显柱南蛇藤根皮氯仿部位化学成分研究
2022-03-24肖本游向家桂王珊珊张昌鹏王发松
肖本游,向家桂,王珊珊,张昌鹏,刘 瑶,王发松**
(1. 湖北民族大学 化学与环境工程学院,湖北 恩施 445000;2. 湖北民族大学 生物资源保护与利用湖北省重点实验室,湖北 恩施 445000;3. 湖北民族大学 医学部,湖北 恩施 445000)
显柱南蛇藤(Celastrus stylosusWall)系卫矛科(Celastraceae)南蛇藤属(Celastrus)植物,该属植物包含50 多个种,分布于世界各地,常见的有灯油藤、独子藤、苦皮藤、显柱南蛇藤等,中国约有30 种分布于各地[1]. 目前研究发现,南蛇藤属植物萜类成分较为丰富,从其属植物的果实和根皮中共发现倍半萜类70 个,三萜类67 个和二萜类化合物2 个[2],其中倍半萜主要以β-二氢沉香呋喃型为主,三萜类化合物大多为五环三萜,如木栓烷型、齐墩果烷型、羽扇豆烷型等. 南蛇藤属植物的叶子中还含有大豆素、山奈酚、槲皮素等常见黄酮类化合物[3]. 此外,南蛇藤属植物还含有一些常见的脂肪酸、酚酸类化合物,如棕榈酸、肉桂酸等[4]. 现代药理学研究发现,该属植物具有良好的抗肿瘤、降压、抗炎、抗氧化、抗菌、抗病毒[5]等药理活性. 为进一步开发和利用显柱南蛇藤,阐明其药效发挥的物质基础,本实验对显柱南蛇藤根皮85%乙醇提取物氯仿部位进行化学成分研究,并采用多种分离技术和波谱学鉴定方法,从其根皮氯仿部位分离鉴定15 个化合物(如图1),所有化合物均为首次从该种植物中分离得到.
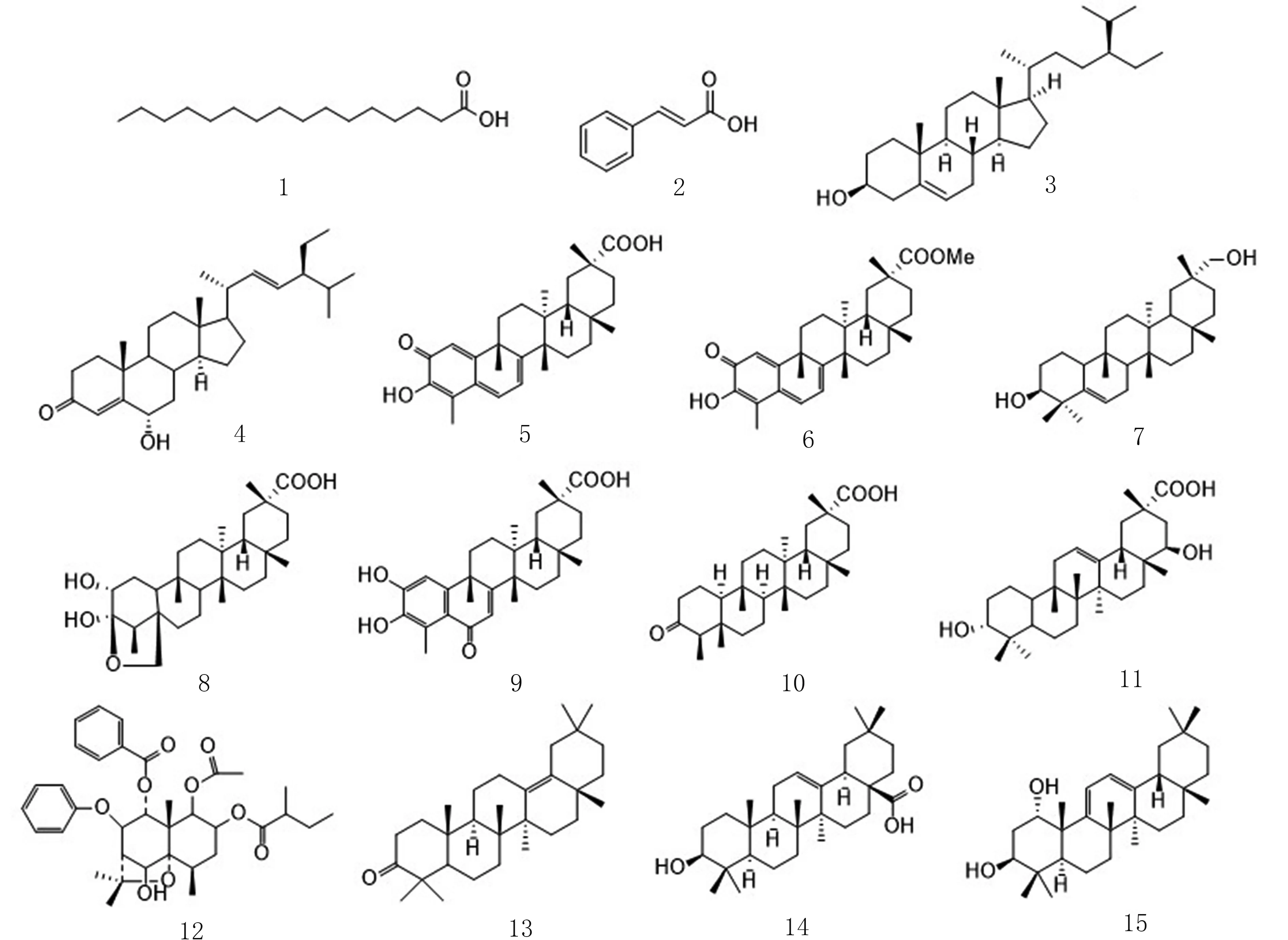
图1 化合物1~15 结构Fig. 1 Chemical structures of compounds 1-15
1 仪器与材料
核磁共振仪(Bruker AV-400 型,德国Bruker 公司),高效液相色谱-质谱联用(Agilent 公司),中压制备液相色谱 (苏州汇通),柱层析硅胶(0.054~0.077 mm,200~300 目,青岛海洋化工厂),制备薄层板(烟台江友硅胶开发有限公司),Sephadex LH-20葡聚糖凝胶(吉泰科技生物),ODS 柱色谱填料(C18, 15 μm, Merck 公司).
试剂:石油醚(60~90 ℃),分析纯;乙酸乙酯,分析纯;氯仿,分析纯;甲醇,色谱纯;乙醇,分析纯.以上试剂均为国药集团化学试剂有限公司生产.
显柱南蛇藤根皮采收于湖北省恩施市,由湖北民族大学王发松教授鉴定为显柱南蛇藤(Celastrus stylosusWall).
2 提取与分离
显柱南蛇藤干燥根皮15.0 kg 粉碎过筛,85%乙醇60 ℃(50 L × 3)超声提取,合并浓缩得1.1 kg总浸膏. 总浸膏用60 ℃水(10 L)分散制成混悬液,分别用石油醚、氯仿和正丁醇萃取. 合并氯仿萃取液,浓缩得氯仿部位浸膏160 g. 氯仿部位湿法上样,减压硅胶柱层析,石油醚-乙酸乙酯梯度洗脱. 经TLC 检识得4 个组分Fr.1~ Fr.4.
Fr.1(20 g)湿法上样,减压硅胶柱层析,石油醚-乙酸乙酯(体积比50∶1→10∶1)梯度洗脱,TLC检识合并得3 个亚组分Fr.1-1~ Fr.1-3. 其中,Fr.1-1、Fr.1-3 静置有晶体析出,反复重结晶得化合物1(11 mg),化合物2 (16 mg). Fr.1-2 经 Sephadex LH-20 凝胶柱层析(甲醇-氯仿,体积比1∶1)分离,TLC 检识合并3 个亚组分Fr.1-2-1~Fr.1-2-3. Fr.1-2-2静置有晶体析出,反复重结晶得化合物3 (15 mg).Fr.1-2-3 经制备薄层板分离,得化合物4(10 mg).
Fr.2(23 g)组分湿法上样,减压硅胶柱层析,石油醚-乙酸乙酯(体积比40∶1→1∶1)梯度洗脱,TLC 检识合并得3 个亚组分Fr.2-1~ Fr.2-3. 其中,Fr.2-1、Fr.2-2 静置有晶体析出,反复重结晶得化合物5(14 mg),化合物6(17 mg). Fr.2-3 经Sephadex LH-20 凝胶柱层析(甲醇-氯仿,体积比1∶1)分离纯化,得化合物7(13 mg).
Fr.3(13 g)组分湿法上样,减压硅胶柱层析,石油醚-乙酸乙酯(体积比30∶1→0∶1)梯度洗脱,TLC 检识合并得4 个亚组分Fr.3-1~Fr.3-4. Fr.3-1湿法上样,减压硅胶柱层析,石油醚-乙酸乙酯(体积比30∶1)等度洗脱,得化合物8 (33 mg). Fr.3-2湿法上样,减压硅胶柱层析,石油醚-乙酸乙酯(体积比25∶1)等度洗脱,得化合物9 (26 mg). Fr.3-3静置有晶体析出,反复重结晶得化合物10 (14 mg).Fr.3-4 经Sephadex LH-20 凝胶柱层析(甲醇-氯仿,体积比1∶1)分离纯化,得化合物11 (13 mg).
Fr.4(8 g)组分经中压制备色谱(甲醇-水,体积比85∶15→65∶35)梯度洗脱,得4 个亚组分Fr.4-1~Fr.4-4. Fr.4-1 进行中压制备色谱等度洗脱(甲醇-水,体积比80∶20),得化合物12 (9 mg).Fr. 4-2 进行中压制备色谱等度洗脱(乙腈-水,体积比80∶20),制备薄层板分离,得化合物13 (17 mg).Fr.4-3 进行Sephadex LH-20 凝胶(甲醇-氯仿,体积比1∶1)柱色谱分离,反复重结晶,得化合物14 (14 mg).Fr.4-4 进行中压制备色谱(甲醇-水,体积比3∶1)梯度洗脱,经Sephadex LH-20 凝胶(甲醇-氯仿,体积比1∶1)柱色谱分离,反复重结晶,得化合物14(17 mg)、15 (12 mg).
3 结构鉴定
化合物1 无色针状结晶(石油醚).1H NMR(400 MHz,CDCl3)δ:2.35 (2H,t,J=7.3 Hz,H-2),1.63(2H,m,H-3) ,1.25 (24H,brs,H4-H15),0.88 (3H,t,J=8.0 Hz,H-16);13C NMR(100 MHz,CDCl3)δ:180.11(C-1),34.18 (C-2),24.83 (C-3),29.22 (C-4),29.40 (C-5),29.52 (C-6),29.59 (C-7),29.75 (C-8),29.86 (C-9),29.86 (C-10),29.86 (C-11),29.86 (C-12),29.86 (C-13),29.22 (C-14),22.85 (C-15),14.27 (C-16). 以上数据与文献[6]基本一致,故鉴定为palmitic acid.
化合物2 无色针状结晶(乙酸乙酯).1H NMR(400 MHz,MeOD)δ:7.66 (1H,d,J=16.0 Hz,H-7),7.54 (2H,dd,J=6.8,2.8 Hz,H-2,H-6),7.42~7.31(3H,m,H-4,H-3,H-5),6.46 (1H,d,J=16.0 Hz,H-8);13C NMR (100 MHz,MeOD)δ:135.71 (C-1),129.13(C-2), 129.94 (C-3), 131.35 (C-4), 129.94 (C-5),129.13 (C-6),146.30 (C-7),119.27 (C-8),170.33 (C-9).以上数据与文献[7]基本一致,故鉴定为cinnamic acid.
化合物3 无色针状结晶(乙酸乙酯).1H NMR(400 MHz,CDCl3)δ:5.34 (1H,t,J=4.0 Hz,H-6),3.52 (1H,m,H-3),1.00 (3H,s,H-19),0.92 (3H,d,J=6.6 Hz,H-21),0.87~0.78 (9H,m,H-26,H-27,H-29),0.67 (3H,s,H-18);13C NMR (100 MHz,CDCl3)δ:37.39 (C-1),32.04 (C-2),71.94 (C-3),42.46 (C-4),140.90 (C-5),121.86 (C-6),31.79 (C-7),32.04 (C-8),50.27 (C-9),36.64 (C-10),21.22 (C-11),39.91 (C-12),42.43 (C-13),56.90 (C-14),24.44 (C-15),28.39 (C-16),56.19 (C-17),12.12 (C-18),19.54 (C-19),36.29 (C-20),18.92 (C-21),34.08 (C-22),26.21 (C-23),45.97 (C-24),29.28 (C-25),19.96 (C-26),19.17 (C-27),23.20 (C-28),12.00 (C-29). 以上数据与文献[8]基本一致,故鉴定为β-sitosterol.
化合物4 白色粉末(石油醚-乙酸乙酯).1H NMR(400 MHz,CDCl3)δ:6.17 (1H,d,J=1.3 Hz,H-4),5.14 (1H,dd,J=15.2,8.5 Hz,H-22),5.02 (1H,dd,J=15.2,8.6 Hz,H-23),4.32 (1H,ddd,J=12.1,5.5,1.7 Hz,H-6),2.41 (2H,ddd,J=15.1,13.4,4.6 Hz,H-2),1.18(3H,s,H-19),1.02 (3H,d,J=6.6 Hz,H-21),0.84 (3H,d,J=6.4 Hz,H-26),0.81 (3H,d,J=7.2 Hz,H-29),0.79 (3H,s,H-27),0.73 (3H,s,H-18);13C NMR (100 MHz,CDCl3)δ: 39.21 (C-1),33.94 (C-2),199.65 (C-3),119.81 (C-4),171.71 (C-5),68.84 (C-6),41.63 (C-7),34.30 (C-8),53.93 (C-9),36.42 (C-10),21.17 (C-11),39.50 (C-12),42.49 (C-13),56.01 (C-14),24.39 (C-15),28.94 (C-16),55.83 (C-17),12.28 (C-18),18.43 (C-19),40.59 (C-20),21.23 (C-21),138.14 (C-22),129.75 (C-23),51.39 (C-24),32.01 (C-25),21.30 (C-26),19.14(C-27),25.54 (C-28),12.38 (C-29). 以上数据与文献[9]基本一致,故鉴定为6-hydroxystigmasta-4,22-dien-3-one.
化合物5 红色针状结晶(丙酮),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:7.07 (1H,d,J=6.9 Hz,H-6),6.50 (1H,s,H-1),6.33 (1H,d,J=7.1 Hz,H-7),2.21 (3H,s,H-23),1.42 (3H,s,H-25),1.27 (3H,s,H-26),1.24 (3H,s,H30),1.08 (3H,s,H-28),0.56 (3H,s,H-27);13C NMR (100 MHz,CDCl3)δ:120.86 (C-1),178.31 (C-2),120.73 (C-3),127.63 (C-4),147.18 (C-5),135.7 (C-6),118.46 (C-7),165.21 (C-8),40.04 (C-9),173.43 (C-10),28.84 (C-11),29.39 (C-12),39.43 (C-13),43.26 (C-14),29.58 (C-15),32.51 (C-16),45.49 (C-17),44.37 (C-18),33.91 (C-19),31.18 (C-20),34.59 (C-21),36.46 (C-22),10.62 (C-23),38.49 (C-25),21.54 (C-26),18.80 (C-27),31.58 (C-28),182.70 (C-29),30.78 (C-30).以上数据与文献[10]基本一致,故鉴定为celastrol.
化合物6 橙红色针状结晶(丙酮),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:6.99(1H,d,J=8.3 Hz,H-6),6.52 (1H,d,J=1.1 Hz,H-1),6.33 (1H,d,J=7.2 Hz,H-7),3.54 (3H,s,OCH3),2.20(3H,s,H-23),1.44 (3H,s,H-25),1.25 (3H,s,H-26),1.16(3H,s,H-30),1.08 (3H,s,H-28),0.52 (3H,s,H-27);13C NMR (100 MHz,CDCl3)δ:119.69 (C-1),178.45(C-2),146.14 (C-3),117.23 (C-4),127.52 (C-5),134.17(C-6),118.25 (C-7),170.17 (C-8),43.04 (C-9),164.87(C-10),33.68 (C-11),29.77 (C-12),39.52 (C-13),45.16(C-14),28.75 (C-15),36.49 (C-16),30.65 (C-17),44.41(C-18),31.01 (C-19),40.52 (C-20),29.99 (C-21),34.91(C-22),10.37 (C-23),38.39 (C-25),21.74 (C-26),18.45(C-27),31.71 (C-28),178.85 (C-29),32.80 (C-30),51.67(C-31). 以上数据与文献[11]基本一致,故鉴定为pristimerin.
化合物7 白色粉末(丙酮),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:5.62 (1H,d,J=5.8 Hz,H-6),3.52 -3.41 (1H,m,H-3),3.27(2H,q,J=10.3 Hz,H-28),1.36(3H,s,H-25),1.20 (3H,s,H-23),1.14 (3H,s,H-24),1.12(3H,s,H-27),1.04 (3H,s,H-28),1.02 (3H,s,H-29),0.99 (3H,s,H-24),0.84(3H,s,H-26);13C NMR (100 MHz,CDCl3)δ:18.36(C-1),27.95 (C-2),76.47 (C-3),40.95 (C-4),141.75(C-5),122.11 (C-6),23.71 (C-7),48.01 (C-8),34.98(C-9),49.81 (C-10),34.76 (C-11),35.92 (C-12),37.83(C-13), 39.71 (C-14), 32.72 (C-15), 30.70 (C-16),30.62 (C-17),42.10 (C-18),29.09 (C-19),33.28 (C-20),28.09 (C-21),39.48 (C-22),25.59 (C-23),29.67 (C-24),16.21 (C-25),20.51 (C-26),18.19 (C-27),32.13(C-28),74.58 (C-29),26.19(C-30). 以上数据与文献[12]基本一致,故鉴定为29-hydroxyglutionl.
化合物8 白色粉末(丙酮),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,C5D5N)δ:4.43 (1H,d,J=3.8 Hz,H-2),4.25 (1H,d,J=8.0 Hz,H-24a),3.80(1H,d,J=8.0 Hz,H-24b),1.45 (3H,s,H-30),1.25(3H,d,J=7.1 Hz,H-23),1.22 (3H,s,H-28),1.14 (3H,s,H-27),0.94 (3H,s,H-25),0.85 (3H,s,H-26);13C NMR (100 MHz,C5D5N)δ:29.25 (C-1),74.68 (C-2),108.55 (C-3),47.32 (C-4),47.98 (C-5),34.48 (C-6),20.14 (C-7),50.92 (C-8),37.84 (C-9),53.78 (C-10),35.18 (C-11),29.95 (C-12),39.76 (C-13),40.07 (C-14), 30.21 (C-15), 37.19 (C-16), 30.96 (C-17),45.25 (C-18),31.42 (C-19),41.26 (C-20),8.83 (C-23),72.57 (C-24),17.39 (C-25),17.29 (C-26),18.50 (C-27),32.59 (C-28),181.99 (C-29),32.84 (C-30). 以上数据与文献[13]基本一致,故鉴定为orthosphenic acid.
化合物9 白色粉末(丙酮),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,DMSO)δ:6.83 (1H,s,H-1),6.01 (1H,s,H-7),2.46 (3H,s,H-23),1.44(3H,s,H-25),1.25 (3H,s,H-26),1.11 (3H,s,H-30),1.07 (3H,s,H-28),0.65 (3H,s,H-27);13C NMR (100 MHz,DMSO)δ:108.51 (C-1),141.93 (C-2),149.40(C-3), 121.04 (C-4), 124.74 (C-5), 186.03 (C-6),125.37 (C-7),170.31 (C-8),39.52 (C-9),149.27 (C-10),33.66 (C-11),29.29 (C-12),38.52 (C-13),43.99 (C-14),28.10 (C-15),36.09 (C-16),32.32 (C-17),43.66 (C-18),30.11 (C-19),39.42 (C-20),29.57 (C-21),34.52 (C-22),13.61 (C-23),37.48 (C-25),20.73 (C-26),18.11 (C-27),31.35 (C-28),179.48 (C-29),32.05 (C-30). 以上数据与文献[14]基本一致,故鉴定为wiforol A.
化合物10 无色针状结晶(甲醇),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,C5D5N)δ:1.46 (3H,s,H-30),1.29 (3H,s,H-28),1.16 (3H,s,H-27),0.95 (3H,d,J=6.7 Hz,H-26),0.86 (3H,s,H-24),0.79(3H,s,H-25),0.67 (3H,s,H-23);13C NMR (100 MHz,C5D5N) δ:22.74 (C-1),41.87 (C-2),212.04 (C-3),58.26 (C-4),42.32 (C-5),41.55 (C-6),18.80 (C-7),50.98 (C-8),37.93 (C-9),59.63 (C-10),35.85 (C-11),31.30 (C-12),39.89 (C-13),40.03 (C-14),30.87 (C-15),37.09 (C-16),30.90 (C-17),45.25 (C-18),30.19 (C-19),41.11 (C-20),29.96 (C-21),37.81 (C-22),7.56 (C-23),15.03 (C-24),18.48 (C-25),18.83 (C-26),16.87 (C-27),32.50 (C-28),181.77 (C-29),32.73 (C-30). 以上数据与文献[15]基本一致,故鉴定为polpunonic acid.
化合物11 白色粉末(氯仿),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,C5D5N)δ:5.43 (1H,brs,H-12),4.10~4.00 (1H,m,H-22),3.62 (1H,brs,H-3),1.81 (3H,s,H-30),1.29 (3H,s,H-28),1.25 (3H,s,H-24),1.17 (3H,s,H-27),1.10 (3H,s,H-26),1.03(3H,s,H-25),0.95 (3H,s,H-23);13C NMR (100 MHz,C5D5N)δ:34.28 (C-1),26.77 (C-2),75.65 (C-3),38.41,(C-4),49.76 (C-5),19.15 (C-6),33.77 (C-7),40.67 (C-8),48.37 (C-9),38.51 (C-10),24.35 (C-11),123.54 (C-12),144.78 (C-13),43.05 (C-14),26.89 (C-15),29.54 (C-16),37.95 (C-17),45.34 (C-18),43.14 (C-19),42.19 (C-20),38.41 (C-21),75.93 (C-22),29.76 (C-23),23.27 (C-24),16.25 (C-25),17.79 (C-26),25.90 (C-27),21.43 (C-28),183.02(C-29), 25.65 (C-30). 以上数据与文献[16]基本一致,故鉴定为3α,22β-dihydroxyolean-12-en-29-oic acid.
化合物12 白色粉末(乙酸乙酯),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:5.58 (1H,d,J=3.8 Hz,H-1),5.66 (1H,t,J=3.4 Hz,H-2),1.78 (1H,m,H-3),2.46 (1H,m,H-4),4.98 (1H,brs,H-6),2.51 (1H,d,J=3.2 Hz,H-7),5.54 (1H,d,J=3.3 Hz,H-8),5.10 (1H,s,H-9),1.45 (3H,d,J=7.6 Hz,H-12),1.72 (3H,s,H-13),1.55 (3H,s,H-14),1.57 (3H,s,H-15),1.56 (3H,s,AcO),7.59 (2H,dt,J=14.9,8.0 Hz,Ar-H),7.51~7.40 (4H,m,Ar-H),7.51 ~7.40 (4H,m,Ar-H),0.90 (3H,t,J=7.4 Hz),1.14 (3H,d,J=7.0 Hz),1.49 (1H,dd,J=7.4,6.2 Hz),1.69~1.62 (1H,m),2.43~2.37 (1H,m);13C NMR (100 MHz,CDCl3)δ:71.52 (C-1),69.60 (C-2),31.47 (C-3),33.36 (C-4),91.74(C-5), 73.35 (C-6), 55.31 (C-7), 77.12 (C-8),77.16(C-9),48.96 (C-10),82.47(C-11),19.28 (C-12),20.51 (C-13),26.14 (C-14),31.47 (C-15),20.96,169.57(AcCO),128.47,128.47,128.69,128.69,128.95,129.86,129.86,130.32,130.32,133.57,133.63,133.96,164.86,165.03 (2 × PhCO),11.60 (CH3),16.89 (CH3),26.74(CH2),41.86 (CH),175.91 (-COO-). 以上数据与文献[17]基 本 一 致,故 鉴 定 为1β-acetoxy-8β-,9αdibenzoyloy-6α-hydroxy-2β(α-methylbutanoyloxy)-βdihydroagarofuran.
化合物13 白色粉末(乙酸乙酯),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:5.20 (1H,s,H-12),2.54 (1H,m,H-2β),2.36 (1H,m,H-2α),1.14 (3H,s,H-27),1.09 (3H,s,H-23),1.07(3H,s,H-25),1.05 (3H,s,H-24),1.02 (3H,s,H-26),0.87 (6H,s,H-29,30),0.84 (3H,s,H-28);13C NMR(100 MHz,CDCl3)δ:39.43 (C-1),34.35 (C-2),217.61(C-3),47.00 (C-4),55.44 (C-5),19.79 (C-6),32.30(C-7),39.92 (C-8),47.61 (C-9),36.81 (C-10),23.82(C-11),121.64 (C-12),145.41 (C-13),41.99 (C-14),28.56 (C-15),26.61 (C-16),32.66 (C-17),47.44 (C-18),46.91 (C-19),31.23 (C-20),34.85 (C-21),37.23 (C-22),26.26 (C-23),21.65 (C-24),15.37 (C-25),16.87 (C-26),26.02 (C-27),27.06 (C-28),33.47 (C-29). 以上数据与文献[18]基本一致,故鉴定为β-amyrenone.
化合物14 白色粉末(乙酸乙酯),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:5.27(1H,t,J=3.5 Hz,H-12),3.22 (1H,dd,J=11.0,4.6 Hz,H-3α),2.81 (1H,dd,J=13.7,4.6 Hz,H-18β),1.12 (3H,s,H-27),0.98 (3H,s,H-23),0.92 (3H,s,H-29),0.91(3H,s,H-24),0.90 (3H,s,H-30),0.76 (3H,d,J=6.4 Hz,H-25),0.74 (3H,s,H-26);13C NMR (100 MHz,CDCl3)δ:38.53 (C-1),27.31 (C-2),79.18 (C-3),38.89(C-4),55.35 (C-5),18.43 (C-6), 32.58 (C-7),39.41(C-8),47.77 (C-9),37.22 (C-10),23.05 (C-11),122.77(C-12),143.74 (C-13),41.72 (C-14),27.82 (C-15),23.54 (C-16),46.01 (C-17),41.10 (C-18),46.67 (C-19),30.82 (C-20),33.94 (C-21),32.74 (C-22),28.24 (C-23),15.46 (C-24),15.68 (C-25),17.27 (C-26),26.08 (C-27),183.62 (C-28),23.72 (C-29),33.21 (C-30). 以上数据与文献[19]基本一致,故鉴定为oleanolic acid.
化合物15 无色针状晶体(石油醚-乙酸乙酯),10%硫酸乙醇显色呈紫红色.1H NMR (400 MHz,CDCl3)δ:5.71 (1H,d,J=5.7 Hz,H-11),5.55 (1H,d,J=5.7 Hz,H-12),4.15 (1H,t,J=2.9 Hz,H-1),3.78(1H,dd,J=12.0,4.8 Hz,H-3),1.21 (3H,s,H-25),1.14 (3H,s,H-26),1.06 (3H,s,H-23),1.01 (3H,s,H-27),0.90 (3H,s,H-29),0.88 (3H,s,H-28),0.88 (3H,s,H-30), 0.82 (3H, s, H-24);13C NMR (100 MHz,CDCl3)δ:72.96 (C-1),31.82 (C-2),73.08 (C-3),39.18(C-4),44.47 (C-5),17.56 (C-6),32.54 (C-7),42.64(C-8), 150.37 (C-9), 45.18 (C-10), 117.14 (C-11),120.05 (C-12), 149.14 (C-13), 40.85 (C-14), 25.75(C-15), 27.10 (C-16), 32.29 (C-17), 45.88 (C-18),46.87 (C-19), 31.25 (C-20), 34.69 (C-21), 37.08(C-22),28.85 (C-23),15.72 (C-24),25.80 (C-25),21.10(C-26), 20.47 (C-27), 28.35 (C-28), 23.83 (C-29),33.32 (C-30). 以上数据与文献[20]基本一致,故鉴定为juglangenin A.
4 结果与讨论
查询国内外文献,发现目前只有对显柱南蛇藤果实石油醚部位化学成分的研究报道[2]. 显柱南蛇藤作为传统中药,其根皮化学成分未详细研究. 本研究从显柱南蛇藤根皮氯仿部位分离鉴定15 个化合物,包括2 个有机酸(1, 2)、2 个甾醇(3, 4)、1 个倍半萜(5),10 个三萜(6~15). 对比发现,根皮与果实化学成分有显著差异,果实部位主要以倍半萜类化合物为主,根皮部位主要以三萜类化合物为主. 南蛇藤属植物的活性报道大多集中在抗肿瘤抗菌等方面,李嘉琪[21]等研究发现南蛇藤素(化合物5)能呈浓度依赖性抑制乳腺癌细胞的增殖、迁移,促进细胞凋亡. 姚正盛[22]研究发现扁蒴藤素(化合物6)对鼻咽癌细胞具有增殖抑制及诱导凋亡的作用. 周永林[23]研究发现齐墩果酸(化合物14)可显著降低由细菌性溶血素对机体造成的损伤和显著恢复β-内酰胺类抗生素的体内外抗菌活性.Vonshak 等[24]发现南蛇藤提取物对红色毛癣菌具有抑制作用. 丁丽娜[25]研究发现,短柄南蛇藤果壳、假种皮、种子的部分提取物对大肠埃希菌有抑制作用. 南蛇藤属植物化学成分丰富,但活性研究大都只停留在初步的活性筛选中,特别是抗菌的作用机制还需要更加深入研究. 本研究所得化合物可用于进一步相关活性筛选,以期进一步阐明显柱南蛇藤根皮的药效物质基础.
