ICIs治疗NSCLC致免疫相关不良反应与疗效相关性的Meta分析
2022-03-29赵倩陈瑞祥温瑾代伟刘晓波何姣张清树
赵倩 陈瑞祥 温瑾 代伟 刘晓波 何姣 张清树
中图分类号 R979.1 文献标志码 A 文章编号 1001-0408(2022)06-0745-09
DOI 10.6039/j.issn.1001-0408.2022.06.17
摘 要 目的 系统评价免疫检查点抑制剂(ICIs)治疗非小细胞肺癌(NSCLC)致免疫相关不良反应(irAEs)与疗效的相关性,为ICIs的临床应用及安全性评估提供循证参考。方法 计算机检索PubMed、Embase、Cochrane图书馆、Web of Science、中国知网、万方数据、维普网、中国生物医学文献服务系统,收集ICIs治疗NSCLC致irAEs与疗效相关性的前瞻性或回顾性队列研究,检索时限均为各数据库建库起至2021年6月30日。筛选文献、提取资料后,采用纽卡斯尔-渥太华量表对纳入文献质量进行评价;采用RevMan 5.3软件进行Meta分析及发表偏倚分析;采用Stata 15.0软件进行敏感性分析。结果 共纳入31项研究,共计7 957例患者。Meta分析结果显示,irAEs组患者的客观反应率(ORR)[RR=2.34,95%CI(1.98,2.76),P<0.000 01]、无进展生存期(PFS)[HR=0.49,95%CI(0.44,0.55),P<0.000 01]、总生存期(OS)[HR=0.45,95%CI(0.39,0.53),P<0.000 01],以及该组第6周时的ORR[RR=1.88,95%CI(1.57,2.25),P<0.000 01]、PFS[HR=0.59,95%CI(0.50,0.69),P<0.000 01]、OS[HR=0.58,95%CI(0.48,0.70),P<0.000 01]均显著高于或长于非irAEs组。按irAEs器官特异性、irAEs严重程度及数量进行的亚组分析结果显示,皮肤、胃肠道、内分泌系统、轻度(1~2级)及发生1种或≥2种irAEs與患者PFS、OS的改善相关(P<0.05),肝、肺和重度(≥3级)irAEs与患者PFS、OS的改善无相关性(P>0.05)。敏感性分析结果显示,所得结果较稳健。发表偏倚分析结果显示,本研究可能存在一定的发表偏倚。结论 对于接受ICIs治疗的NSCLC患者,irAEs的发生可能与其更好的预后有关;irAEs可能是ICIs疗效的预测因子。
关键词 非小细胞肺癌;免疫检查点抑制剂;免疫相关不良反应;疗效;相关性;Meta分析
Meta-analysis of the correlation between immune-related adverse events and efficacy of immune checkpoint inhibitors in the treatment of non-small cell lung cancer
ZHAO Qian1,2,CHEN Ruixiang2,WEN Jin2,DAI Wei2,LIU Xiaobo1,HE Jiao1,2,ZHANG Qingshu1,2(1. College of Pharmacy, Dali University, Yunnan Dali 671000, China; 2. Dept. of Pharmacy, Yunnan Third People’s Hospital, Kunming 650011, China)
ABSTRACT OBJECTIVE To systematically evaluate the relationship between immune-related adverse events (irAEs) and efficacy of immune checkpoint inhibitors (ICIs) in the treatment of non-small cell lung cancer (NSCLC), and to provide evidence-based reference for clinical application of ICIs and safety evaluation. METHODS PubMed, Embase, Cochrane Library, Web of Science, CNKI, Wanfang database, VIP and CBM were searched to collect prospective or retrospective cohort studies on the correlation between irAEs and efficacy of ICIs in the treatment of NSCLC. The retrieval time was from the inception to June 30th, 2021. After literature screening and data extraction, Newcastle-Ottawa scale was used to evaluate the quality of included literatures. Meta-analysis and publication bias analysis were performed by using RevMan 5.3 software; Stata 15.0 software was used for sensitivity analysis. RESULTS A total of 7 957 patients were included in 31 studies. Meta-analysis showed that the objective response rate (ORR) [RR=2.34,95%CI(1.98,2.76),P<0.000 01], progression-free survival (PFS) [HR=0.49,95%CI(0.44,0.55),P<0.000 01] and overall survival (OS) [HR=0.45,95%CI(0.39,0.53),P<0.000 01] of irAEs group as well as ORR[RR=1.88,95%CI(1.57,2.25),P<0.000 01], PFS [HR=0.59,95%CI(0.50,0.69),P<0.000 01] and OS [HR=0.58,95%CI(0.48,0.70),P<0.000 01] of this group at 6th week were all significantly higher or longer than non irAEs group. According to organ specificity, severity and quantity of irAEs, subgroup analysis showed that skin, gastrointestinal and endocrine system, mild irAEs (grade 1-2) and one or more than 2 kinds of irAEs were significantly correlated with the improvement of PFS and OS (P<0.05), while liver and lung, severe irAEs (≥ grade 3) were not significantly correlated with the improvement of PFS and OS (P>0.05). Sensitivity analysis results showed that the results of the above-mentioned meta-analysis were relatively robust. The results of publication bias showed that there was may be some possibility of publication bias in this study. CONCLUSIONS For NSCLC patients treated with ICIS, the occurrence of irAEs may be related to their good prognosis. The irAEs may be a predictor of the efficacy of ICIs.
KEYWORDS non-small cell lung cancer; immune checkpoint inhibitors; immune-related adverse events; efficacy; correlation; meta-analysis
肺癌是最常见的恶性肿瘤之一,也是全球癌症相关死亡的主要原因[1]。非小细胞肺癌(non-small cell lung cancer,NSCLC)是肺癌最常见的病理类型,占肺癌患者总数的85%以上[2]。由于早期症状不明显,70%的NSCLC患者在确诊时已为晚期或出现远处转移,难以接受手术治疗,导致其预后较差;化疗及放疗虽为NSCLC的主要治疗手段,但效果有限,患者的5年生存率仅约5%[3-4]。
近年来,随着肿瘤细胞免疫逃逸机制的阐明,免疫治疗在肿瘤治疗中显示出独特优势,成为肿瘤患者多线治疗失败后的新希望[5]。有研究认为,包括程序性死亡蛋白1(programmed death-1,PD-1)及其配体(programmed death ligand-1,PD-L1)、细胞毒性T淋巴细胞相关抗原4(cytotoxic T-lymphocyte-asscociated antigen 4,CTLA-4)在內的免疫检查点抑制剂(immune checkpoint inhibitors,ICIs)可阻断PD-1/PD-L1、CTLA-4信号通路,从而恢复机体免疫细胞杀死肿瘤细胞的能力[6]。ICIs增强了宿主细胞的免疫能力,但其介导的免疫相关不良反应(immune-related adverse events,irAEs)发生频繁,虽然大部分的irAEs为轻度,但也存在一些较严重的irAEs,可能会导致患者免疫治疗终止或加用免疫抑制剂,甚至引发严重或致死性的毒性反应[7]。irAEs可累及全身任一器官或系统,以皮肤、胃肠道、呼吸及内分泌系统最为常见,给免疫治疗带来了极大的挑战[8]。目前,irAEs发生的机制尚不清楚,但其发生可能在某种程度上提示机体免疫系统被激活[9]。免疫系统的激活是否与更好的ICIs疗效有关?基于这个假设,Horvat等[10]、Dupont等[11]对irAEs与ICIs疗效的相关性进行了研究,前者研究结果显示,irAEs与临床结局并无关联,而后者则认为发生了irAEs的肿瘤患者的总体缓解率更高。可见,ICIs疗效与irAEs的关系尚无定论。基于此,本研究拟对NSCLC患者使用ICIs治疗后发生irAEs与疗效的相关性研究进行Meta分析,以评估irAEs是否与更好的ICIs疗效有关,并探索不同器官特异性irAEs、irAEs严重程度及数量对患者无进展生存期(progression free survival,PFS)和总生存期(overall survival,OS)的影响,评价irAEs对ICIs疗效的预测价值,旨在为ICIs的临床应用及安全性评估提供循证参考。
1 资料与方法
1.1 纳入与排除标准
1.1.1 研究类型 国内外公开发表的前瞻性或回顾性队列研究;语种限定为中文或英文。
1.1.2 研究对象 经组织病理学和(或)细胞学诊断为NSCLC,肿瘤分期不限;年龄>18岁。
1.1.3 干预措施 所有患者至少接受过1种ICIs治疗,如帕博利珠单抗、纳武利尤单抗、阿特珠单抗等,并根据是否发生irAEs将患者分为irAEs组和非irAEs组。
1.1.4 结局指标 结局指标包括:(1)客观反应率(objective response rate,ORR);(2)PFS;(3)OS。
1.1.5 排除标准 排除标准包括:(1)无法对NSCLC患者数据进行提取的文献;(2)仅报道了OS、PFS,未提供其风险比(hazard ratio,HR)及95%置信区间(confidence interval,CI)的文献;(3)样本量小于50的文献;(4)评论、综述、动物研究、调查或指南。
1.2 文献检索策略
计算机检索PubMed、Embase、Cochrane图书馆、Web of Science、中国知网、万方数据、维普网、中国生物医学文献服务系统。中文检索词为“非小细胞肺癌”“免疫检查点抑制剂”“帕博利珠单抗”“纳武利尤单抗”“阿特珠单抗”“度伐利尤单抗”“伊匹木单抗”“特瑞普利单抗”“卡瑞利珠单抗”“西米普利单抗”“信迪利单抗”“阿维单抗”“西木单抗”“免疫相关不良反应”“疗效”“结局”;英文检索词为“non-small lung cancer”“NSCLC”“immune checkpoint inhibitors”“PD-1”“PD-L1”“CTLA-4”“pembrolizumab”“nivolumab”“atezolizumab”“durvalumab”“ipilimumab”“toripalimab”“camrelizumab”“cemiplimab”“sintilimab”“avelumab”“tremlimumab”“immune-related adverse events”“irAEs”“efficacy”“outcome”。采用主题词与自由词相结合的检索方式。检索时限均为各数据库建库起至2021年6月30日。
1.3 文献筛选与数据提取
由两名研究者根据纳入与排除标准对文献进行筛选,如遇分歧,则双方协商或请求第三名研究者协助解决。若研究同时包含多变量和单变量分析结果,优先提取多变量数据。提取信息包括:第一作者、发表年份、国家、样本量、患者年龄、患者性别、研究类型、干预措施,中位随访时间、发生irAEs的样本量及结局指标等。
1.4 文献质量评价
采用纽卡斯尔-渥太华量表(Newcastle-Ottawa scale,NOS)对纳入文献的质量进行评价,具体内容包括:选择(暴露组的代表性、非暴露组的代表性、暴露的确定、研究开始前是否有研究对象发生结局事件)、队列的可比性(暴露组与非暴露组间的可比性)、结果的评估(结局指标的评估、随访时间足够长以及随访的完整性)。以上条目,满足1项为1分,满分为9分;<5分为低质量,5~<8分为中等质量,8~9分为高质量[12]。
1.5 统计学方法
采用RevMan 5.3软件进行Meta分析。ORR采用相对危险度(risk ratio,RR)及其95%CI表示,PFS、OS采用HR及其95%CI表示。以Q检验和I 2检验评估研究间的异质性,若P>0.1且I 2<50%,表示各研究间无统计学异质性,采用固定效应模型进行分析;反之则采用随机效应模型进行分析。分别以器官特异性irAEs、irAEs严重程度[irAEs 严重程度按美国国家癌症研究所不良反应通用术语标准(common terminology criteria adverse events,CTCAE)4.0版进行分级,≥3级为重度irAEs[13]]、irAEs数量进行亚组分析;采用Stata 15.0软件进行敏感性分析;采用RevMan 5.3软件绘制倒漏斗图进行发表偏倚分析。检验水准α=0.05。
2 结果
2.1 文献检索结果
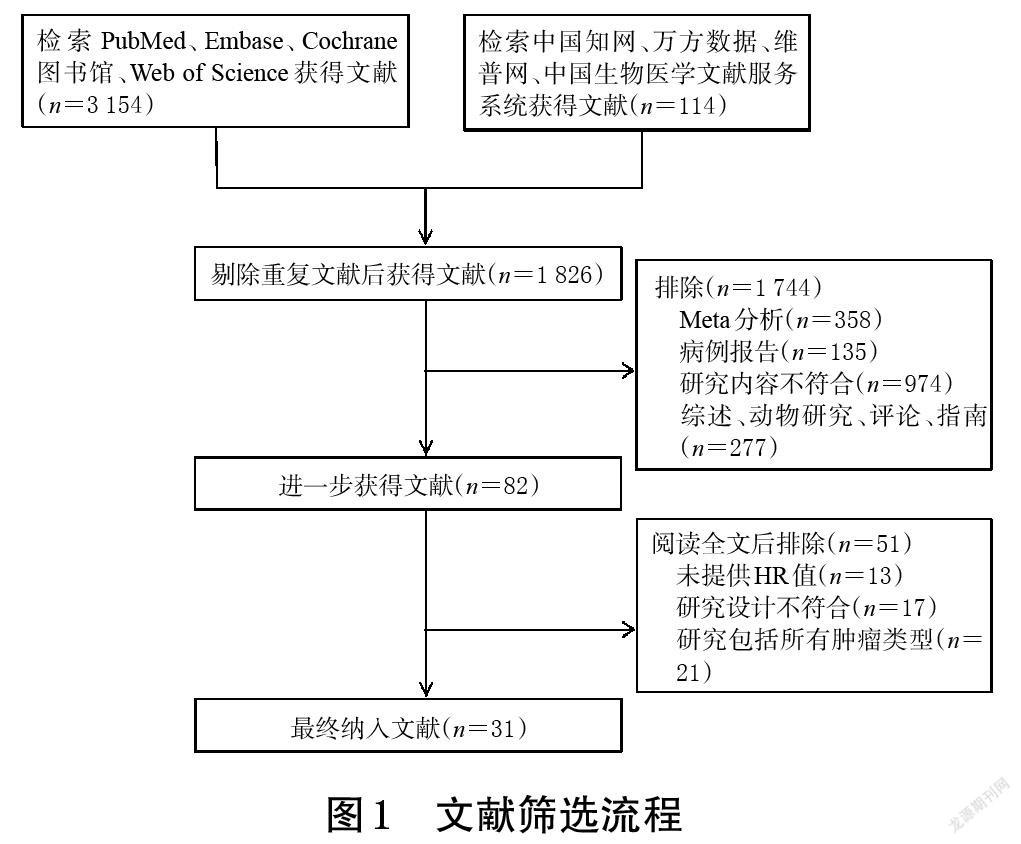
初检共获得相关文献3 268篇,经阅读标题、摘要和全文后,最终纳入31篇文献[14-44],共计7 957例患者。其中,29篇文献为回顾性队列研究[14-27,29-33,35-44],2篇文献为前瞻性队列研究[28,34]。文献筛选流程见图1,纳入研究的基本信息见表1。
2.2 纳入研究的质量评价
31篇文献中,7篇文献评分为6分[16,18,21,26,33,37,43],12篇文献评分为7分[14,20,22-24,27,30,32,36,40,42,44],9篇文献评分为8分[15,17,25,28-29,31,34-35,39],3篇文献评分为9分[19,38,41]。纳入研究的质量评价结果见表1。
2.3 Meta分析结果
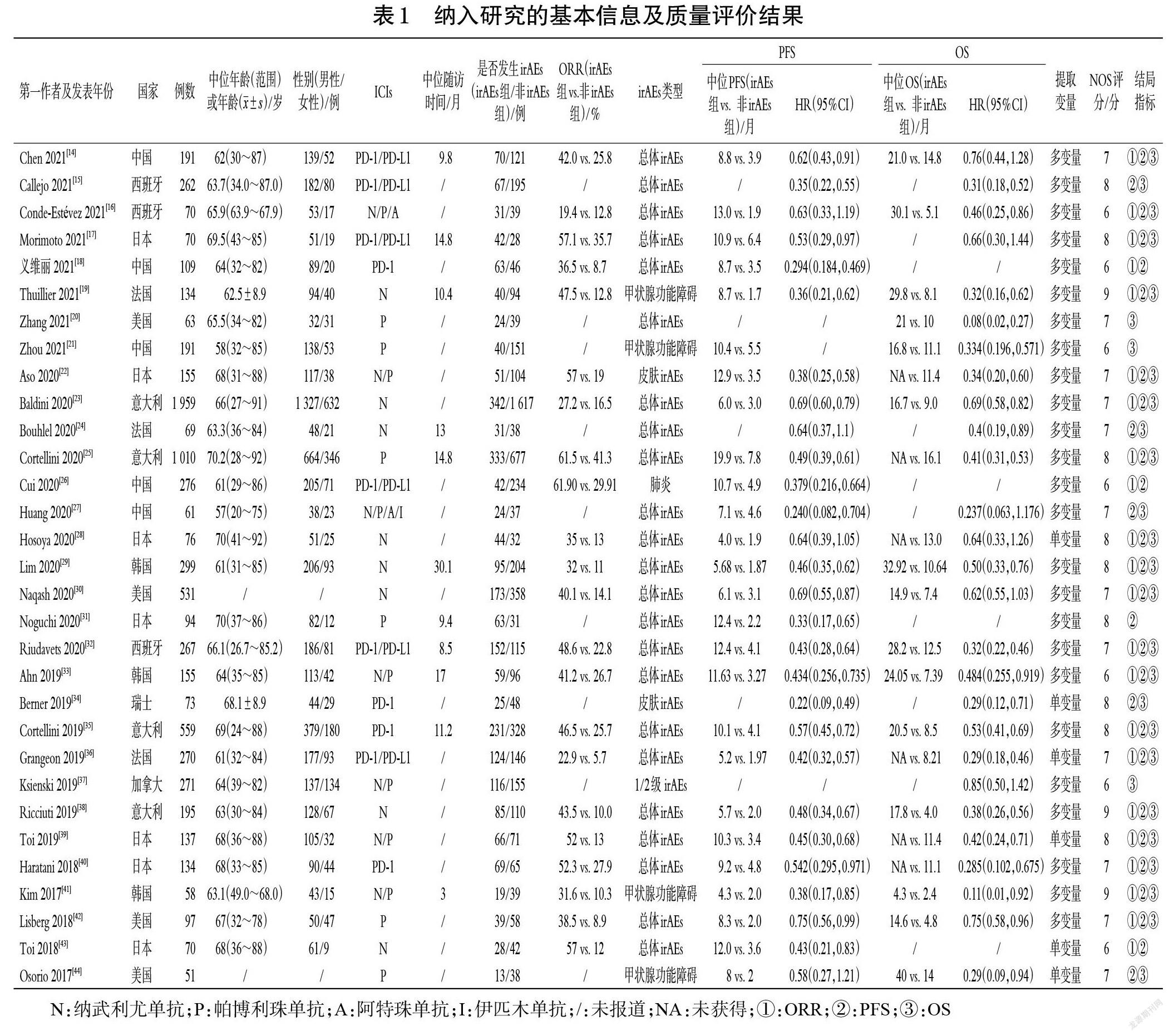
2.3.1 ORR 22项研究报道了ORR[14,16-19,22-23,25-26,28-30,32-33,35-36,38-43],各研究间有统计学异质性(P<0.000 01,I 2=67%),采用随机效应模型进行Meta分析,详见图2(因原始文献中有患者退出,在分析ORR时未纳入最初的所有患者,故文献[14,25,33,35-36,40]中的两组患者例数与表1不同)。Meta分析结果显示,irAEs组患者的ORR显著高于非irAEs组[RR=2.34,95%CI(1.98,2.76),P<0.000 01]。对第6周时两组患者的ORR进行评估(图略,因接受ICIs治疗所致irAEs的发生时间不固定,可在接受ICIs治疗的任何时间出现,加之纳入原始文献的部分数据不足,因此仅对第6周进行评估,下同),结果显示,irAEs组患者的ORR显著高于非irAEs组[RR=1.88,95%CI(1.57,2.25),P<0.000 01]。
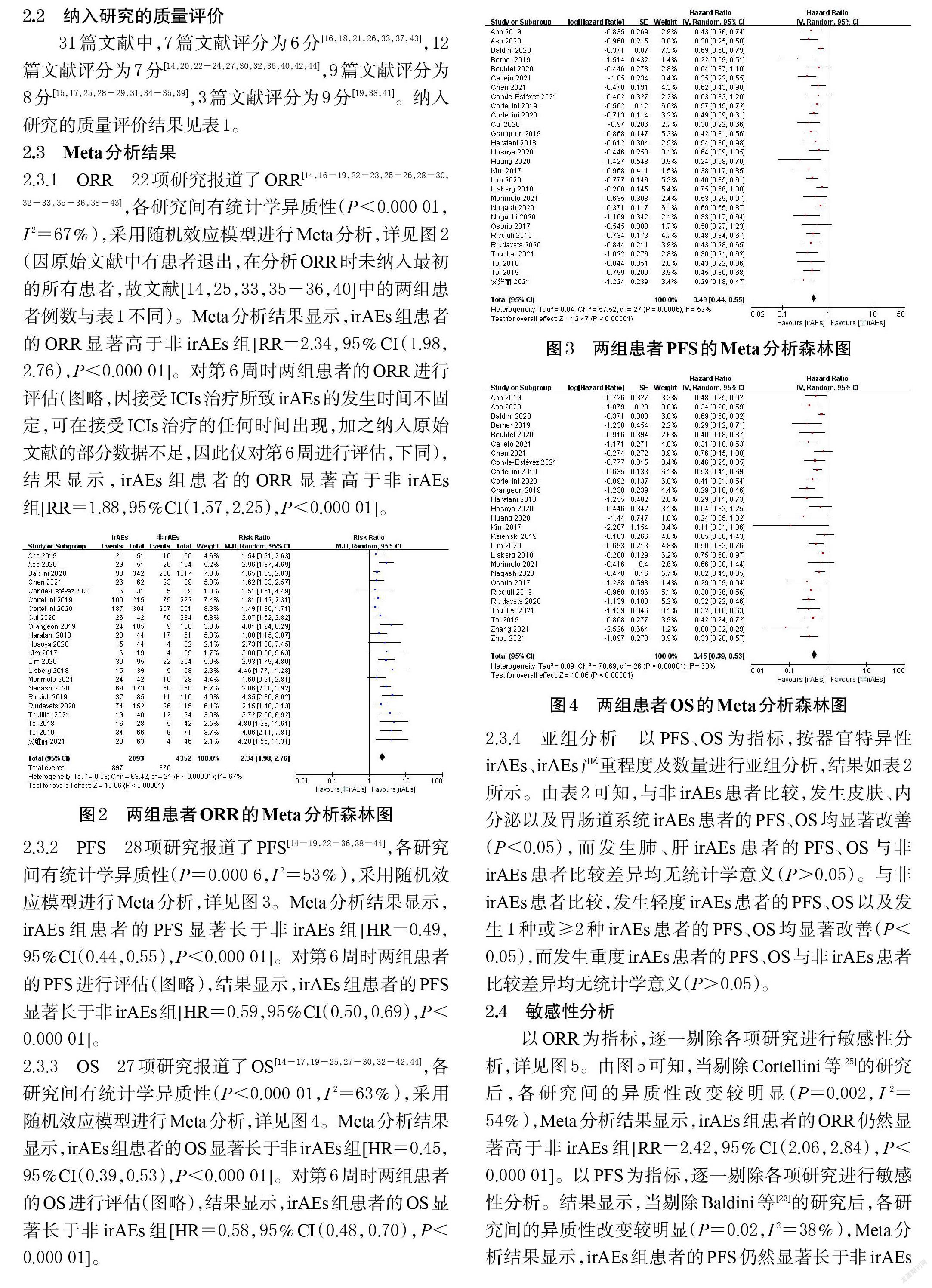
2.3.2 PFS 28项研究报道了PFS[14-19,22-36,38-44],各研究间有统计学异质性(P=0.000 6,I 2=53%),采用随机效应模型进行Meta分析,详见图3。Meta分析结果显示,irAEs组患者的PFS显著长于非irAEs组[HR=0.49,95%CI(0.44,0.55),P<0.000 01]。对第6周时两组患者的PFS进行评估(图略),结果显示,irAEs組患者的PFS显著长于非irAEs组[HR=0.59,95%CI(0.50,0.69),P<0.000 01]。
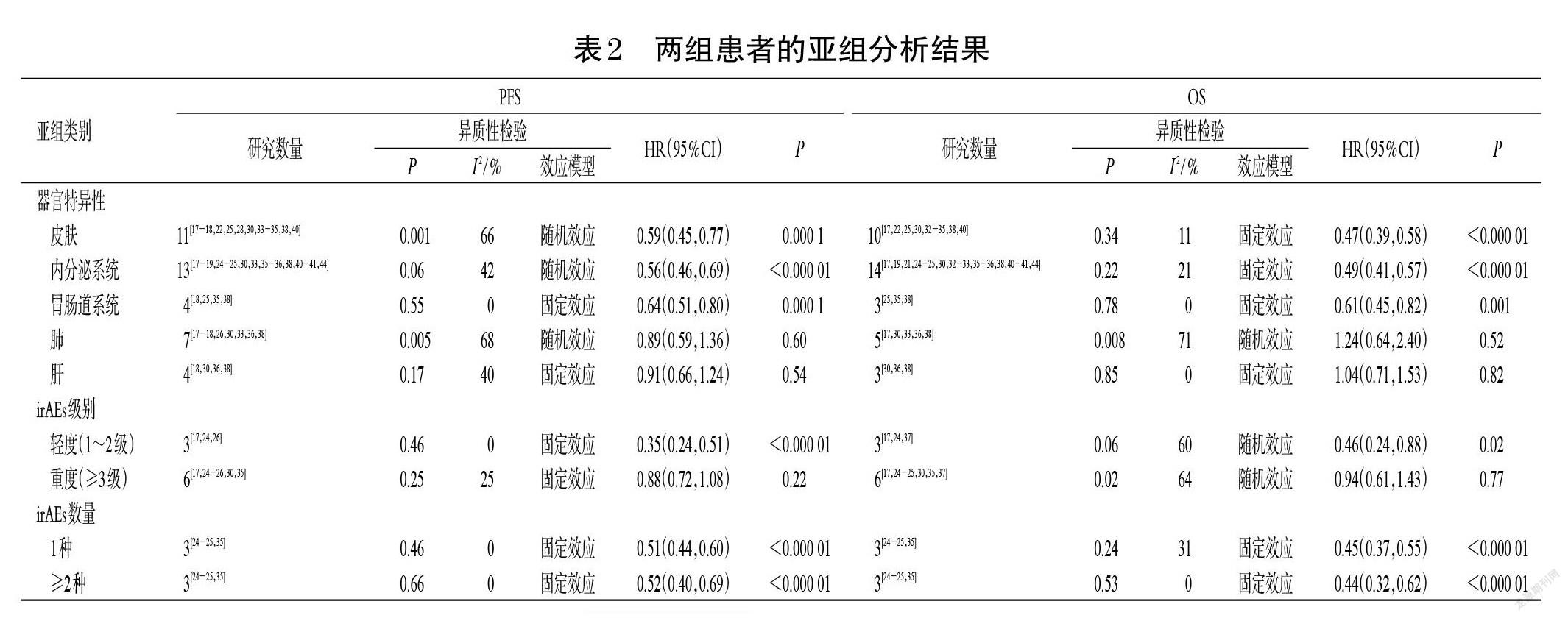
2.3.3 OS 27项研究报道了OS[14-17,19-25,27-30,32-42,44],各研究间有统计学异质性(P<0.000 01,I 2=63%),采用随机效应模型进行Meta分析,详见图4。Meta分析结果显示,irAEs组患者的OS显著长于非irAEs组[HR=0.45,95%CI(0.39,0.53),P<0.000 01]。对第6周时两组患者的OS进行评估(图略),结果显示,irAEs组患者的OS显著长于非irAEs组[HR=0.58,95%CI(0.48,0.70),P<0.000 01]。
2.3.4 亚组分析 以PFS、OS为指标,按器官特异性irAEs、irAEs严重程度及数量进行亚组分析,结果如表2所示。由表2可知,与非irAEs患者比较,发生皮肤、内分泌以及胃肠道系统irAEs患者的PFS、OS均显著改善(P<0.05),而发生肺、肝irAEs患者的PFS、OS与非irAEs患者比较差异均无统计学意义(P>0.05)。与非irAEs患者比较,发生轻度irAEs患者的PFS、OS以及发生1种或≥2种irAEs患者的PFS、OS均显著改善(P<0.05),而发生重度irAEs患者的PFS、OS与非irAEs患者比较差异均无统计学意义(P>0.05)。
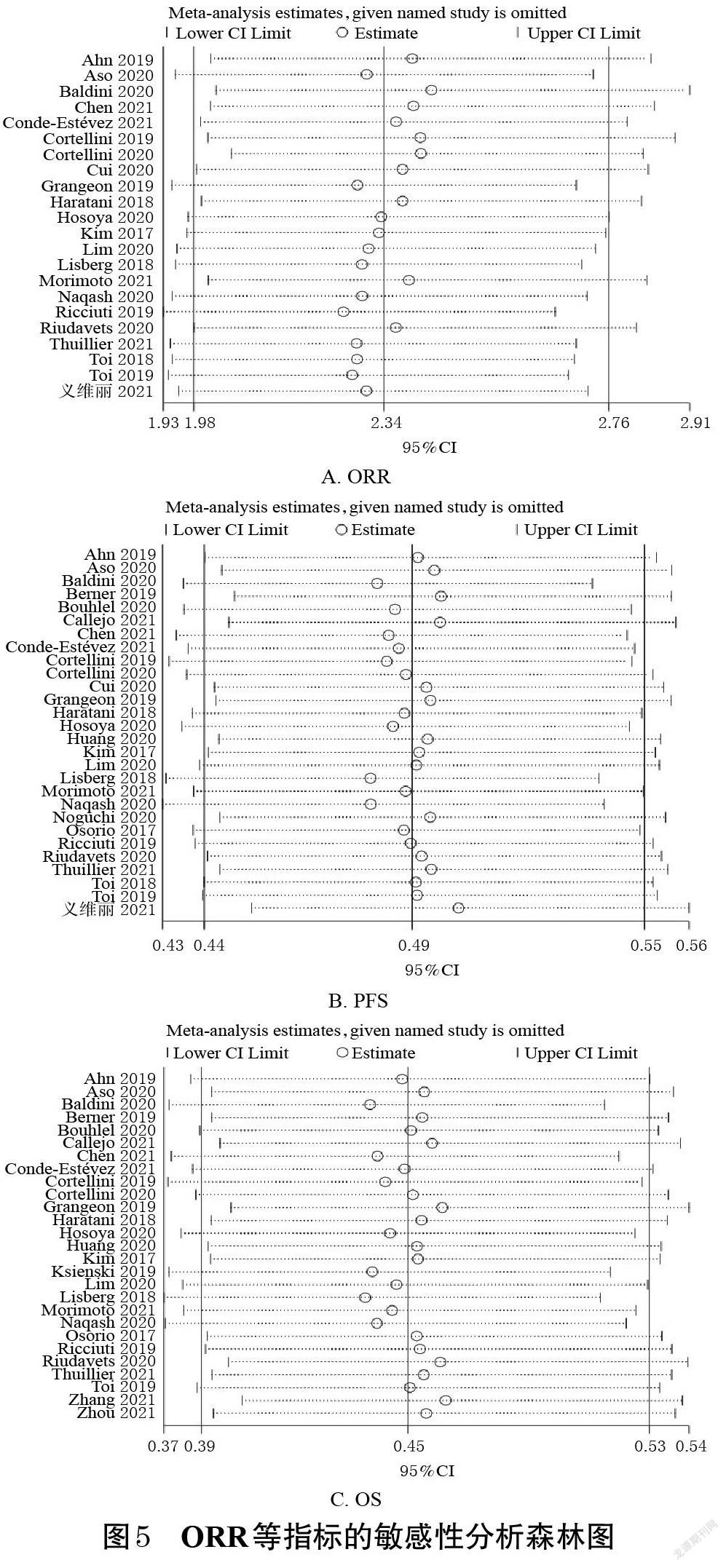
2.4 敏感性分析
以ORR为指标,逐一剔除各项研究进行敏感性分析,详见图5。由图5可知,当剔除Cortellini等[25]的研究后,各研究间的异质性改变较明显(P=0.002,I 2=54%),Meta分析结果显示,irAEs组患者的ORR仍然显著高于非irAEs组[RR=2.42,95%CI(2.06,2.84),P<0.000 01]。以PFS为指标,逐一剔除各项研究进行敏感性分析。结果显示,当剔除Baldini等[23]的研究后,各研究间的异质性改变较明显(P=0.02,I 2=38%),Meta分析结果显示,irAEs组患者的PFS仍然显著长于非irAEs组[HR=0.48,95%CI(0.44,0.54),P<0.000 01]。以OS为指标,逐一剔除各项研究进行敏感性分析。结果显示,当剔除Baldini等[23]的研究后,各研究间的异质性改变较明显(P=0.000 3,I 2=55%),Meta分析结果显示,irAEs组患者的OS仍然显著长于非irAEs组[HR=0.44,95%CI(0.38,0.51),P<0.000 01],提示本研究所得结果较稳健。
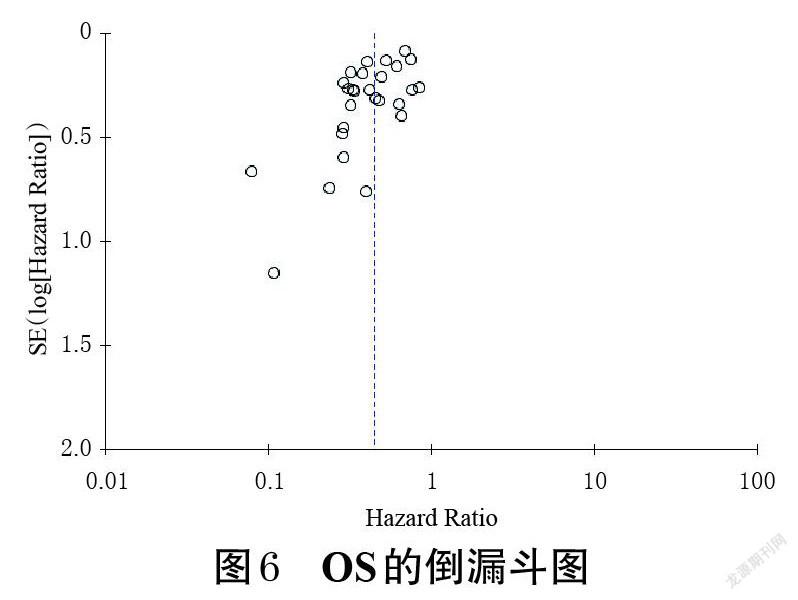
2.5 发表偏倚分析
以OS为指标,绘制倒漏斗图进行发表偏倚分析。结果显示,各研究散点位于倒漏斗图的中上方,倒漏斗图左右两侧不对称,提示可能存在一定的发表偏倚,详见图6(以其余指标进行的发表偏倚分析所得结果类似,故图略)。
3 讨论
ICIs可改变肿瘤治疗的结局,显示出较好的抗肿瘤作用,但在实际临床应用中,ICIs的疗效存在较大差异[45]。因此,探索预测ICIs疗效的标志物一直是学界研究的焦点之一。目前,ICIs疗效预测的常见标志物有PD-L1的表达水平、肿瘤突变负荷、肿瘤微卫星不稳定性等[46],但由于肿瘤自身异质性、耐药性及检测方法不完善等原因,使得现有标志物并不能很好地预测ICIs的疗效,且不同肿瘤用何种预测指标、用单一指标还是联合指标预测仍未有标准[47]。有研究发现,irAEs可能是ICIs疗效的预测因子[48],但关于irAEs是否与较好的ICIs疗效有关尚无定论。因此,本研究系统评价了接受ICIs治疗的NSCLC患者发生irAEs与ICIs疗效的相关性,结果显示,irAEs组患者的ORR、PFS、OS均显著高于或长于非irAEs组。亚组分析结果显示,皮肤、胃肠道、内分泌系统irAEs与患者PFS、OS的改善相关,而肝、肺irAEs与其PFS、OS的改善无关。这可能与肝、肺irAEs的发生率较低,而病死率较高有关[49-50]。此外,本研究在分析肝、肺irAEs与ICIs疗效相关性时发现,由于纳入研究的数据较少,可能会使得该亚组分析结果存在一定偏倚。irAEs的发生被认为与免疫系统的激活有关,这似乎意味着重度irAEs患者能比轻度irAEs患者获得更好的结局[9]。但本研究结果显示,轻度irAEs与患者PFS、OS的改善相关,而重度irAEs与其PFS、OS的改善无关,这可能是由于重度irAEs的病情严重程度较高,临床治疗存在一定难度,可能导致重度irAEs患者的预后较差[51]。既往研究表明,发生多器官或多系统irAEs的患者可能具有更好的疗效,这种相关性与irAEs 的数量有关[52-53]。本研究结果显示,发生1种或≥2种irAEs患者的PFS、OS均得到显著改善,可能与irAEs数量无关。因此,关于irAEs数量对ICIs疗效的影响尚有待进一步探索。
irAEs与患者生存获益相关的潜在机制尚未完全阐明,但学界普遍认为,irAEs的发生在一定程度上提示了宿主免疫系统被激活,从而使得机体对肿瘤细胞和正常组织均具有较强的免疫作用,进而使患者获得更好的疗效[9]。但本研究结果发现,并非所有的irAEs都与更好的ICIs疗效有关,因此识别具有预测能力的irAEs就显得尤为重要。在以irAEs预测ICIs疗效时,有两个问题值得注意:(1)并非所有的irAEs都具有预测患者临床获益的能力。在本研究中,皮肤、胃肠道及内分泌系统irAEs与患者更好的预后相关,而肝、肺irAEs却无类似结果;轻度irAEs与患者更好的预后相关,而重度irAEs则与预后无关,因此识别哪些irAEs有助于患者临床获益是非常重要的。(2)应关注使用类固醇治疗irAEs对临床获益的影响。相关研究表明,irAEs与患者临床获益的相关性并没有受到类固醇的影响,应适当使用类固醇来减少自身免疫性炎症反应的发生,防止更高级别irAEs的发展[54]。然而,在基线或治疗的早期阶段使用类固醇可能与较差的治疗结局有关[55-56]。因此,何時开始使用类固醇、类固醇用药剂量、维持时间以及能否使用其他药物代替是值得深入探索的问题。
Hussaini等[57]、Petrelli等[58]的研究纳入了黑色素瘤、NSCLC、肾细胞癌等多种肿瘤,结果虽证明了irAEs与ICIs疗效的改善有关,但患者的基线信息存在较大差异,对结果可能存在一定影响,并且不能确定irAEs在具体某种肿瘤中是否具有同样的作用。本研究纳入了NSCLC患者,并增加了新的文献,所得结果与同类研究结果基本一致[57-59],均表明irAEs与ICIs疗效的提升显著相关。不同的是,本研究分析了irAEs的器官特异性、严重程度及数量与ICIs疗效的关系,并以第6周作为时间点评估irAEs与ICIs间的关系是否受时间的影响,所得结果能够全面地解释irAEs与NSCLC患者疗效改善的关系,对免疫治疗以及irAEs的临床管理提供了一定的参考。
综上所述,对于接受ICIs治疗的NSCLC患者,irAEs的发生可能与其更好的预后有关,irAEs可能是ICIs疗效的预测因子。虽然本研究在提取数据时,尽量从多变量分析中提取HR值以避免其他混杂因素的影响,并且对第6周的生存结局进行了分析以减少时间对评估结果的影响,但仍存在如下局限性:(1)纳入文献大多为回顾性研究,患者的基线信息如样本量、年龄、性别、肿瘤分期、ICIs治疗、irAEs的判定方法等均存在差异,可能会影响Meta分析的结果;(2)纳入的文献具有一定的发表偏倚,可能与本研究报告的结果均为阳性有关,可在一定程度上导致本次Meta分析的结果被放大;(3)由于数据有限,本研究未分析ICIs种类以及联合用药对irAEs与疗效相关性的影响;(4)本研究仅能提示发生irAEs可能与患者更好的ICIs疗效有关,并不能表明irAEs与ICIs疗效之间的因果关系,也不能阐明其具体的机制,故此结论尚需大规模、高质量的前瞻性研究进一步验证。
参考文献
[ 1 ] SUNG H,FERLAY J,SIEGEL R L,et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249.
[ 2 ] OSMANI L,ASKIN F,GABRIELSON E,et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma(NSCLC):moving from targeted the- rapy to immunotherapy[J]. Semin Cancer Biol,2018,52:103-109.
[ 3 ] MOLINA J R,YANG P,CASSIVI S D,et al. Non-small cell lung cancer:epidemiology,risk factors,treatment,and survivorship[J]. Mayo Clin Proc,2008,83(5):584- 594.
[ 4 ] SIEGEL R L,MILLER K D,JEMAL A. Cancer statistics,2020[J]. CA Cancer J Clin,2020,70(1):7-30.
[ 5 ] HARGADON K M,JOHNSON C E,WILLIAMS C J. Immune checkpoint blockade therapy for cancer:an overview of FDA-approved immune checkpoint inhibitors[J]. Int Immunopharmacol,2018,62:29-39.
[ 6 ] SANMAMED M F,CHEN L P. A paradigm shift in cancer immunotherapy:from enhancement to normalization[J]. Cell,2018,175(2):313-326.
[ 7 ] LIU Y H,ZANG X Y,WANG J C,et al. Diagnosis and management of immune related adverse events(irAEs) in cancer immunotherapy[J]. Biomed Pharmacother,2019,120:109437.
[ 8 ] SI X Y,SONG P,NI J,et al. Management of immune checkpoint inhibitor-related adverse events:a review of case reports[J]. Thorac Cancer,2020,11(3):498-504.
[ 9 ] LEE D J,LEE H J,Jr,FARMER J R,et al. Mechanisms driving immune-related adverse events in cancer patients treated with immune checkpoint inhibitors[J]. Curr Cardiol Rep,2021,23(8):98.
[10] HORVAT T Z,ADEL N G,DANG T O,et al. Immune- related adverse events,need for systemic immunosuppression,and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering cancer center[J]. J Clin Oncol,2015,33(28):3193-3198.
[11] DUPONT R,BÉRARD E,PUISSET F,et al. The progno- stic impact of immune-related adverse events during anti- PD1 treatment in melanoma and non-small-cell lung cancer:a real-life retrospective study[J]. Oncoimmunology,2019,9(1):1682383.
[12] 艾飛玲,胡葵茹,石钰霖,等.基于纽卡斯尔-渥太华量表对中国吸烟队列研究文献的质量评价[J].中华疾病控制杂志,2021,25(6):722-729.
[13] 皋文君,刘砚燕,袁长蓉.国际肿瘤化疗药物不良反应评价系统:通用不良反应术语标准4.0版[J].肿瘤,2012,32(2):142-144.
[14] CHEN X L,NIE J,DAI L,et al. Immune-related adverse events and their association with the effectiveness of PD-1/PD-L1 inhibitors in non-small cell lung cancer:a real- world study from China[J]. Front Oncol,2021,11:607531.
[15] CALLEJO A,FRIGOLA J,IRANZO P,et al. Interrelations between patients’ clinicopathological characteristics and their association with response to immunotherapy in a real-world cohort of NSCLC patients[J]. Cancers,2021,13(13):3249.
[16] CONDE-ESTÉVEZ D,MONGE-ESCARTÍN I,RÍOS-HOYO A,et al. Prognostic factors and effect on survival of immune-related adverse events in patients with non-small- cell lung cancer treated with immune checkpoint blo- ckage[J]. J Chemother,2021,33(1):32-39.
[17] MORIMOTO K,YAMADA T,TAKUMI C,et al. Immune-related adverse events are associated with clinical benefit in patients with non-small-cell lung cancer treated with immunotherapy plus chemotherapy:a retrospective study[J]. Front Oncol,2021,11:630136.
[18] 義维丽,赵文成,黄东宁,等. PD-1单抗治疗非小细胞肺癌相关不良反应及其与疗效的相关性分析[J].中国癌症杂志,2021,31(3):203-211.
[19] THUILLIER P,JOLY C,ALAVI Z,et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associa- ted with a better progression-free survival and overall survival in non-small cell lung cancer:an original cohort study[J]. Cancer Immunol Immunother,2021,70(7):2023-2033.
[20] ZHANG M,RODRIGUES A J,POLLOM E L,et al. Improved survival and disease control following pembrolizumab-induced immune-related adverse events in high PD-L1 expressing non-small cell lung cancer with brain metastases[J]. J Neurooncol,2021,152(1):125-134.
[21] ZHOU Y W,XIA R L,XIAO H L,et al. Thyroid function abnormality induced by PD-1 inhibitors have a positive impact on survival in patients with non-small cell lung cancer[J]. Int Immunopharmacol,2021,91:107296.
[22] ASO M,TOI Y,SUGISAKA J,et al. Association between skin reaction and clinical benefit in patients treated with anti-programmed cell death 1 monotherapy for advanced non-small cell lung cancer[J]. Oncologist,2020,25(3):e536-e544.
[23] BALDINI E,LUNGHI A,CORTESI E,et al. Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab:the Italian NSCLC expanded access program[J]. Lung Cancer,2020,140:59-64.
[24] BOUHLEL L,DOYEN J,CHAMOREY E,et al. Occurrence and number of immune-related adverse events are independently associated with survival in advanced non-small-cell lung cancer treated by nivolumab[J]. Bull Cancer,2020,107(9):946-958.
[25] CORTELLINI A,FRIEDLAENDER A,BANNA G L,et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥50% and their relationship with clinical outcomes[J]. Clin Lung Cancer,2020,21(6):498-508.e2.
[26] CUI P F,HUANG D,WU Z Z,et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer[J]. Ther Adv Med Oncol,2020,12:1758835920922033.
[27] HUANG L,LI L,ZHOU Y X,et al. Clinical characteri- stics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer[J]. J Cancer,2020,11(24):7137-7145.
[28] HOSOYA K,FUJIMOTO D,MORIMOTO T,et al. Association between early immune-related adverse events and clinical outcomes in patients with non-small cell lung cancer treated with immune checkpoint inhibitors[J]. Clin Lung Cancer,2020,21(4):e315-e328.
[29] LIM S M,KIM S W,CHO B C,et al. Real-world experience of nivolumab in non-small cell lung cancer in Korea[J]. Cancer Res Treat,2020,52(4):1112-1119.
[30] NAQASH A R,RICCIUTI B,OWEN D H,et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with ni- volumab:a pooled exploratory analysis from a global cohort[J]. Cancer Immunol Immunother,2020,69(7):1177-1187.
[31] NOGUCHI S,SUMINAGA K,KAKI T,et al. Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non-small cell lung cancer[J]. Lung Cancer(Auckl),2020,11:53-57.
[32] RIUDAVETS M,MOSQUERA J,GARCIA-CAMPELO R,et al. Immune-related adverse events and corticosteroid use for cancer-related symptoms are associated with efficacy in patients with non-small cell lung cancer receiving anti-PD-(L)1 blockade agents[J]. Front Oncol,2020,10:1677.
[33] AHN B C,PYO K H,XIN C F,et al. Comprehensive ana- lysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice[J]. J Cancer Res Clin Oncol,2019,145(6):1613-1623.
[34] BERNER F,BOMZE D,DIEM S,et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer[J]. JAMA Oncol,2019,5(7):1043-1047.
[35] CORTELLINI A,CHIARI R,RICCIUTI B,et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients[J]. Clin Lung Cancer,2019,20(4):237-247.e1.
[36] GRANGEON M,TOMASINI P,CHALEAT S,et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer[J]. Clin Lung Cancer,2019,20(3):201-207.
[37] KSIENSKI D,WAI E S,CROTEAU N,et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events:a retrospective multicenter analysis[J]. Clin Lung Cancer,2019,20(1):e97-e106.
[38] RICCIUTI B,GENOVA C,DE GIGLIO A,et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivo- lumab:long-term outcomes from a multi-institutional analysis[J]. J Cancer Res Clin Oncol,2019,145(2):479- 485.
[39] TOI Y,SUGAWARA S,SUGISAKA J,et al. Profiling preexisting antibodies in patients treated with anti-PD-1 the- rapy for advanced non-small cell lung cancer[J]. JAMA Oncol,2019,5(3):376-383.
[40] HARATANI K,HAYASHI H,CHIBA Y,et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer[J]. JAMA Oncol,2018,4(3):374-378.
[41] KIM H I,KIM M,LEE S H,et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer[J]. Oncoimmunology,2017,7(1):e1375642.
[42] LISBERG A,TUCKER D A,GOLDMAN J W,et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center[J]. Cancer Immunol Res,2018,6(3):288- 294.
[43] TOI Y,SUGAWARA S,KAWASHIMA Y,et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab[J]. Oncologist,2018,23(11):1358-1365.
[44] OSORIO J C,NI A,CHAFT J E,et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer[J]. Ann Oncol,2017,28(3):583-589.
[45] MEYERS D E,BANERJI S. Biomarkers of immune checkpoint inhibitor efficacy in cancer[J]. Curr Oncol,2020,27(Suppl 2):S106-S114.
[46] PRELAJ A,TAY R,FERRARA R,et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer[J]. Eur J Cancer,2019,106:144-159.
[47] 石元朝,關泉林.非小细胞肺癌免疫治疗疗效预测的研究进展[J].现代肿瘤医学,2022,30(2):360-364.
[48] AKAMATSU H,MURAKAMI E,OYANAGI J,et al. Immune-related adverse events by immune checkpoint inhi- bitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer[J]. Oncologist,2020,25(4):e679-e683.
[49] 王汉萍,郭潇潇,周佳鑫,等.免疫检查点抑制剂相关肺炎的临床诊治建议[J].中国肺癌杂志,2019,22(10):621-626.
[50] WANG D Y,SALEM J E,COHEN J V,et al. Fatal toxic effects associated with immune checkpoint inhibitors:a systematic review and meta-analysis[J]. JAMA Oncol,2018,4(12):1721-1728.
[51] WANG W,GU X,WANG L,et al. The prognostic impact of mild and severe immune-related adverse events in non- small cell lung cancer treated with immune checkpoint inhibitors:a multicenter retrospective study[J]. Cancer Immunol Immunother,2021:2021Nov24.
[52] FREEMAN-KELLER M,KIM Y,CRONIN H,et al. Ni- volumab in resected and unresectable metastatic melanoma:characteristics of immune-related adverse events and association with outcomes[J]. Clin Cancer Res,2016,22(4):886-894.
[53] SHANKAR B,ZHANG J J,NAQASH A R,et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer[J]. JAMA Oncol,2020,6(12):1952-1956.
[54] SHAFQAT H,GOURDIN T,SION A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy[J]. Semin Oncol,2018,45(3):156-163.
[55] ARBOUR K C,MEZQUITA L,LONG N,et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer[J]. J Clin Oncol,2018,36(28):2872-2878.[PubMed]
[56] RICCIUTI B,DAHLBERG S E,ADENI A,et al. Immune checkpoint inhibitor outcomes for patients with non-small- cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications[J]. J Clin Oncol,2019,37(22):1927-1934.
[57] HUSSAINI S,CHEHADE R,BOLDT R G,et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors:a systematic review and meta-analysis[J]. Cancer Treat Rev,2021,92:102134.
[58] PETRELLI F,GRIZZI G,GHIDINI M,et al. Immune-related adverse events and survival in solid tumors treated with immune checkpoint inhibitors:a systematic review and meta-analysis[J]. J Immunother,2020,43(1):1-7.
[59] WANG D,CHEN C,GU Y,et al. Immune-related adverse events predict the efficacy of immune checkpoint inhibitors in lung cancer patients:a meta-analysis[J]. Front Oncol,2021,11:631949.
(收稿日期:2021-08-26 修回日期:2022-02-07)
(編辑:陈 宏)
