Preparation of Polyaniline/Cellulose Nanofiber Aerogel for Efficient Removal of Cr(VI) from Aqueous Solution
2022-03-08LIJiaqiang李加强LIAOYaozu廖耀祖WeiYUJunrong于俊荣
LI Jiaqiang (李加强), LIAO Yaozu (廖耀祖), LÜ Wei (吕 伟)*, YU Junrong (于俊荣)*
1 State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai 201620, China2 College of Materials Science and Engineering, Donghua University, Shanghai 201620, China
Abstract: Aerogel composed of polyaniline/cellulose nanofiber (PANI/CNF) was synthesized by a two-step method and used as an adsorbent to remove Cr(VI) from wastewater in this study. Adsorption results showed that PANI/CNF aerogel adsorbent exhibited a high adsorption capacity of 298.5 mg·g-1 for Cr(VI) (318 K, pH=2.0). Meanwhile, the adsorption process can be fitted well with the pseudo-second-order model and the Freundlich model. Moreover, the Cr(VI) removal efficiency of the adsorbent remained above 85% after ten recycling experiments, indicating that PANI/CNF aerogel had excellent stability and reusability. Therefore, PANI/CNF aerogel showed a potential application in the treatment of chromium-containing wastewater.
Key words: polyaniline(PANI); cellulose; nanofiber aerogel; Cr(VI); adsorption
Introduction
In recent years, the rapid development of industry has led to the continuous deterioration of the environment. Various heavy metal ions [e.g., Cr(VI), Cu(II), Pb(II), Cd(II), and Hg(II)] in industrial wastewater are discharged into the water system[1-3]. Among these ions, Cr(VI) has been considered as a typical heavy metal pollutant, which is mainly released from electroplating, pharmaceuticals, leather tanning, and printing and dyeing[4-6]. Because of its high toxicity, carcinogenicity and teratogenicity, Cr(VI) is one of the heavy metal pollutants that need to be strictly controlled[7-10]. In order to remove Cr(VI) from wastewater, the traditional treatment techniques including chemical precipitation[11-12], ion exchange[13-14], membrane filtration[15-16], and adsorption[17-18]have been studied. Among these techniques, the adsorption method has been widely used for Cr(VI) removal due to its easy operation, low cost, and high efficiency[19-22].
Conventional materials such as activated carbon, molecular sieves, and clay minerals, have been reported as adsorbents for the removal of Cr(VI) from aqueous solution and wastewater[23-29]. However, the insufficient adsorption capacity of traditional adsorption materials is still a problem. It is therefore necessary to develop new and highly efficient adsorbents for the removal of Cr(VI)[30]. Polyaniline (PANI) has been extensively applied in the removal of organic pollutants because of its simple synthesis, stable properties, abundant active groups, and reversible acid-base doping-dedoping characteristics[31-34]. Recently, PANI-based composites have been widely studied due to the synergistic effect of PANI and composite materials on the adsorption of heavy metal ions[3]. For example, Hsinietal.[35]prepared PANI@Almond shell (PANI@AS) biocomposite with an excellent adsorption capacity of 335.25 mg·g-1for Cr(VI) at pH of 2.0 by a simple in-situ interfacial oxidative polymerization. Zuoetal.[36]designed a composite material formed by Fe3O4@mSiO2@CS grafted by PANI (FSCP) with the adsorption capacity for Cr(VI) reached around 249.6 mg·g-1. And the adsorbent could still show a removal rate of more than 90% and excellent magnetic separation ability after eight adsorption-desorption cycles. Lüetal.[37]prepared 3D hierarchical coral-like PANI (CL-PANI) and the corresponding magnetic nanocomposites (CL-PANI@Fe3O4and Fe3O4@CL-PANI) via a simple chemical oxidation method. The Fe3O4@CL-PANI showed that the removal capability of Cr(VI) was better than that of CL-PANI@Fe3O4, and the magnetic adsorbents could be easily separated by the external magnetic field. Nevertheless, the high aggregation and the difficulty in processing are still the drawbacks which limit the application of PANI magnetic powders in industry.
The gel state endows PANI-based materials with the advantages of easy operation and high porosity. Cellulose nanofiber (CNF) can be extracted from most abundant natural fiber materials[38-40]. And CNF aerogels with interconnected pores, high specific surface, and high strength could be prepared by freeze-drying technology, which have a wide range of applications in the field of organic pollutant treatment[41-44]. In our previous study, a PANI/CNF aerogel used for the efficient adsorption of acid red G(ARG) and methylene blue(MB) dyes was prepared by a two-step method[45]. Herein, we systematically investigated the adsorption capacity of the PANI/CNF aerogel for Cr(VI), including adsorption isotherms and adsorption kinetics. Besides, the adsorption behavior and mechanism of Cr(VI) onto PANI/CNF aerogel, and the regeneration performance of PANI/CNF aerogel were analyzed.
1 Experiments
1.1 Materials
Carboxylated CNF powders were purchased from Qihong Co., Ltd., Guilin, China. Aniline (C6H5NH2) was obtained from J&K Chemical Ltd., Shanghai, China. FeCl3, KOH, and H2SO4were purchased from Sinopharm group chemical reagent Co., Ltd., Shanghai, China. All chemicals were used as received. The deionized water was used in all experiments.
1.2 Preparation of PANI/CNF aerogel
PANI/CNF aerogel was prepared as reported in our previous study[45]. In brief, the CNF powder aqueous solution with a mass fraction of 12% was stirred at room temperature for 3 min. Subsequently, 0.1 mmol·L-1FeCl3and 1.0 mmol·L-1aniline were added under the rapid stirring. After 5 min, the sample was put into the -20 ℃ freezer for 5 h and thawed at room temperature to obtain the hydrogel. Then, the hydrogel was placed into the 0.5 mol·L-1H2SO4aqueous solution containing 3.0 mmol·L-1FeCl3to further induce the polymerization for 24 h. Finally, the hydrogel was washed with deionized water and ethanol, and then freeze-dried to obtain the PANI/CNF aerogel.
1.3 Characterization
The morphologies of the samples were characterized by a Hitachi SU8010 ultra-high resolution scanning electron microscopy (SEM) which was equipped with energy-dispersive X-ray spectroscopy(EDS). Fourier transform infrared (FT-IR) spectra were performed on a Nicolet 670 spectrometer (Madison, American) within the spectra range of 400-4 000 cm-1. Zeta potentials of the samples were tested on an Anton Paar Litesizer 500 apparatus (Graz, Austria). X-ray photoelectron spectra (XPS) were obtained from the ESCALAB 250Xi apparatus (Shanghai, China) with C 1s signal at 284.8 eV as the reference.
1.4 Adsorption experiment
The adsorption experiments were investigated in a constant temperature shaker at the speed of 300 r/min under the temperatureTof 25-45 ℃. The as-prepared adsorbent with a dosage of 0.5 -2.5 g·L-1was added into Cr(VI) solution with initial concentration of 50-700 mg·L-1and then stirred. The initial pH of solution was adjusted from 2.0 to 10.0, using 0.1 mol·L-1H2SO4solution and 0.1 mol·L-1KOH solution. After 24 h, the adsorbent was separated by a 0.45 μm filter membrane, and a UV-Vis spectrophotometer (UV-2600i, Shimadzu, Kyoto, Japan) was used to determine the concentration of Cr(VI) remaining in solution by the 1, 5-diphenylcarbohydrazide spectrophotometry method[46]. Equations (1) and (2) were used to calculate the adsorption rateR(%) and the adsorption capacityQt(mg·g-1):
(1)
(2)
whereC0andCtare the initial concentration of Cr(VI) and the concentration of Cr(VI) at any timet, respectively;VandMare the volume of Cr(VI) solution and the mass of the used adsorbent, respectively.
2 Results and Discussion
2.1 Adsorption performance of PANI/CNF aerogel


Fig. 1 Effects of (a) adsorbent dosage and (b) pH on the adsorption capacities of Cr(VI)
The ability to achieve adsorption equilibrium in a short period time is an important evaluation of the performance to an excellent adsorbent material. Three different initial Cr(VI) concentrations (50, 100, and 200 mg·L-1) were therefore selected to investigate the effect of contact time between the adsorbent and Cr(VI),i.e., adsorption kinetics. The pseudo-first-order shown in Eq. (3), pseudo-second-order shown in Eq. (4) and Elovich shown in Eq. (5) models were used to fit the experiment data shown in Fig. 2(a) and Table 1.

Fig. 2 Kinetics plots of (a) Cr(VI) adsorption at different initial concentrations; adsorption isotherms for Cr(VI) adsorption on PANI/CNF aerogel at temperatures of (b) 298 K, (c) 308 K, and (d) 318 K, respectively

Table 1 Kinetics parameters for Cr(VI) adsorption at different initial concentrations
Qt=Qe(1-e-K1t),
(3)
(4)
(5)
whereQe(mg·g-1) is the adsorption capacity for Cr(VI) at equilibrium;K1(min-1) andK2(g·mg-1·min-1) are the rate constants for the pseudo-first-order and the pseudo-second-order, respectively;α(mg·g-1·min-1) is the initial dye adsorption rate for the Elovich model andβ(g·mg-1) is the Elovich adsorption constant.
The fitting results suggested that the values of determination coefficientR2calculated from the pseudo-second-order model were significantly higher than those of pseudo-first-order and Elovich models, indicating that the chemisorption was the rate controlling step in the adsorption process[37].
The isothermal adsorption of Cr(VI) on PANI/CNF aerogel was then studied at different temperatures (298, 308, and 318 K), and Langmuir shown in Eq. (6), Freundlich shown in Eq. (7) and Temkin shown in Eq. (8) models were used to fit the experiment data shown in Figs. 2(b)-2(d) and Table 2.

Table 2 Langmuir, Freundlich and Temkin isotherm parameters for Cr(VI) adsorption
(6)
(7)
(8)
whereQmax(mg·g-1) is the maximum adsorption capacity;Ce(mg·L-1) is the equilibrium concentration of Cr(VI) in the solution;KL(L·mg-1) is the Langmuir isotherm constant related to the adsorption heat;KF[(mg·g-1)·(mg·L-1)-n] is the Freundlich isotherm constant describing the adsorption density, andnis the adsorption intensity;KT(L·mg-1) is the Temkin isotherm constant;bT(J·mol-1) represents the Temkin isotherm constant that relates to adsorption heat;T(K) is the absolute temperature andR(8.314 J·mol-1·K-1) is the universal gas constant.
It can be seen that the Freundlich model is more suitable for describing the adsorption process of Cr(VI) due to the higherR2. The obtained results suggest that the adsorption process is the chemical heterogeneous adsorption. The maximum adsorption capacity of Cr(VI) on PANI/CNF aerogel was calculated around 232.8, 272.9, and 298.5 mg·g-1at 298, 308, and 318 K, respectively, indicating that the increase of temperature is beneficial to the adsorption process.
Figures 3(a) and 3(b) show SEM images of the PANI/CNF aerogel before and after Cr(VI) adsorption. It can be seen that PANI/CNF nanofibers are completely covered after adsorption. The results of EDS shown in Fig. 3(d) further confirmed the PANI/CNF nanofibers were covered by Cr(VI), indicating that Cr(VI) was successfully adsorbed onto aerogel. In order to analyze the mechanism of Cr(VI) removal by PANI/CNF aerogel, FT-IR spectra were used to character the aerogel before and after adsorption. As shown in Fig. 3(c), after the adsorption of Cr(VI) solution, the increase of the intensity ratio (IQ/IB) of peaks at 1 560 cm-1(the C═C stretching vibrations of quinoid ring) and 1 481 cm-1(the C═C stretching vibrations of benzenoid ring) indicated that PANI might be oxidized and Cr(VI) might be reduced to Cr(III) during the adsorption process[37].

Fig. 3 SEM images of PANI/CNF aerogel (a) before and (b) after Cr(VI) adsorption; (c) FT-IR spectra before and after adsorption; (d) EDS results after adsorption
In addition, XPS of PANI/CNF aerogel after adsorption was studied to further explore the mechanism. As shown in Fig. 4(a), compared with the aerogel before Cr(VI) adsorption, a new peak located around 575.7 eV appeared in the full-range XPS spectra of aerogel after loading Cr(VI), which proved that Cr was successfully adsorbed onto the aerogel. The Cr 2p core-level spectra of PANI/CNF aerogel after Cr(VI) adsorption was divided into the binding energies of Cr 2p3/2and Cr 2p1/2shown in Fig. 4(b) . Besides, the peaks at around 575.6, 576.5, 585.3, and 586.4 eV could be observed, attributing to Cr(III) 2p3/2, Cr(VI) 2p3/2, Cr(III) 2p1/2and Cr(VI) 2p1/2, respectively. This suggested that Cr(VI) existed on the aerogel after adsorption and partially reduced to Cr(III) (52.04%). The N 1s core-level spectra of aerogel before and after adsorption were shown in Fig. 4(c), which could be associated to ═N— (about 398 eV), —NH— (about 399 eV) and —N—+(about 401 eV). It could be seen that the content of ═N— increased during the adsorption process, suggesting that the redox occurred between PANI and Cr(VI).

Fig. 4 XPS spectra of PANI/CNF aerogel before and after adsorption: (a) full-range XPS spectra; (b) Cr 2p core-level spectra; (c) N 1s spectra
The regeneration ability and recycling of adsorbent were essential for its practical application. To evaluate the potential of the aerogel, the Cr(VI)-loaded adsorbent was eluted by 0.1 mol·L-1KOH for 0.5 h. Then 0.1 mol·L-1H2SO4was used as an active agent to restore the activity of adsorbent. It could be found that the aerogel still maintained a high removal efficiency of 85% after ten cycles (shown in Fig. 5), suggesting that PANI/CNF aerogel was an efficient and reusable adsorbent for removal of Cr(VI).
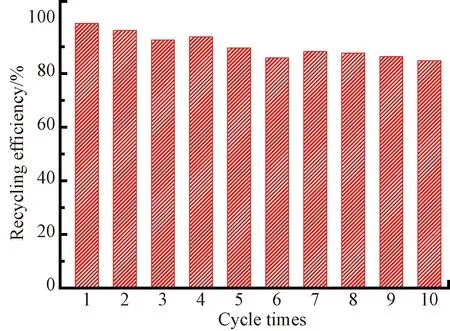
Fig. 5 Adsorption cycles of PANI/CNF aerogel for removal of Cr(VI)
3 Conclusions
In this study, PANI/CNF aerogel composed of PANI nanofiber and CNF was used to remove heavy metal ions Cr(VI), of which the maximum adsorption capacity achieved 298.5 mg·g-1at 318 K, and pH of 2.0. Mechanism studies suggested that the electrostatic attraction and the redox reaction occurred during the adsorption process. Moreover, after ten cycles, the adsorption rate of PANI/CNF aerogel still maintained over 85%, indicating that the aerogel showed good industrial application potential in the treatment of wastewater containing heavy metalion pollution.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Diradical Character and Controllable Magnetic Properties in Chitin Through Radical Modification
- Recombinant Pyriform Spider Silk Expression and Wet-Spinning
- Experimental Study on Grinding Force of Electrostatic Coated Grinding Wheel
- Influence of Three Sizes of Sliding Windows on Principle Component Analysis Fault Detection of Air Conditioning Systems
- Consensus for High-Order Linear Multi-Agent Systems with Unknown but Bounded Measurement Noises
- Theoretical Calculation and Analysis of Muffler Based on Multilayer Sound Absorbing Material
