approximately 35–50 min),with the aim of preserving arterial patency for further cycles of treatment(Figure 3).Negative predictive factors for response to TACE treatment in GEP-NETs are represented by impaired liver function(ascites,bilirubin ≥ 2 mg/dL,albumin ≤ 3.5 mg/dL),tumor burden ≥ 70% and previous treatment with three or more systemic lines of therapy[61].In the case of bilobar liver involvement,a sequential approach with multiple selective or lobar TACE treatment sessions is recommended,usually at a 6-8 wk interval,with assessments for patient tolerance and response after each course.Possible complications include portal vein narrowing or thrombosis,bile duct dilatation leading to biloma formation,and liver necrosis with the possible development of abscesses.Caution is therefore recommended especially in the case of bilio-enteric anastomoses,when initial bile duct dilatation or segmental portal vein thrombosis is detected by pretreatment imaging,representing relative contraindications to the performing of TACE.
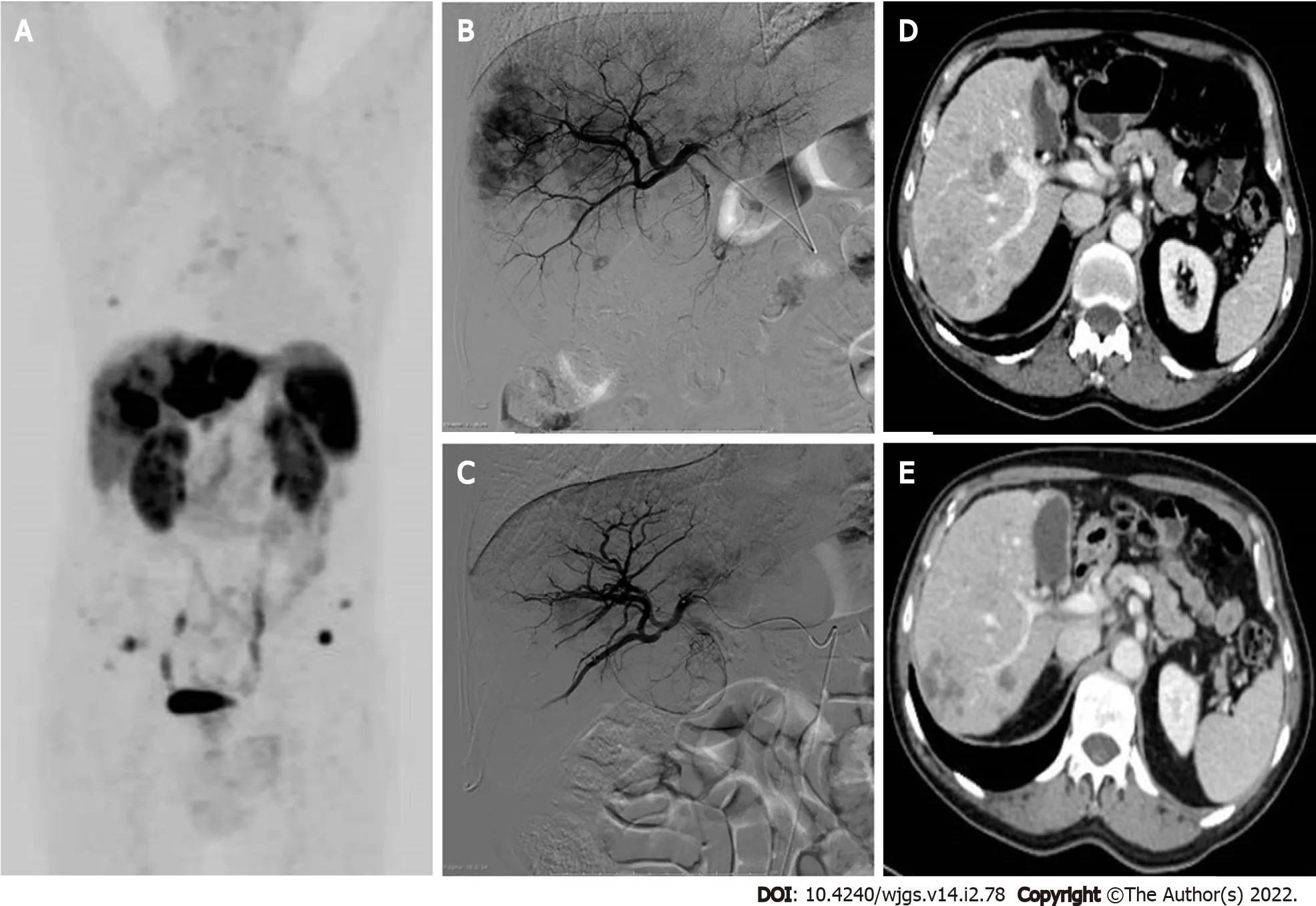
Figure 3 Locoregional treatments for neuroendocrine liver metastases-presentation of a clinical case referred to our hospital,with progressive liver disease after multiple systemic treatments.A:68Ga-DOTATOC-positron emission tomography/computed tomography(CT)whole-body maximum intensity projection image reveals multiple liver metastases involving both hepatic lobes,the left lobe being almost completely replaced by tumor.Bone and lymph nodal small metastases are also evident;B:Selective angiography of the right hepatic artery performed before lobar chemoembolization shows multiple hypervascular liver lesions;C:Selective angiography of the right hepatic artery performed 1 mo after two sessions of degradable starch microsphere transarterial chemoembolization(DSM-TACE).A marked reduction of the liver metastases enhancement is visible,preserved patency of the arterial intra-hepatic branches;D:Portal-phase CT scan before arterial chemoembolization:Multiple confluent hypodense lesions compared to liver parenchyma are detected in the right liver lobe;E:Portal-phase CT scan control after two DSM-TACE:Partial response of the liber metastases,which appear reduced in size and without contrast enhancement.Right portal vein branch narrowing represents an initial sign of liver/biliary toxicity.
Another technique,TARE with90Y-loaded microspheres,has a more favorable safety profile than TACE or TAE,with fewer AEs(pain,post-embolization syndrome,liver/biliary toxicity)in the early post-treatment period;however,hepatic cirrhosis with portal hypertension may appear as a long-term complication,especially in the case of bilobar treatment[66].Patients should undergo preprocedural evaluation for hepatopulmonary shunts to ensure that no more than 20% of the blood flow is diverted to the lungs to avoid radiation pneumonitis.
A recent meta-analysis revealed that patients treated with TACE had significantly better OS than those treated with TARE[67].TARE proved to be more effective than TAE/TACE when Ki67 ≥ 3%,whereas Ki67 < 3% predicts a greater benefit with TACE[68].TARE is indicated in the case of TACE failure or in patients at risk for TACE including major portal vein thrombosis,bilio-enteric anastomoses,and heart problems contraindicating doxorubicin administration.The costperprocedure for TARE is nearly double that of TACE;however,there is usually no need for multiple treatment sessions[61].The ArTisaN study will provide data on the efficacy of TARE in metastatic NETs in a phase II-designed study(Supplementary Table 1).
Future perspectives and open questions:Studies also including GEP-NETs G3 might explore the efficacy of locoregional treatments for liver metastases in these patients,especially cases with a lower proliferative index(e.g.,Ki67 < 55%).The LUTIA trial will investigate the efficacy of the intraarterial administration of177Lu-DOTATATE in patients with neuroendocrine liver metastases,and the impact on intra-hepatic biodistribution(Supplementary Table 1).The synergistic effect of liver directed-therapies with immunotherapy represents a further interesting approach to be investigated[69].
SSAs
Indications,efficacy,and safety:The expression of SSTRs is the prerequisite for benefiting from SSAs.These drugs bind with high affinity to the G protein-coupled transmembrane SSTR2 and with moderate affinity to SSTR5.They are usually adopted at the first-line stage in advanced GEP-NETs,with good tolerability.They have a double effect:Clinical syndrome control in functionally active NENs(i.e.carcinoid syndrome or duodenopancreatic functioning tumors),and antiproliferative effect[13].
Different formulations are available.The short-acting Octreotide is administered subcutaneously,usually to test the tolerability of the therapy.Long-acting formulations for antiproliferative treatment include Octreotide LAR(10,20,or 30 mg)with intramuscular injection,and Lanreotide autogel(60,90,or 120 mg)with deep subcutaneous injection.Pasireotide will not be discussed in this review,due to the limited and controversial results regarding its role as an antineoplastic treatment.
The antiproliferative effect of SSAs compared to placebo has been proved by two double-blind RCTs:The PROMID study[70]for Octreotide LAR and the CLARINET trial[71]for Lanreotide.Thanks to these publications,Octreotide LAR was registered for intestinal NETs and NETs of unknown primary tumor site,whereas Lanreotide autogel for intestinal NETs,Pan-NETs,or for cases with unknown primary tumor site.The recommended dosage for the antiproliferative use is the maximum available(Octreotide LAR 30 mg or Lanreotide autogel 120 mg,administered every 4 wk)[13].
The cumulative antineoplastic effect of Octreotide and Lanreotide compared to placebo has been assessed by a meta-analysis with an overall population of 289 patients,showing a reduction of DP risk of 41% by adopting SSAs compared to placebo[HR:0.41;95% confidence interval(CI):0.29-0.58,P<0.01][72].The meta-analysis also showed no statistically significant difference in terms of serious AEs(SAEs)between the two arms.However,a higher frequency of biliary stones occurred in the treatment arm(10.5%vs2.7%,respectively)[72].The elective prophylactic cholecystectomy in advanced GEP-NETs undergoing primary tumor resection represents a possible,but still debated,option in case SSAs are required.
Other possible side effects observed during treatment with SSAs are hypo/hyperglycemia,gastrointestinal symptoms(abdominal pain and diarrhea),and pancreatic insufficiency,which can be confirmed by fecal elastase test,and treated by pancreatic enzyme supplementation[73].
SSAs for highly proliferating Pan-NETs:Focusing on Pan-NETs,the available data regarding the efficacy of SSAs as antineoplastic treatment are limited to the CLARINET study,which however included only G2 cases with Ki67 < 10%[71].Thus the question regarding their use in the case of higher proliferative index remains open.A recent cooperative real-world study analyzed the antiproliferative effect of SSAs when adopted at the first-line stage for non-functioning,metastatic Pan-NETS with Ki67 ≥10%[74].The total population of 73 patients also included five Pan-NETs G3.The median PFS was 11.9 mo(95%CI:8.6,14.1),but a higher efficacy was shown in G2 patients and with limited hepatic tumor involvement.In detail,the median PFS was 12.4 mo in G2 patientsvs4 mo in G3 cases(P< 0.01).Patients with liver load ≤ 25% had a median PFS of 15 movs9.7 mo in the case of higher hepatic tumor load(P= 0.04).
Dose escalation:After the occurrence of DP during the treatment with SSAs,GEP-NET patients receive more aggressive and less tolerable drugs.A possible alternative option to this approach is a dose escalation of SSAs.A recent systematic review regarding this therapeutic strategy has reported a disease control rate(DCR)of 30%-100%,and a median PFS of 6.8-32 mo.These wide ranges are probably due to the heterogeneity of the included studies,as they are both retrospective and prospective and they adopt different SSA formulations and at different disease statuses[75].
The NETTER-1 study evaluated the administration of Octreotide 60 mg every 4 wk,but in clinical practice,the dose increase is usually performed by shortening the time interval between injections[76].The CLARINET FORTE study recently investigated,for the first time in a prospective setting,the potential benefit of this strategy in a series of Sb-NETs G1-G2 or Pan-NETs(NCT02651987)[77].After experiencing DP during monthly injections of Lanreotide 120 mg,patients were treated with the same dosage but every 2 wk,respectively for 48 and 24 cycles.The results were presented at the last ESMO Conference 2020,showing a duration of stable disease of 13.8 mo for Sb-NETs and 8.3 mo for Pan-NETs.The DCR after 48 wk was 33.3% and 22.9%,respectively.Toxicity was similar to the data observed in the CLARINET trial[71],additionally highlighting the good safety profile of SSAs also after dose escalation,with rare Grade 3 side effects.Considering the efficacy,the good safety profile and the absence of deterioration of quality of life with SSA dose escalation,this approach might represent a valid option for progressive NENs,as it can delay the switch to other potentially more toxic drugs.
Novel biomarkers:Measuring the transcript profile of blood in NET patients is more sensitive and specific than chromogranin A or other blood tests available,and might overcome the limits of imaging tests in assessing the tumor response.The “NETest” represents a transcriptomic signature of NETs,being a multianalyte algorithm analysis PCR-based test.It evaluates,using peripheral blood real-time PCR,the tumor biological activity by measuring the expression of 51 genes,which are associated with neoplastic behavior.In a prospective study,its role in predicting tumor progression during SSAs for GEP-NETs was assessed,showing an earlier prediction of DP than chromogranin A,with an accuracy of 80%-100%[15].Besides the potential applications of the NETest both for NET diagnosis and follow-up,this test is currently only experimental and it is unavailable in daily clinical practice[78].
Future perspectives and open questions:Besides the use of SSAs at the first-line stage in advanced GEP-NETs G1-G2,the role of these drugs in maintaining therapy is being explored.The REMINET trial is assessing whether Lanreotide 120 mg can maintain a stable disease in duodenopancreatic NETs G1-G2,after response to first-line chemotherapy.The preliminary results were presented at the last ENETS Conference 2021,but a phase III trial is needed for their validation(Supplementary Table 1).The TNEIDC-COLE trial is evaluating,in a prospective randomized setting,the potential benefit of prophylactic cholecystectomy in advanced GEP-NETs receiving SSAs(Supplementary Table 1).The indication of SSAs in G3 cases needs to be further investigated,as well as the potential benefit of SSAs in cases with low or heterogeneous expression of SSTRs.Prospective studies assessing the role of NETest in predicting response to SSAs,as well as other therapeutic options,are needed for validation of this test in clinical practice.
Interferon
Interferon alpha(IFN-α)is licensed in Europe for functioning GEP-NETs,but it can also control tumor growth.This latter function is based both on a direct antiproliferative effect(influencing the cell cycle,the production of growth factors,and angiogenesis),and an indirect immunomodulatory effect.Several prospective studies have investigated its efficacy as antineoplastic therapy,with conflicting results.
Bajettaet al[79]prospectively enrolled 53 patients affected by progressive,metastatic NETs.Patients received IFN-α-2a with the following scheme:3 × l06IU for the first 3 d,progressively increased to 6 ×l06IU for 8 wk,and then three timesperweek.After a median treatment duration of 6 mo,64% of patients showed partial or complete tumor regression,lasting 1-11 mo.Less enthusiastic results were reported by Faisset al[80],showing no benefit in terms of PFS adopting in naïve GEP-NETs the association of IFN-α/Lanreotide alone.Regarding comparison with chemotherapy,a study showed,in naïve patients with functioning tumor,a better DCR with IFN-α than with streptozotocin(STZ)/5-fluorouracil(5-FU)(P< 0.01)[81].
The most common clinical AEs that occur during IFN-therapy(nearly 50% of the patients)are:Flulike syndrome(fatigue,fever),which can be prevented by paracetamol,neurological disorders(depression),weight loss,abdominal pain,alopecia,pain at the injection site,and headache.Biochemical toxicity includes:Impaired liver functional test(one third of patients),leukopenia,autoimmune diseases(thyroiditis)in 20% of cases,anemia(31%),thrombocytopenia,hyper/hypoglycemia,and the production of neutralizing interferon antibodies.Considering the balance of pros and cons,and the fact that we currently have several alternative options for unresectable GEP-NENs,IFN therapy is currently reserved for only very select cases,mostly syndromic[13].Regarding the increase in dosage of IFN at DP,as well as its use in G3 patients,no consistent data are available in the literature.
PRRT
Indications,efficacy,and safety:PRRT is based on radiolabeled somatostatin receptor agonists binding SSTRs on tumor cells.After binding,they are internalized and stored in lysosomes,thereby delivering the radioactivity to the tumor cells.The target of PRRT is DNA damage induced by radiation and suboptimal repair,and this effect is more active during mitosis.Before PRRT begins,a basal Octreoscan®,68Ga-DOTA-PET/CT or64Cu-DOTA-PET/CT is mandatory in order to obtainin vivomapping of all lesions expressing SSTRs.Suitable patients for PRRT have strong SSTR expression,whereas extensive hepatic and/or bone disease,as well as decreased renal function,may limit its indication.According to ENETS Consensus Guidelines “PRRT is a therapeutic option in progressive SSTR-positive NET with homogenous SSTR expression(all lesions are positive)”[82].
Radiolabeled DOTA pharmaceuticals include90Y- or177Lu-DOTATOC,and currently,177Lu-DOTATATE(LutaThera®),which was approved for GEP-NETs by the United States Food and Drug Administration in 2018.Due to the high renal toxicity,90Y is now used for the locoregional treatments of liver metastases.The usual schedule for PRRT comprises four cycles of177Lu-DOTATATE over 6-8 mo,achieving total radioactivity of 25-30 GBq.Toxicity includes myelotoxicity,which can be mitigated with extracorporeal affinity adsorption treatment.This side effect is usually mild and reversible;however,up to 10% of patients may develop WHO Grade 3/4 hematotoxicity,and rarely myelodysplastic syndrome or leukemia[10,83].Nephrotoxicity may also be caused by PRRT,as the radiopeptides accumulate in the renal interstitium;however,this AE can be reduced by administering a positively charged amino acid infusion.Nausea,vomiting,or(rarely)carcinoid crisis may also occur with PRRT[10].
After a long series of retrospective studies investigating PRRT and proving its ability to inhibit tumor growth in 50%-70% of GEP-NETs[84],the first phase III RCT(the NETTER-1 study)[76]was published.It included 229 patients affected by progressive,unresectable,Sb-NETs G1-G2,and showed an improved outcome with Lutathera®+ best supportive care(including Octreotide 30 mg)than with Octreotide 60 mg administered every 4 wk.More specifically,PFS rates at month 20 were 65.2% in the177Lu-DOTATATE group and 10.8% in the control group,and a benefit was also observed in terms of quality of life[85].Based on this trial,Lutathera®has been registered for advanced,progressive GEPNETs(although Pan-NETs had not been included in this RCT).Further analysis of the NETTER-1 results showed that in the PRRT arm,PFS was not significantly affected by tumor shrinkage,suggesting that this treatment prolonged PFS even when tumor objective response was not detected at imaging[86].A delayed response to PRRT was indeed observed 3 years after PRRT in a patient participating in this trial[87].These encouraging results have been strengthened by a meta-analysis of 22 RCTs investigating the efficacy of Lu-DOTATATE/DOTATOC in a cumulative population of 1758 advanced/inoperable NETs[88].The pooled disease RR was 25.0%-35.0%,while the pooled DCR was around 80.0%,proving the efficacy of PRRT as an antineoplastic treatment in these patients.
In a recent consensus,the indication for PRRT was confirmed as a second-line treatment for GEPNETs with68Ga-DOTA-SSA-uptake in all lesions,in NET G1-G2 at DP,and in a subset of NETs G3 when all lesions are positive at68Ga-DOTA-PET/TC[89].Regarding the efficacy of PRRT in improving OS,the data are still scarce.A new analysis from the NETTER-1 trial,presented at the American Society of Clinical Oncology conference 2021,has however observed no significant benefit from PRRT compared to high-dose SSAs in terms of OS[90].
PRRT for G3 patients:The data regarding the use of PRRT in GEP-NENs G3 are derived from retrospective series,suggesting the potential active role of this treatment for highly proliferating cases.A recent review of the literature with the same topic has shown a median PFS of 19 mo when adopting PRRT in NETs G3 patientsvs11 mo for NECs with Ki67 < 55%,and only 4 mo for NECs with higher Ki67[91].Based on these results,PRRT can be considered for patients with increased uptake on somatostatin-based imaging tests,both in GEP-NETs G3 and NECs,but with a Ki67 < 55%,inoperable disease,life expectancy of at least 3–6 mo,and reasonable performance status(Karnofski Score > 50%)[82].A potential role for highly proliferating NEC patients might be reserved to very selected cases,and probably a dual tracer using somatostatin-based imaging tests and18Fluorine-fluorodeoxyglucose(18FFDG)PET/CT might be necessary for these patients.
Novel biomarkers and potential role of 18F-FDG-PET/CT:DP during PRRT is reported in 15%–30% of patients,and the lack of predictive biomarkers helping identify respondersvsnon-responders represents an open issue for NEN management.Proposed tests are the PRRT prediction quotient(PPQ),which is a blood-based assay for eight genes useful to predict PRRT efficacy with an accuracy of 97%,and the NETest,showing an accuracy of 98% in assessing response to PRRT.Trends of NETest correlate with PPQ prediction,but no tests can predict toxicity[92,93].The18F-FDG-PET/CT might also help select patients who are candidate for PRRT.It is commonly used in many tumors,but its value for NENs had been initially reserved only for poorly differentiated cases.The recent International Consensus regarding the role of theragnostic in NENs considered it suitable to employ18F-FDG PET/CT in NECs,in NETs G3 and also in NETs G1-G2,in order to identify the mismatched(18F-FDG-PET/CT-positive/68Ga-DOTA-SSA-negative)lesions[89].Indeed,as up to 45% of patients referred to PRRT may present heterogeneous SSTR expression,18F-FDG PET/CT might differentiate GEP-NETs G1-G2 disease into low- and high-risk patients of poor response to PRRT[94].
Re-treatment with PRRT:The opportunity to perform a second PRRT regimen,in patients already undergoing this therapy,is currently being discussed.Rudisileet al[95]re-treated 35 patients,who had previously received four cycles with177Lu-DOTATATE,obtaining a stable disease in 26 patients(81.3%).They concluded that salvage therapy with177Lu-DOTATATE is safe and effective,even in patients with extensive previous multimodal therapies during DP.The experience from Denmark reports a better response for G1-G2 cases than G3,but shorter survival outcomes upon retreatment(median PFS 19 mo,median OS 54 mo)[96].In 2021,a meta-analysis of seven studies regarding PRRT re-treatment in 414 patients with advanced NETs showed a median PFS of 12.52 mo,with a safety profile similar to the initial PRRT treatment[97].These encouraging data have been recently supported by a consensus on theragnostic in NENs,proposing PRRT rechallenge in patients with a stable disease for at least 1 year following therapy completion[89].
Neoadjuvant PRRT:The use of pre-surgical PRRT,aimed at obtaining disease downstaging,primarily derives from small retrospective series.The largest series includes 57 GEP-NETs with unresectable primary tumor due to vascular involvement,with or without liver metastases.After receiving preoperative177Lu-DOTATATE,resectable primary tumor was observed in 15(26.3%)cases.The estimated PFS rate at 2 years was 90%-95%,and OS accounted for 92.1%.A better response was observed in the case of:Duodenal NETs,GEP-NETs with no regional lymph node involvement,primary tumor < 5 cm,liver lesions ≤ 1.5 cm,number of liver lesions ≤ 3,and18FDG-uptake as a maximum standard uptake value < 5 in the primary tumor[98].Regarding Pan-NETs,neoadjuvant PRRT seems to reduce the size of the primary tumor,the size of metastatic lymph nodes,and the risk of pancreatic fistula,maintaining the same post-operative survival outcomes[99].
Future perspectives and open questions:Besides the available data supporting PRRT as a second-line treatment after SSA-failure,the efficacy of PRRT at first line will be evaluated by the NETTER-2 study,which adopts Lutathera®in combination with long-acting Octreotide in advanced GEP-NETs G2-G3 compared to high-dose(60 mg)long-acting Octreotide(Supplementary Table 1).The RCT is including both naïve patients and cases previously treated with SSAs in the absence of DP.The study will also provide more data regarding the use of PRRT in the treatment of GEP-NETs G3,probably also at first line.The identification of novel biomarkers helping select the right candidates for PRRT from the NENs would pave the way for the application of precision medicine in this field.The NeoLuPaNET trial will assess the role of neoadjuvant PRRT in resectable Pan-NETs at high risk of disease recurrence.The study endpoints will include post-operative 90-d morbidity and mortality rates,and objective RRs(Supplementary Table 1).Somatostatin receptor antagonists rather than agonists,labeled with radionuclides,are being investigated and seem to provide a longer tumor residence time of the administered dose.New alpha,beta,gamma,and Auger electron-emitting radionuclides are being investigated.In particular,212Pb-DOTAMTATE seems to be a possible alternative to177Lutethium(NCT03466216).The first results from a dose-escalation study on 6 patients were presented at the NANETS 2020 Conference[100],and the results are promising.
Targeted therapies:Everolimus
Indications,efficacy,and safety:Everolimus is an inhibitor of the mammalian target of rapamycin,which is an intracellular protein kinase downstream of the phosphoinositide 3-kinase(PI3K)/Akt pathway involved in tumorigenesis.It has been approved as an antineoplastic drug for progressive GEP-NETs as a result of several trials and also “real-life” experiences.It is usually prescribed at a standard dosage of 10 mg/d as continuous oral intake,but in the case of toxicity it can be reduced to 5 mg/d or interrupted(in the case of Grade 3 or 4 side effects).
Focusing on metastatic Pan-NETs,the phase II trial RADIANT-1 proved the efficacy in tumor control after chemotherapy failure of both everolimus alone(10 mg/d)and combined with Octreotide LAR,led to a median PFS of 9.7 mo and 16.7,respectively[101].The subsequent phase III RADIANT-3 study assessed tumor control by everolimus in 140 progressive Pan-NETs,and showed a significantly different median PFS compared to placebo:11.0 movs4.6 mo,respectively(P< 0.01)[102].
Regarding non-pancreatic NETs,the RADIANT-4 RCT evaluated the efficacy of everolimus 10 mg/d compared to placebo in progressive,well-differentiated,non-functioning lung and non-pancreatic digestive NETs[103].A significantly higher PFS was observed in the treatment arm compared to placebo(11 movs3.9 mo;P< 0.001),with a rate of disease stabilization respectively of 81%vs64%.The efficacy of everolimus was also proved in terms of OS,with a 36% reduction in the risk of death(HR:0.64;P=0.037).However,a recent meta-analysis of all available trials adopting everolimus for NENs confirmed the benefit in terms of PFS,but not in terms of OS[104].
The efficacy of everolimus and the good safety profile in advanced progressive GEP-NETs were also confirmed in the real-world setting.In 169 patients receiving this drug for compassionate use,the median PFS was 12 mo and the median OS was 32 mo.The results of the study also suggested the use of everolimus before chemotherapy and PRRT,as the subgroup of patients previously treated with these therapies had suffered due to higher toxicity[105].
Reported toxicity during treatment with everolimus includes:Stomatitis(up to 67% of cases),skin rash(29%–49%),fatigue(33%),infections(20%),diarrhea(30%),cytopenias(< 20%),pulmonary toxicity(10.4%),metabolic impairment(hyperglycemia 5%-13%,increased triglyceride and cholesterol levels 39%-66%,hypophosphatemia 40%),peripheral oedema(13%-20%),and renal impairment(rare and transient)[13,106,107].Regarding stomatitis,a systematic review observed a longer PFS when it occurs within 8 wk from the start of therapy[106].
Everolimus for G3 patients:A potential antiproliferative effect of everolimus in NENs G3 far been reported in well-differentiated cases.A median PFS of 6 mo and a median OS of 28 mo were observed in a small,retrospective cohort of 15 cases with Ki67 20%-55%[108].In this series,disease stabilization was maintained in 40% of cases for at least 1 year.Focusing on prospective studies,the NECTOR study(a phase II multicenter trial)has evaluated the safety and efficacy of everolimus after failure of platinumcontaining chemotherapy in Pan-NECs,providing discouraging results[109].In the enrolled 25 patients,the median PFS was only 1.2 mo and median OS was 7.5 mo.Disease control was obtained in 39.1% of cases,with no objective response.
Resistance to everolimus:The antiproliferative effect of everolimus may be limited by primary and secondary drug resistance.In detail,patients showing DP at their first evaluation after starting treatment are primary refractory,whereas cases facing DP after an initial tumor response are patients with acquired resistance[110].Several strategies are being investigated to overcome the resistance to everolimus.Retreatment after a pause might be an option,but this strategy is only supported by clinical experience and not by published data.A possibility reported in the literature is represented by BEZ-235,which is a dual inhibitor for PI3K and mammalian target of rapamycin(mTOR)(PI3K/mTOR kinase inhibitors),and has a potential synergistic effect when adopted in combination with everolimus.Passing from preclinical to clinical studies,about 250 patients affected by several tumor types were treated with this drug.Since the patients experienced high toxicity of the gastrointestinal tract and bone marrow,as well as early progression,the trials including Pan-NETs were prematurely stopped[111,112].
Future perspectives and open questions:The EVINEC study is currently enrolling patients with G3 neuroendocrine disease,after platinum-based chemotherapy failure,to be treated with everolimus(Supplementary Table 1).This trial will provide further data regarding the use of this therapy in NEC patients.The possibility to retreat patients with everolimus,alone or in combination with other drugs,has never been investigated but may represent another option to be evaluated in future studies.This strategy might also help overcome the resistance to everolimus.
Targeted therapies:Sunitinib
Indications,efficacy,and safety:Sunitinib is an oral multikinase inhibitor competing with ATP for binding within the intracellular domain of various wild-type and/or mutated receptor tyrosine kinases.This antiangiogenetic drug acts against vascular endothelial growth factor receptors,platelet-derived growth factor receptors,KIT,fms-like tyrosine kinase 3,and RET.It has been registered for advanced progressive Pan-NETs at a standard oral daily dose of 37.5 mg,based on a double-blind phase III RCT including 171 well-differentiated,advanced,progressive Pan-NETs receiving sunitinib or placebo[113].The trial was interrupted early due to the significantly different outcomes and toxicity observed in the two arms:Median PFS 11.4 mo with sunitinibvsonly 5.5 mo in the placebo arm(P< 0.01),OS at 6 mo 92.6%vs85.2%,respectively(P= 0.02).A re-analysis of this study[114]showed no significant difference in terms of quality of life between the two arms,with the exception of a worsening of diarrhea observed in the treated patients(P< 0.05).Reported toxicity observed during treatment with sunitinib generally includes gastrointestinal symptoms(diarrhea,nausea,vomiting)in 33%-59% of cases,and fatigue(41%of patients).Other possible side effects can be hypertension,headache,the hand-foot syndrome,and neutropenia(Grade 3-4 in 12%).Treatment discontinuation due to side effects occurs in 15% of patients,and 31% require a dose reduction[13].Experiences from the real-world setting reported,in 62 Pan-NETs receiving Sunitinib for a median time of 165 d,objective response in 13.7% of patients,but the need for dose reduction in 41.9%[115].In an Italian retrospective study[116]of 80 pre-treated Pan-NETs receiving sunitinib,the median PFS was very close to the results of the trial by Raymondet al[113](10 mo),with 7.5% of patients stopping the treatment due to toxicity.The data concerning the efficacy of sunitinib in non-pancreatic NENs are scare and disappointing.One study from Korea[117]adopted sunitinib in 10 non-pancreatic patients,observing a disease stabilization in 50% of the series,but a poorer median PFS than in cases treated with everolimus:1.7 movs14.7 mo,respectively(P< 0.01).
Sunitinib for G3 patients:Regarding G3 disease,data regarding the use of sunitinib are scarce.Mizunoet al[118]observed,in 15 unresectable Pan-NENs G3 receiving sunitinib,a significantly better outcome for Pan-NETs G3 than Pan-NECs(P< 0.05),and no significant difference between Pan-NETs G3 and G1-G2 cases.A tumor response in G3 cases treated with sunitinib was also observed by Pellatet al[119]in an open-label study,who described in 31 GEP-NENs G3 a median PFS of 42 d,and median OS of 181 d.However,this study was primarily focused on biomarkers,and did not report further details regarding survival.
Future perspectives and open questions:Prospective studies should assess the efficacy of sunitinib in non-pancreatic,digestive NENs,as well as in GEP-NENs G3.
Targeted therapies:Surufatinib
Surufatinib is an oral tyrosine kinase inhibitor targeting immune cells and angiogenesis.To date,few data are available on its efficacy in GEP-NENs,but they are encouraging.Results of the SANET-ep RCT[120]enrolling 198 patients with progressive,unresectable or metastatic,well differentiated,extrapancreatic NETs showed a better median PFS for the surufatinib arm compared to placebo(9.2 movs3.8 mo,respectively,P< 0.01).The SANET-p trial included 172 progressive,advanced,Pan-NETs,receiving surufatinib or placebo.The median PFS rates were 10.9 movs3.7 mo,respectively(P< 0.01)[121].Based on these results,surufatinib might represent a possible further therapeutic option for advanced GEPNENs,but it also needs to be evaluated in a real-life setting to draw definitive conclusions,especially if we consider the reported toxicity.The two available trials[120,121],in fact,showed more frequent AEs,the occurrence of Grade 3 or worse hypertension,proteinuria,and hypertriglyceridemia.SAEs were reported in 22%-25% of cases in the surufatinib group,and death was observed in 3 patients in both trials.
Chemotherapy
According to the ENETS Guidelines[9],chemotherapy in general represents a valid option for progressive or advanced Pan-NETs and GEP-NENs G3.Besides these indications,it may also be considered in other particular situations,such as GEP-NENs G2 with high Ki67,in the case of rapidly progressive disease,after the failure of other treatments,or even in cases not expressing SSTRs.
Chemotherapy:STZ
Indications,efficacy,and safety:STZ is generally adopted in advanced/metastatic Pan-NETs G1-G2 with high tumor burden,with the aim of obtaining an objective response.STZ is an alkylating agent,usually administered intravenously as a daily regimen for a 6-wk schedule,by rapid injection or short(15–30 min)infusion with a maximum single dose of 1500 mg/m2.The data concerning its efficacy are controversial,and this drug is not available in some European countries(including Italy).A retrospective study from Germany adopted STZ/5-FU in 96 Pan-NETs,including 56.3% naïve patients,and 6.3% G3.Objective response was reached in 42.7% of patients and stable disease in 40.6%.The median time to progression and OS were 19.4 and 54.8 mo,respectively.A better outcome was observed for Pan-NETs with Ki67 < 15%[122].Besides the association with 5-FU,an alternative combination of STZ with doxorubicin(or even the STZ/5-FU/doxorubicin regimen)has been investigated,and a better response was observed compared to STZ/5-FU;however,the application of these regimens was limited by a significant cardiotoxicity[123,124].
The most frequent AEs caused by STZ are renal toxicity(dose-related and cumulative),gastrointestinal symptoms(nausea,vomiting,diarrhea),glucose intolerance,liver dysfunction,and hematotoxicity.STZ is mutagenic and carcinogenic and its extravasation causes necrotic tissue lesions[9].With regard to toxicity,a Japanese retrospective,multicenter study[125]reported in 110 patients the same efficacy adopting a dailyvsweekly administration of STZ-based chemotherapy,and with monotherapyvscombination therapy,but with a significantly better tolerability when STZ was adopted as a monotherapy.The objective response observed in the overall population was 21.8%,with median PFS of 9.8 mo.Schraderet al[126]proposed maintaining therapy with STZ/5-FU,using an extended cycle protocol.After the 6-wk protocol,resulting in a median PFS of 21 mo and a median OS of 69 mo,13 of the 28 included patients were switched to an extended 3-mo cycle protocol for maintaining therapy.This treatment provided an additional median PFS of 23 mo.
Future perspectives and open questions:The use of STZ for non-pancreatic GEP-NETs needs to be further investigated,and might represent a potential option as a neoadjuvant treatment.There are a few studies that evaluate a potential role of STZ in the management of G3 cases and these provided conflicting results[122,127,128].This option should be further investigated in a prospective setting.Therapy combination with PRRT might be explored as a possible additional therapeutic option for GEPNETs.
Chemotherapy:Temozolomide and capecitabine
Indications,efficacy,and safety:Temozolomide is an oral alkylator,whereas capecitabine is an oral prodrug for 5-FU.Their association(CAPTEM)usually follows a scheme consisting of capecitabine 750 mg/m2twice daily(days 1–14)and temozolomide 200 mg/m2once daily at bedtime(days 10–14)every 28 d[129].Chemotherapy with CAPTEM has been initially adopted in advanced Pan-NETs G1-G2,based on retrospective studies showing a synergistic effect of these two drugs against tumor proliferation.A randomized phase II study(NCT01824875)including Pan-NETs has definitely proved its superiority in disease control compared to only temozolomide,observing a median PFS of 22.7 movs14.4 mo,respectively(P= 0.023),whereas median OS was not reachedvs38 mo[130].
The cumulative antineoplastic effect of CAPTEM regimen has been calculated by a recent metaanalysis including 15 studies and a total population of 384 NENs:Median OS was at least 12 mo and DCR was 72.89%[131].The efficacy of CAPTEM has also been assessed at first line for Pan-NETs,resulting in an objective response in 70% of patients and median PFS of 18 mo[129].Regarding toxicity,most frequent AEs due to temozolomide are gastrointestinal symptoms(vomiting,mild nausea,constipation,anorexia),rash,headache,and fatigue,but convulsions may also occur.Grade 3-4 events have been observed in more than 40% of cases after 4 mo of therapy,and may remain in more than 30%for 12 mo following the stopping of treatment.They include thrombocytopenia(3.36%),neutropenia(0.69%),lymphopenia(0.65%),anemia(0.59%),mucositis(0.57%),and transaminase elevation(0.13%)[9,131].Capecitabine is associated with hand-foot syndrome and liver toxicity(usually hyperbilirubinemia).Less frequently,hematological toxicity may also occur.Side effects are usually reversible and do not require permanent drug discontinuation,but only a dose reduction[9].
CAPTEM for non-pancreatic GEP-NETs and G3 patients:Some series report the use of CAPTEM regimen also for non-pancreatic GEP-NETs.Ostwalet al[132]included in their series of 29 NENs G2-G3 also 12 Sb-NENs,obtaining a median PFS for the overall cohort of 33.7 mo.Spadaet al[133]analyzed data regarding 170 NETs treated with temozolomide-based chemotherapy,including 21 gastrointestinal primary cases and G1 cases.Objective response of the overall population was 28%,median OS 35.6 mo,and median PFS 14.7 mo.The efficacy and safety of CAPTEM regimen have also been proven after prolonged administration in a retrospective study from Israel[134]including 79 NENs with median treatment duration of 12.1 mo(range 0.6-55.6).The median PFS was 10.1 mo and median OS 102.9 mo,with DCR achieved in 59.5% patients.SAEs were rare,with a low discontinuation rate.Regarding the use of temozolomide-based therapy for NENs G3,data from the literature describes CAPTEM as the most commonly used treatment for NETs G3,with a DCR of 65%(35% objective response)and a median PFS of 9.4-12 mo[135].Instead,the few data available regarding the use of this temozolomide-based regimen in GEP-NECs report poorer disease control in this subset of patients compared to all NETs(HR:2.70)[136],with a median PFS of 1.8 mo and a median OS of 7.8 mo observed in unresectable extrapulmonary NECs after platinum-based chemotherapy failure[137].
Neoadjuvant use of TEMCAP:Only two series sought to exploit the downstaging effect of TEMCAP as a neoadjuvant treatment.In a series of 30 Pan-NETs with advanced disease or hepatic metastases,partial response after CAPTEM was achieved in 43% of cases[138].Another report from the United States adopted in six Pan-NETs with borderline criteria for resectability the CAPTEM regimen +/-radiotherapy before surgery,and obtained in all the patients a radiologic response,and a R0 resection in four[139].
Future perspectives and open questions:Alkylating agents(temozolomide,dacarbazine,DTZ)transfer methyl adducts on DNA bases.Of these,O6-methylguanine accounts for many of their cytotoxic effects and can be repaired by the O6-methylguanine-methyltransferase(MGMT).Approximately half of Pan-NETs are MGMT-deficient,as determined by impaired tumor MGMT expression or by MGMT promoter methylation[133].An open issue is whether the MGMT deficiency may be a relevant biomarker for increased response and improved survival in these patients.Prospective studies evaluating this possibility and attempting to standardize the assessment of MGMT status are needed.Prospective studies investigating the potential benefit from neoadjuvant CAPTEM for advanced GEPNETs would provide data regarding a possible further cytotoxic role of this chemotherapy regimen.
Chemotherapy:Platinum-based regimens
Indications,efficacy,and safety:Platinum-based chemotherapies are considered the standard of care for unresectable GEP-NECs[9].Sorbyeet al[140]showed a better outcome for advanced GEP-NENs G3 adopting palliative chemotherapy compared to best supportive care.Median OS was indeed 11 movs1 mo,respectively.Patients with Ki67 < 55% had a lower RR to the treatment(15%vs42%,P< 0.001)but a better OS than cases with higher Ki67(14 movs10 mo,P< 0.001).Furthermore,the analysis identified negative prognostic factors for survival a poor performance status,a primary tumor colorectal site,and elevated platelets or lactate dehydrogenase levels.
A recent study performed a reclassification of G3 patients previously treated with platinum-based chemotherapy based on the new WHO classification[6].In this analysis,a higher RR was observed for NECs with Ki67 ≥ 55%(44%)than NECs with Ki67 < 55%(25%)or NETs G3(24%).Median PFS was instead 5 mo for all the subgroups[141].The cisplatin-etoposide regimen(or alternatively carboplatinetoposide,or irinotecan-cisplatin)is usually adopted at first line in these neoplasms,with an expected RR of 30%-70% and high toxicity.An adequate organ function and performance status are thereby required to receive this systemic treatment[9,142,143].
Cisplatin is usually administered by intravenous infusion,after intensive pre- and post-treatment intravenous hydration +/- osmotic diuretic(to prevent renal toxicity)[9].Etoposide is usually administered by intravenous infusion,but oral formulation is also available[144].Regarding toxicity,cisplatin is contraindicated in the case of renal impairment or allergic reactions against platinum compounds,whereas dose reduction is not needed in the case of liver function impairment.Side effects(involving at least 10% of patients)include gastrointestinal symptoms(anorexia,nausea,vomiting,and diarrhea),hematotoxicity(leukopenia,thrombocytopenia,and anemia),renal disorders,hearing impairment,fever,and peripheral sensory neurotoxicity(transient or permanent)[9].The data concerning the possibility to adopt carboplatin in the case of renal failure as an alternative to cisplatin are still scarce;however,AEs,including liver failure,may occur also with carboplatin[9].Etoposide is carcinogenic and mutagenic.The dose-limiting effect of etoposide is myelosuppression.Impaired hepatic or renal function may increase etoposide concentration in tissue.Gastrointestinal symptoms may also occur,as well as stomatitis and temporary hair loss[9].The association of oxaliplatin-based chemotherapy with 5-FU,leucovorin,and oxaliplatin(FOLFOX)or with capecitabine(XELOX)are usually adopted for NECs as a second or further line of therapy,with an expected DCR of 62%-84%[145,146].Some series have shown activity also in GEP-NENs G1-G2[145,147],and a retrospective series has reported promising results with FOLFOX also adopted at first line for GEP-NETs G2 and GEP-NENs G3[148].Toxicity from FOLFOX includes hematotoxicity(84.1%),chemotherapy-induced peripheral sensory neuropathy,renal toxicity,and infections[148].
Platinum-based chemotherapy for GEP-NETs G3:The efficacy of platinum-based chemotherapy in unresectable GEP-NETs G3 is uncertain.A recent retrospective series analyzed the efficacy of platinumbased treatment,regardless of tumor differentiation and grading[149].The data regarding 50 Pan-NETs and 29 Pan-NECs were collected,observing partial response in 20% and 41%,respectively.Median OS was 10.9 movs29.2 mo,respectively,and no statistically significant difference in terms of PFS was observed.A potential role of cisplatin-etoposide and FOLFOX-regimens in NETs G3 have been suggested,but with a short-lived response[135].These data also suggest a potential role of platinumbased regimen in Pan-NETs,but patient selection still represents a critical issue.Some molecular markers have been proposed to help select patients(e.g.,retinoblastoma protein,KRAS,and TP53 mutations),but the data are still scarce and only based on retrospective series[150-152].
Future perspectives and open questions:Prospective studies investigating new biomarkers predicting tumor response to platinum-based chemotherapy would help select the right candidates for this treatment,including a subgroup of GEP-NETs G3.
Prospective studies adopting FOLFOX at first line in GEP-NENs G3 would definitely assess the potential efficacy of this regimen in these aggressive neoplasms.
Chemotherapy:Fluorouracil,leucovorin,and irinotecan
Regarding GEP-NECs,chemotherapy with fluorouracil,leucovorin,and irinotecan(FOLFIRI)is a possible second-line option for GEP-NENs after cisplatin-etoposide failure.The series published to date are small and retrospective[153].A randomized,non-comparative,multicenter phase II trial(the SENECA study)will assess the efficacy of CAPTEMvsFOLFIRI in GEP-NECs as a second-line treatment after failure of platinum-based therapy(Supplementary Table 1).
Immunotherapy
Indications,efficacy,and safety:In the last decade,immunotherapy has revolutionized the prognosis of many solid tumors,such as melanoma and non-small cell lung cancer.However,the efficacy of immune checkpoint inhibitors(ICIs)in NENs is disappointing.The reasons for this failure might be related to their tumor biology,since NETs are usually characterized by a slow growth rate,a relatively low tumor mutational burden and a rare microsatellite instability[154,155].Instead,although NECs are highly aggressive neoplasms with high tumor mutational burden,ICIs have not achieved the expected results with these patients[154,156,157].
One of the first ICIs tested in NENs is pembrolizumab,a highly selective,humanized monoclonal antibody blocking the interaction of programmed cell death protein 1(PD-1)with its ligands[programmed death-ligand 1(PD-L1)and PD-L2].The multicohort,single-arm,phase 1 KEYNOTE-028 basket trial evaluated the safety and efficacy of pembrolizumab monotherapy across 20 tumor cohorts,including a cohort of 25 non-pancreatic NETs and a cohort of 16 Pan-NETs.Patients had a PD-L1-positive tumor and were mostly heavily pre-treated.The median follow-up was 20 mo and the overall RR 12.0% in non-pancreatic NETs and 6.3% in Pan-NETs,respectively.The range of response duration was 6.9-17.6 mo.No complete response was observed[158].In the subsequent phase II KEYNOTE-158 basket trial,pembrolizumab was administered in a cohort of 107 progressive NETs.Patients were enrolled regardless of PD-L1 expression.Objective(only partial)response was achieved in 3.7% of patients,and they all had PD-L1-negative neoplasms.The treatment provided disease stabilization se in 57% of cases,a median PFS of 4.1 mo and a median OS of 24.2 mo.Although these results seem encouraging,they need to be read with caution,as NETs are characterized by a slow growth[159].
ICI for G3 patients:The role of ICIs was also analyzed in NENs G3.Vijayvergiaet al[160]published a joint analysis of two prospective,non-randomized trials with pembrolizumab in 29 advanced NENs G3 after failure of platinum-based treatment.In 1 patient(3.4%),an objective response was observed,while 6(20.7%)achieved stable disease.The median PFS was 8.9 wk,with no significant differences between the PD-L1-positive and PD-L1-negative groups.Similar and no clinically relevant results were obtained with avelumab[161].Another humanized anti-PD-1 antibody,spartalizumab,was evaluated in a phase II,multicenter,single-arm study of 95 patients including 55 GEP-NETs and 21 GEP-NECs[162].All patients were progressive at study entry and had received prior treatment for advanced disease.The DCR was 64.2% in the NET group and 19% in the GEP-NEC group,with a better outcome observed for thoracic NETs.However,this study was formally negative because the primary endpoint(objective response > 10%)was not reached.
Combination immunotherapy:Studies of combination immunotherapy with dual blockade of PD-1 and cytotoxic T-lymphocyte antigen 4(CTLA-4)have shown more promising results.In the DART SWOG 1609 basket trial,ipilimumab was adopted in combination with nivolumab[163].The cohort of rare tumors also included 32 extra-pancreatic NENs(18 with high-grade disease).One patient obtained complete response(3%),whereas 7(22%)achieved a partial response,with a better outcome achieved by NECs than NETs(P= 0.004).The combination of durvalumab with tremelimumab in patients with progressive NETs was investigated in the phase II DUNE trial.This study recruited 123 patients,including GEP-NENs after the failure of standard therapies.The immune-related RECIST objective response was 0% for gastrointestinal NETs,6.3% for Pan-NETs,and 9.1% for GEP-NENs G3[164].
Future perspectives and open questions:Considering the poor results obtained by adopting immunotherapy in NENs,compared to other solid cancers,new biomarkers able to identify the right candidates for immunotherapy are needed.New prospective trials investigating further immunotherapy combination are also needed to provide further therapeutic options to progressive,heavily pretreated patients.More data concerning immunotherapy for GEP-NECs are also needed,as therapeutic options for these aggressive cases are still scarce.
NEW FRONTlERS
The concept of new frontiers in the field of therapy for GEP-NENs can have several interpretations.Besides the introduction of novel medications,new perspectives also include:Endoscopic ablation for Pan-NETs,medical therapy combination,and the optimization of therapy sequences.
Endoscopic ablation for Pan-NETs
The development of specifically designed accessories and suitable technologies for locoregional treatments with EUS guidance has made it possible to perform tumor ablations in Pan-NETs not eligible for surgery,resulting in a lower morbidity rate.
These treatments include EUS-guided radiofrequency ablation(EUS-RFA),which is utilized for the treatment of small functional Pan-NETs to improve symptoms without serious complications[165].The same technique has also been used to treat non-functional asymptomatic Pan-NETs,with complete response obtained in 71.4%-85.7%[166].
Another technique that has demonstrated good safety and reproducible results is ablation by ethanol injection.Multiple case reports and case series have been published with a procedure success rate ranging from 50% to 60% for non-functioning Pan-NETs and 93% for functioning Pan-NETs[167].Similar results were achieved in a larger cohort by Choiet al[168],treating 33 Pan-NETs with a mixture of 1:1 ethanol and lipidiol.Complete ablation was observed in 60% of the lesions,with complete necrosis at the 3-year follow-up in 41%.
The RAPNEN trial is currently recruiting patients with Pan-NENs to be treated with EUS-RFA,and will investigate the efficacy of this treatment in both functioning and non-functioning cases,including G3 patients(Supplementary Table 1).
Therapy combination
Several studies have investigated the antiproliferative effect of therapy combinations,thereby exploiting the potential synergistic effect of the different treatments.This approach must however be investigated with caution,also considering the possible superior toxicity compared to single treatments.
Therapy combination with PRRT:In one phase II clinical trial[169],177Lu-DOTATATE PRRT was implemented with capecitabine and temozolomide in advanced low-grade NETs,achieving DCR in 71%of patients,a median PFS of 31 mo and median OS not reached.AEs were mild to moderate,and most frequently represented by nausea,thrombocytopenia and neutropenia.In another phase II study[170],the efficacy and toxicity of PRRT with177Lu-DOTATATE were assessed in combination with metronomic capecitabine,as a radio-sensitizer agent,in advanced,progressive18FDG-positive GEP NETs with Ki67 <55%.The DCR was obtained in 85% of cases,with a median PFS of 31.4 mo,and a median OS not reached.No renal toxicity was observed in this series.
The combination with CAPTEM was also investigated as a sandwich chemo-PRRT treatment.More specifically,within 2 wk after PRRT,CAPTEM was administered followed by a 2-wk rest period;the next cycle of CAPTEM was repeated similarly and followed by 1 mo break,and the next cycle of PRRT was administered at about 3 mo.Two cycles of CAPTEM were therefore sandwiched between two cycles of PRRT.With this treatment schedule,DCR was observed in 84% of cases,while the median PFS and OS were not reached at a median follow-up of 36 mo[171].Regarding first-line PRRT,a series from India investigated its efficacy in association with Capecitabine in 45 consecutive unresectable NETs,with favorable outcomes.In detail,partial response was observed in 30% of cases,and the median PFS was 48 mo[172].
Therapy combination with everolimus:Bajettaet al[173]adopted the everolimus + LAR Octreotide combination regimen in naïve advanced NETs,and obtained positive conclusions.More specifically,18% and 74% of the cases showed objective response and disease stabilization for at least 6 mo,respectively.The EVERLAR study[174]reported prospective data on everolimus in combination with SSAs in non-functioning gastrointestinal NETs,with encouraging results in terms of both safety and efficacy.Indeed,the 24-mo PFS rate was 43.6%,with objective response achieved in 2.3% and stable disease in 58.1%.The median OS was not reached after 24 mo.Focusing on chemotherapy,the combination of everolimus and temozolomide offered interesting results in advanced Pan-NETs.In 40 patients treated with these two therapies for 6 mo,no synergistic toxicities were observed and 40% of patients experienced a partial response.The median PFS rate was 15.4 mo,whereas the median OS was not reached[175].A single-arm trial(NCT02248012)will show the potential synergy of everolimus and temozolomide in NET G3 patients with Ki67 ranging from 20% to 55%(Supplementary Table 1).
Therapy combination with sunitinib:The combination of sunitinib and SSAs adopted in 50 NET patients lead to a "not reached" median PFS,with DCR of 86%.These results come from real-world studies and may be limited by retrospective design and heterogeneous population[176].Sunitinib was also investigated to potentiate tumor control after TAE in 23 NETs,administered for 1 year after the procedure,achieving a median PFS of 15.2 mo and RR of 72%[177].
Chemotherapy combination:A recent retrospective study has evaluated response to treatment with 5-FU,doxorubicin and STZ(FAS)in Pan-NETs.Median PFS was 20 mo and median OS 63 mo.A better outcome was observed when adopting the FAS regimen at first line,without significant safety concerns[178].The BEVANEC trial is currently recruiting GEP-NEC patients,after failure of platinum-based chemotherapy,to receive a combination of bevacizumab with FOLFIRIvsFOLFIRI alone(Supplementary Table 1)[179].
Therapy sequence
Although the therapeutic landscape for NENs offers several options,the correct therapy sequence to be adopted is so far unknown.Several trials are attempting to compare different sequences in order to understand,on the basis of benefit and toxicity,which alternative option should be preferred.The safety and efficacy of everolimus after prior treatment with PRRT was investigated in a multicenter study including 24 GEP-NETs[180].Major clinical AEs during treatment with everolimus were hyperglycemia(20.8%),thrombocytopenia(8.3%),fatigue(8.3%)and elevated alanine transaminase levels(8.3%).The median PFS was 13.1 mo,longer than observed in previous trials,suggesting that pretreatment with PRRT might not affect response to everolimus.A retrospective series of Pan-NETs pretreated with chemotherapy followed by PRRT showed that previous treatment with more than one chemotherapy line was a negative prognostic factor for survival outcome,whereas resection of the primary tumor had a positive impact on survival[181].The COMPETE trial is currently recruiting unresectable,progressive GEP-NETs G1-G2 to receive treatment by PRRT with177Lu-Edotreotidevseverolimus(Supplementary Table 1).The SEQTOR study,which has been developed in Europe,aims instead to investigate the optimal sequence for everolimus and chemotherapy(Supplementary Table 1).
CONCLUSlON
In summary,as GEP-NENs represent a highly heterogeneous disease treated with therapeutic protocols that are not fully standardized,a multidisciplinary approach is mandatory for their management.Great advances have been made in the last decade in terms of treatments.Current trials will help answer the open questions regarding therapies for these patients,offering new perspectives in terms of novel drugs,therapy sequence and therapy combination.These data,together with molecular profiling and the application of radiomics in the understanding of tumor features and behavior,will also pave the way for precision medicine in this oncological field.
FOOTNOTES
Author contributions:All authors performed the literature research,wrote the manuscript,and read and approved the final manuscript.
Conflict-of-interest statement:All Authors declare no conflict of interest related to this publication.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Elettra Merola 0000-0001-9553-7684;Andrea Michielan 0000-0003-1353-0935;Umberto Rozzanigo 0000-0002-9541-2555;Marco Erini 0000-0002-6314-0196;Sandro Sferrazza 0000-0002-7595-0129;Stefano Marcucci 0000-0001-9144-9038;Chiara Sartori 0000-0003-3137-0082;Chiara Trentin 0000-0002-5213-0208;Giovanni de Pretis 0000-0001-7636-0422;Franca Chierichetti 0000-0002-1898-4736.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Surgery for Cronkhite-Canada syndrome complicated with intussusception:A case report and review of literature
- Status of bariatric endoscopy–what does the surgeon need to know?A review
- lmpact of parenchyma-preserving surgical methods on treating patients with solid pseudopapillary neoplasms:A retrospective study with a large sample size
- Laparoscopic vs open total gastrectomy for advanced gastric cancer following neoadjuvant therapy:A propensity score matching analysis
- Nomograms predicting prognosis of patients with pathological stages T1N2-3 and T3N0 gastric cancer
- Choledocholithiasis characteristics with periampullary diverticulum and endoscopic retrograde cholangiopancreatography procedures:Comparison between two centers from Lanzhou and Kyoto
