Morphological and physiological plasticity response to low nitrogen stress in black cottonwood (Populus deltoides Marsh.)
2022-02-26CunChenYanguangChuQinjunHuangChangjunDingWeixiZhangBoLiJingZhangXiaohuaSu
Cun Chen · Yanguang Chu · Qinjun Huang · Changjun Ding · Weixi Zhang · Bo Li · Jing Zhang · Xiaohua Su,3
Abstract It is important to evaluate nitrogen use effi-ciency and nitrogen tolerance of trees in order to improve their productivity.In this study, both were evaluated for 338 Populus deltoides genotypes from six provenances.The plants were cultured under normal nitrogen (750 μM NH4NO3) and low nitrogen (5 μM NH4NO3) conditions for 3 months.Growth, chlorophyll content and glutamine synthetase activity of each genotype were measured.Under low nitrogen, heights, ground diameter, leaf area, leaf and root biomass, and chlorophyll contents were significantly lower than those under normal nitrogen level.Correlation analysis showed that nutrient distribution changed under different nitrogen treatments.There was a negative correlation between leaf traits and root biomass under normal nitrogen level, however, the correlation became positive in low nitrogen treatment.Moreover, with the decrease of nitrogen level, the negative correlation between leaf morphology and chlorophyll levels became weakened.The growth of the genotypes under the two treatments was evaluated by combining principal component analysis with a fuzzy mathematical membership function; the results showed that leaf traits accounted for a large proportion of the variation in the evaluation model.According to the results of comprehensive evaluation of plants under the two treatments, the 338 P.deltoides genotypes could be divided into nine categories, with wide genotypic diversity in nitrogen use efficiency and low nitrogen tolerance.As a result, 26 N-efficient genotypes and 24 N-inefficient genotypes were selected.By comparative analysis of their morphological and physiological traits under the two treatments, leaf traits could be significant indicators for nitrogen use efficiency and nitrogen tolerance, which is of considerable significance for breeding poplar varieties with high nitrogen use efficiencies.
Keywords Populus deltoides · Nitrogen deficiency · Nitrogen use efficiency · Comprehensive evaluation · Genotypic diversity
Introduction
Nitrogen (N) is an essential mineral element and an important component of many biological macromolecules such as nucleic acids, proteins, hormones and chlorophyll.Nitrogen plays a crucial role in the growth and development of plants (Binkley and Högberg 2016; Rubio-Asensio and Bloom 2017; Cánovas et al.2018).Both inorganic and organic N are absorbed by roots and transported to every part of the plant.Under the action of various enzymes, N is transformed into several organic substances that participate in essential metabolic processes (Rennenberg et al.2010; Tegeder and Masclaux-Daubresse 2018).Plant productivity is closely related to N levels in the soil (Mamashita et al.2015).To increase crop yields, exgenous N application is a common method to increase the N content in the soil.However, N application increases crop production costs and may cause environmental pollution (Robertson and Vitousek 2009; Sutton et al.2011).Tree species have high demands for N but some grow in poor quality soil where the N availability is low (Rennenberg et al.2010; Balasus et al.2012), leading to low forest quality.Undoubtedly, the application of N fertilizer to promote plant growth in such situations will increase costs.Poplar species are widely planted because they are fast-growing, reproduce easily and display strong adaptability to a variety of environments, and thus have important economic and ecological protection values (Jansson and Douglas 2007; Polle et al.2013).However, the growth of poplar species is constrained by nitrogen availability.Therefore, it is important to understand mechanisms underlying poplar adaptation to N limitation in order to enhance N tolerance and cultivate varieties with high nitrogen use efficiency (NUE) (Ren et al.2015; Meng et al.2018).
The response mechanisms of trees to N starvation or limitation have been studied in terms of phenotypic and physiological characteristics (Rennenberg et al.2010).Nitrogen starvation affects the allocation of N within the plant and will stimulate the transport of more nutrients to underground parts to encourage the growth of roots, especially fine roots, to absorb more N from the soil (Gao et al.2015; Kiba and Krapp 2016; Luo et al.2019; Qin et al.2019).Root structure displays high plasticity in different environments with different N levels (Dathe et al.2016; Dong et al.2016; Luo and Luo 2017).Similar to the roots, leaf characteristics, especially leaf area which is closely related to biomass, are sensitive to changes in N availability (Tavarini et al.2016; Zeng et al.2017; Mao et al.2018).Therefore, leaf characteristics are often used as indexes to evaluate site conditions and plant growth (Pérezharguindeguy et al.2013).The composition of soil fauna and microbial communities can be affected by N levels, which plays an important role in the growth of plants, especially roots (Hodge et al.2000; Ouyang et al.2017; Sun et al.2017; Bian et al.2019).
The response of poplar species to low N levels has mainly been studied using a single genotype or two contrasting genotypes.Few studies have been conducted on the adaptability and NUE of natural populations ofPopulus(Kalcsits and Guy 2016).It is well known that selecting poplar genotypes suitable for the site conditions will achieve high productivity (Ghezehei et al.2016, 2019).Populusspecies have been planted over large areas of the world on various site conditions, so it is particularly important to select clones scientifically (Truax et al.2014; Ghezehei et al.2019).
Populus deltoidesMarsh.is a highly suitable short rotation timber species in the middle latitudes of the world.P.deltoidesis often used in hybrid breeding which has important research value (Fahrenkrog et al.2017).P.deltoidesgermplasm resources have high genetic diversity (Fahrenkrog et al.2017; Chen et al.2020).However, the NUE and response to low N stress of a large number of genotypes in theP.deltoidespopulation are unknown.Therefore, in this study, the phenotypic and physiological indexes of 338 genotypes ofP.deltoideswere observed under normal N supply and low N stress, and the growth of each genotype was evaluated.It was hypothesized that the growth of each genotype (roots, stems and leaves) would be inhibited under low N stress and that the response of different genotypes to the change in N levels would be different.This study aimed to evaluate the NUE and low N tolerance of different genotypes.The results could provide a theoretical basis for clonal selection in poplar breeding and lay the foundation for further research on molecular mechanism regulation under low N stress.
Materials and methods
Plant materials
Through international cooperation and exchange of germplasm resources,P.deltoidesgermplasm was collected from main distribution areas in North America.A germplasm bank was established in Ningyang County, Tai’an City, Shandong Province (35°55′39" N, 116°53′59" E) in 2009.Cuttings of 338 genotypes ofP.deltoideswere selected from this bank (Fig.1).These individuals came from six provenances of three different basins.Among them, 18 genotypes were from Iowa (‘Iow’), 10 were from two sampling sites in Missouri (‘Mis’), and 117 and 92 individuals were from eight sampling sites in Louisiana (‘Lou’) and five in Tennessee (‘Ten’), respectively.These provenances were located in the Mississippi River basin.In the Columbia River basin, 16 genotypes were from Washington (‘Was’).In the Saint Lawrence River basin, 85 individuals were from seven sampling sites in Quebec, Canada (‘Que’).These materials were similar to those used in a previous study (Chen et al.2020).

Fig.1 Locations of a the provenances and b the germplasm bank of Populus deltoides.Que, Quebec; Lou, Louisiana; Iow, Iowa; Mis, Missouri; Ten, Tennessee; Was, Washington
Plant cultivation and experimental design
One-year-old stem cuttings (15 cm in length, 1.0-1.5 cm in diameter) of the 338 genotypes were rooted and cultured in nutritional pots (5 cm height, 5 cm diameter) filled with medium (nutrient soil: perlite=9: 1).For every genotype, there were 20 cuttings.The plants were cultivated for 30 days, the root systems carefully washed, replanted in new pots (20 cm height, 10 cm diameter) with vermiculite to hold air, water and nutrients.The plants were cultured with water for 15 days, and then irrigated every other day with 100 mL of one-tenth strength Hoagland nutrient solution containing 0.4 mM Ca(NO3)2·4H2O, 0.5 mM KNO3, 0.1 mM NH4NO3, 0.1 mM KH2PO4, 0.4 mM MgSO4·7H2O, 5 μM Fe-EDTA [pH=5.5], 2.5 nM KI, 50 nM H3BO3, 50 nM MnSO4·4H2O, 15 nM ZnSO4·7H2O, 0.5 nM Na2MoO4·2H2O, 0.05 nM CuSO4·5H2O and 0.05 nM CoCl2.The solution was adjusted to pH 6.0.After 20 days, 12 plants of each genotype with similar growth performance were selected and randomly divided into two groups of six plants, one group as the control (CK) and the other the N stress group (low N; LN).Plants of the LN and CK were cultivated with modified Hoagland nutrient solution (0.4 mM CaCl2·2H2O, 0.5 mM KCl, 0.1 mM KH2PO4, 0.4 mM MgSO4·7H2O, 5 μM Fe-EDTA [pH=5.5], 2.5 nM KI, 50 nM H3BO3, 50 nM MnSO4·4H2O, 15 nM ZnSO4·7H2O, 0.5 nM Na2MoO4·2H2O, 0.05 nM CuSO4·5H2O and 0.05 nM CoCl2) containing 5 μM or 750 μM NH4NO3, respectively, every two days.The treatments were carried out for 3 months until distinct morphological differences were observed and then harvested.All treatments were carried out in the greenhouse of the Chinese Academy of Forestry (40°0′10" N, 116°14′38" E).
Measurements of chlorophyll and glutamine synthetase (GS) activity
After 2 months of treatments, three plants with similar heights (H) were selected for each genotype in each treatment.Three mature functional leaves of each plant were collected, placed into liquid N, and stored at-80 °C for the determination of chlorophyll contents and GS activity.Concentrations of chlorophyll (Chl a, Chl b and total Chl) were analyzed using the 96% ethanol method (Lichtenthaler and Wellburn 1983).The activity of GS was determined using the relevant biochemical kit (GS-1-Y, Cominbio, Suzhou, China).
Determination of leaf morphological characteristics and plant biomass
The initial height (H0) and the ground diameter (GD0) of each plant were determined before the treatments.After treatments, three plants with similar growth performances were selected for each genotype in each treatment for harvesting, and final heights (Hn) and the ground diameters (GDn) were measured.Before harvesting, five mature leaves that had formed during the treatments were collected from each plant to determine leaf morphological characteristics.Fresh weight of the leaves was recorded, and leaf length, width and area were measured using a leaf area meter (Yaxin-1241, Beijing Yaxin Liyi Technology Co., Ltd., Beijing, China).Root systems of each plant were washed and fresh weight was determined.Leaves and roots were then dried at 75 °C for 96 h until constant weight and dry weights of the leaves and roots were recorded.
Verification experiment
Based on the results of the comprehensive evaluation, three genotypes were randomly selected from plants of different N utilization types to verify the evaluation results.The culture method used for the plants was similar to that described previously; however, it differed in that plants of each genotype with similar heights were randomly divided into three groups, with six plants in each group.Plants in each of the three groups were cultivated with the application of modified Hoagland nutrient solution containing 5 μM (LN), 750 μM (CK) or 3000 μM (high nitrogen; HN) NH4NO3, every 2 days.
The measured trait parameters included the height and ground diameter of each plant, the morphological parameters and chlorophyll content of the leaves, and the biomass values of the roots, stems, petioles, and leaves without petioles after the N treatments.In addition, the net photosynthetic rate (Pn) and transpiration rate (Tr) of mature leaves were measured from 09:00 to 11:00 using a portable photosynthesis system (Li-Cor-6400, LI-COR Inc., Lincoln, NE, USA) with an attached light-emitting diode (LED) light source.The light intensity was 1000 μmol m-2s-1, the CO2concentration 400 μmol mol-1and the gas flow rate 500 μmol s-1.
Statistical analyses
All data were recorded using Excel software, and the mean value of every parameter of each genotype was calculated.Correlation analysis between parameters was carried out using the package ‘corrplot’ in R.Heat maps, violin plots and network diagrams of each parameter were drawn using OmicShare tools (www.omics hare.com/ tools).A Tukey test was used for comparing the means of the parameters.For principal component analysis (PCA), the data were standardized and computed using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA).In order to explain the extracted principal components clearly, the factors were rotated using the maximum variance method with Kaiser normalization.Afterwards, based on the result of the PCA, comprehensive analysis of each genotype were conducted using the fuzzy mathematics membership function method.The comprehensive evaluation value was calculated using three equations as follows:

wherey(PCij) is the fuzzy mathematical membership function value of thej-th principal component of thei-th poplar genotypes,PCijis the score of thej-th principal component of thei-th poplar genotypes,PCjminis the minimum value of thej-th principal component score of all genotypes andPCjmaxis the maximum value.

wherewjandrjare the weight and the contribution rate of thej-th principal component, respectively.

wherePCE–iis the comprehensive evaluation value of thei-th poplar genotypes.
Results
Morphological and physiological traits of the genotypes
P.deltoidesgenotypes were sensitive to the change of available N in the culture environment.Compared with plants cultured under normal N supply (CK), growth of plants under low N (LN) was inhibited.Before the N treatment, there was no significant difference in height between LN and CK treatments (Fig.2), and the means were 34.68 and 34.62 cm, respectively (Table S1).Average ground diamters of plants in the LN treatment was 3.41 mm, significantly higher than that of the CK plants (3.34 mm;p< 0.05; Fig.2 and Table S1).After the treatments, heights and ground diameters of plants in the LN treatment were significantly lower than those in the CK treatment (p< 0.01; Fig.2).The mean fresh weight (RFW) and mean dry weight (RDW) of roots of LN treated plants were 1.70 and 1.01 g, respectively.These values were significantly lower than those of the plants in the CK group (p< 0.01; Fig.2 and Table S1).Growth of leaves was obviously inhibited in the LN treatment, and this manifested as decreases in length (LL), width (LW), area (LA), fresh weight (LFW) and dry weight (LDW) (p< 0.01; Fig.2).Under LN treatment, chlorophyll contents also decreased significantly (p< 0.01; Fig.2).However, the change in N concentration had no significant effect on GS activity (Fig.2).

Fig.2 Height (H), ground diameter (GD), root biomass, leaf morphology and biomass, chlorophyll content and glutamine synthetase (GS) activity of Populus deltoides under normal N supply (CK; white) and low N supply (LN; gray) *: p < 0.05, **: p < 0.01
Correlation analysis of morphological and physiological parameters
Under both treatments (CK and LN), there was a significant positive correlation between leaf morphological parameters (p< 0.01; Fig.3a and b).A similar correlation also existed between the physiological parameters (p< 0.01; Fig.3a and b).In addition, there was a significant positive correlation between stem growth and growth of leaves and roots (p< 0.01; Fig.3a and b).There was also a negative correlation between leaf physiological and morphological parameters (Fig.3a and b).
There were differences in the correlation relationships of all parameters under the two treatments (Fig.3c and d), specifically, there were significant positive correlations between RFW and leaf morphological parameters in the LN treatment (p< 0.01; Fig.3a and b).In the controls, there were significant negative correlations between leaf morphological parameters and chlorophyll contents (p< 0.01; Fig.3a and b), but this negative correlations were weak in LN treatment.The same results can be found by comparing the network diagrams of the CK (Fig.3c) and LN (Fig.3d) treatments.

Fig.3 Heat maps and network diagrams of correlation among characteristic parameters of Populus deltoides in a, c normal N supply (CK) and b, d low N supply (LN); *: p < 0.05, **: p < 0.01.In the network diagrams (c, d), red lines represent negative correlations, green lines positive correlation, solid lines p < 0.01, dotted lines p < 0.05; dark-colored lines indicate that the correlation was significantly different between the CK and LN treatments; light-colored lines indicate that the correlation was not significantly different between CK and LN treatments.Hn, the height after treatment; GDn, the ground diameter after treatment; LFW, leaf fresh weight; LDW, leaf dry weight; LA, leaf area; LL, leaf length; LW, leaf width; RFW, root fresh weight; RDW, root dry weight; GS, glutamine synthetase activity; Chl a: chlorophyll-a content; Chl b: chlorophyll-b content; Chl: total chlorophyll content
Principal Component Analysis (PCA) of morphological and physiological parameters
Correlation analysis results show that there was a strong correlation between the measured trait parameters, whether under CK treatment or LN treatment.After removing the parameter Chl (Chl a + Chl b), PCA was performed for 12 parameters after N treatment.The Kaiser-Meyer-Olkin (KMO) test and Bartlett′s test of sphericity were carried out on the parameters under the two treatments, and the results show that they were suitable for principal component analysis (KMO > 0.7,p< 0.001).
For both treatments, four principal components with eigenvalues > 1 after rotation were extracted (Wang et al.2018a, b), and the cumulative variance contribution rates reached 85.7% and 87.1%, respectively (Table 1).In addition, according to the rotation component matrices, the composition and biological interpretation of the four principal components were similar in both treatments (Table 2).The first principal component (PC1) was leaf character factor, upon which the LFW, LDW, LA, LL and LW had a large loading.The second principal component (PC2) was physiological index factor (Chl a, Chl b and GS).The mean root fresh weight (RFW) and mean root dry weight (RDW) were the main load factors of the third principal component (PC3), which was named root character factor.The fourth principal component (PC4), stem character factor, mainly contained the variation information of the fnial heights (Hn) and fnial ground diameters (GDn).The four extracted principal components refelct adequately the growth of eachP.deltoidesgenotype under CK and LN treatments.

Table 1 Eigenvalues and cumulative contribution rate of principal components extracted from growth indexes of Populus deltoides under normal N supply (CK) and low N supply (LN)

Table 2 Rotated component matrix of the principle component analysis of Populus deltoides growth in conditions of normal N supply (CK) and low N supply (LN)
Comprehensive evaluation of plants
Different genotypes varied in response to different N treatments.Therefore, the results of the PCA and fuzzy mathematics membership function method were used to evaluate individuals under CK and LN treatment.
In the control treatment, the equation to calculate the comprehensive evaluation was:

In the LN treatment, the equation used was:

Based on their comprehensive evaluation results, the genotypes were divided into three categories: excellent growth category (PCE>+ SD), medium growth category (-SD < PCE<SD) and poor growth category (PCE<-SD), whereis the mean value of comprehensiveevaluation values and SD the standard deviation of comprehensive evaluation values.
Based on the classification results in the CK and LN treatments, theP.deltoidesgenotypes were divided into nine groups (Fig.4a).Individuals in the excellent growth category (PCE>SD) in both treatments showed high NUE, and were grouped into group A, which included 26P.deltoidesgenotypes (Fig.4a and b).However, there were 24 individuals with low evaluation values (PCE<SD) in both treatments, indicating that their NUE was low and named group C (Fig.4a and b).
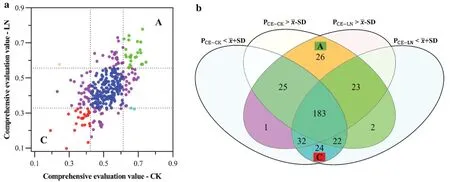
Fig.4 Comprehensive evaluation, a classification and b statistics of Populus deltoides genotypes grown under normal N supply (CK) and low N supply (LN); PCE-CK, comprehensive evaluation value under CK treatment; P CE-LN, comprehensive evaluation value under LN treatment; , the mean value of comprehensive evaluation values; SD, the standard deviation of comprehensive evaluation values.Green symbols, PCE-CK > + SD and PCE-LN > + SD; blue symbols, -SD < PCE-CK < + SD and -SD < PCE-LN < + SD;red symbols, PCE-CK < -SD and PCE-LN < -SD; orange symbols, PCE-CK < -SD and PCE-LN > + SD; light blue symbols, PCE-CK > + SD and PCE-LN < -SD; purple symbols, PCE-CK < -SD and -SD < PCE-LN < + SD, PCE-CK > + SD and -SD < PCE-LN < + SD, -SD < PCE-CK < + SD and PCE-LN < -SD, -SD < PCE-CK < + SD and PCE-LN > + SD.A, N-efficient genotypes; C, N-inefficient genotypes
Comparative analysis of plants with different nitrogen use efficiencies
Based on the comprehensive evaluation, two groups with different NUEs were selected: group A (N-efficient genotypes) and C (N-inefficient genotypes).The scores of the four principal components of group A were higher than those of group C, irrespective of the treatment (Fig.5a and b).Moreover, compared with other principal components, the difference in leaf character (PC1) between the two groups was the largest.The PC1 score was the highest among the four principal components of group A in either treatment, whereas the opposite was true for group C.
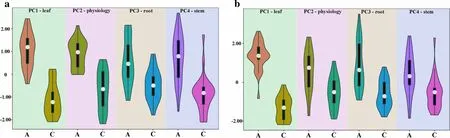
Fig.5 Score of principal components of N-efficient (group A) and N-inefficient (group C) Populus deltoides genotypes under a normal N supply and b low N supply; PC1-leaf, PC2-physiology, PC3-root and PC4-stem represent the principal components of leaf character, physiological index, root character and stem character, respectively
Verification of analysis results
The index parameters of sixP.deltoidesgenotypes were measured under three N-level treatments (LN, CK and HN).These included H, GD, RFW, RDW, LFW, LDW, LA, LL, LW, Chl a, Chl b, Pn, Tr, SFW (stem fresh weight), SDW (stem dry weight), PFW (petiole fresh weight) and PDW (petiole dry weight).The same comprehensive evaluation analysis was used to evaluate the plants, and the results show that in LN, control, HN treatments, the comprehensive evaluation values of the three genotypes selected from group A were higher than those of the three from group C (Fig.6).

Fig.6 Comprehensive evaluation value of Populus deltoides genotypes under low nitrogen (LN2), normal nitrogen levels (CK2) and high nitrogen (HN2) in the verification test.A-1, A-2 and A-3 represent the three N-efficient genotypes; C-1, C-2 and C-3 the three N-inefficient genotypes
Discussion
Effects of low N stress on plant growth
Since N is an essential element for plant growth and development; growth will be significantly inhibited under conditions of low N availability (Kavoosi et al.2014; Xu et al.2017; Li et al.2018).Lu et al.(2019) studied the wood properties ofPopulusduring acclimation to low N (50 μM NH4NO3).They found that the width and number of cell layers in thexylem decreased, and wood fiber content was reduced.It has also been shown that N defciiency affected the accumulation of N, phosphorus and potassium in roots, leaves and stems ofPistacia chinensisBunge seedlings.More nutrients were transported to the root system to promote the growth of fine roots to reduce the negative effects of N deficiency (Song et al.2019).In addition, when subjected to N deficiency (0.25 mM NH4NO3), root biomass ofPopulus cathayanaRehd.increased and stem and leaf biomass decreased.This suggests that, with the change of N levels in the environment, the internal resource allocation by the plant changed adaptively (Luo et al.2019).In addition, chlorophyll concentrations, leaf area, net photosynthetic rates and enzyme activities have been shown to be less under low N conditions, which eventually leads to reductions in biomass (Li et al.2012; Luo et al.2015, 2019; Lu et al.2019; Su et al.2019).
In this study, the growth of 338P.deltoidesgenotypes was evaluated under a normal N supply and low N stress.Compared with the CK treatment, chlorophyll levels of the leaves and the growth of roots, stem, and leaf tissues were significantly lower in LN treatment (p< 0.01; Fig.2).As N levels decreased, the correlation between the trait indicators changed.The negative correlation between the leaf morphological parameters (area, width, length) and chlorophyll content was weaker in the LN treatment (Fig.3).This indicated that the extent of the inhibition caused by N deficiency differed for leaf development and for chlorophyll synthesis.Furthermore, the positive correlation between the root growth parameters (root fresh weight, RFW and root dry weight, RDW) and the leaf parameters (leaf fresh weight, LFW, leaf dry weight, LDW, area, width and length) was stronger under LN conditions (Fig.3).This indicates that under N stress, leaf development was more dependent on the status of the root system to obtain mineral elements and other substances.Simultaneously, the development of the root system required more nutrients provided by photosynthesis.
Contrary to previous studies, low N stress did not promote root growth in this study but significantly inhibited root growth.Comparing the results of different studies, it was found that the growth and development of roots under low N stress was related to treatment time.When the treatment time is relatively short (3-7 weeks), the length, surface area and biomass of the roots, especially of fine roots, are significantly stimulated by N starvation (Li et al.2012; Luo et al.2015, 2019; Meng et al.2018).However, with an increase of stress time (5-6 months), root growth is significantly inhibited, although root to shoot ratios increases (Song et al.2019).The root system absorbs the necessary nutrients (mineral elements, organic matter and water) for plant growth and transports them to the leaves through the xylem.In the leaves, nutrients are transformed into sugars, proteins and other compounds via metabolic processes such as photosynthesis and transpiration.These compounds are then transported to various organs (roots, stems and leaves) to promote growth and development (Nunes-Nesi et al.2010; Xu et al.2012).When under low N stress, roots react to the deficiency, leading to increased transport to the roots of compounds synthesized in the leaves.This promoted the growth of fine roots and thus the absorption of more nutrients to be transported to the leaves.With the prolongation of stress time, compounds produced by leaves could no longer meet the requirements for root growth and growth of the root system would eventually be inhibited.
Evaluation of plant nitrogen use efficiency
Different genotypes of the same species show contrasting adaptabilities to growing conditions, and different tolerances to biotic and abiotic stresses (Guo and Zhang 2010; Liu et al.2015; Zhao et al.2016a, b; Ferreira et al.2018; Ghezehei et al.2019; Zhang et al.2020).Kalcsits and Guy (2016) studied the variability of nitrogen uptake, assimilation and allocation traits of 25Populus balsamiferaL.genotypes from five climatically different provenances.There was extensive genotypic variation in N isotope composition, and a high degree of genotypic variation in N use traits was identified at both the provenance and genotypic level.In this study, the growth performance of a naturalP.deltoidespopulation (338 genotypes) under low and normal N conditions was evaluated using PCA combined with a fuzzy mathematics membership function.According to the evaluation results, all the genotypes could be divided into nine categories (Fig.5), indicating a wide genotypic diversity in NUE and N tolerance.Due to the existence of this genotypic diversity, different genotypes could be selected specifically to study the differences in N absorption, transportation, assimilation and utilization to explain the phenotypic and physiological differences.
At the same time, among the evaluation models established for the two N treatments in this study, the response by leaf traits accounted for the largest proportion of the variability.This was followed by chlorophyll content and the activity of key enzymes such as glutamine synthetase.The contribution of stem growth, which was lower than root growth, was the smallest.These results suggest that changes in leaf characteristics of size, color and biomass could be used as a key factor to evaluate plant nitrogen use efficiencies of plants.Previous studies have also found that leafrelated traits (morphological, physiological and nutritional status) were sensitive to changes in the growth environment (Marron et al.2005; Pérezharguindeguy et al.2013; Tsujii et al.2017; Mao et al.2018; Wang et al.2018a, b; Jiang et al.2019).
Morphological and physiological differences among genotypes with different nitrogen use efficiencies
In previous studies, genotypes with different NUE were used to analyzed characteristics of N utilization and the phenotypic and physiological adaptability of plants to low N stress (Shen et al.2013; Quan et al.2016; Wang et al.2016; Zhu et al.2017).Plant growth was inhibited under low N conditions, and compared with plants with low NUE, aboveground biomass, leaf area and enzyme activities related to N absorption, transportation and assimilation were higher in plants with high NUE.Furthermore, nutritional status (carbon and N) were better than plants with low NUE (Li et al.2012; Luo et al.2013; Meng et al.2018).In this study, the growth of two groups of plants with different NUE were analyzed under different N levels.Growth of N-efficient plants (especially the leaves) was better than that of N-inefficient plants (Fig.6).Maintaining high growth and physiological activities under stress indicates a strong adaptability to adversity (Zhao et al.2016a, b; Liu et al.2020).In addition, a verification experiment was carried out for genotypes with different NUE, and measurement indexes (photosynthesis, transpiration, stem biomass and petiole biomass) and an excess N treatment were added.The results show that the previous evaluation results were reliable.
It has been shown that gene expression of plants changes during N starvation.There are some differences in gene expression between N-efficient and N-inefficient genotypes (Wang et al.2016; Li et al.2020).In the present study, the nitrogen use efficiency of 338P.deltoidesgenotypes had been preliminarily evaluated, and the adaptability to N stress had also been analyzed.As a result, genotypes with different NUE were selected to study the molecular mechanism of different N use strategies, which is of great significance for breeding poplar varieties for high NUE.
Conclusions
The growth (roots, stems and leaves) and chlorophyll synthesis of 338P.deltoidesgenotypes were significantly inhibited under N starvation.The correlation between the traits, especially between root and leaf traits, changed under low N conditions.This indicated that nutrient transport and distribution changed to adapt and to reduce the negative effects of a low N environment.In addition, based on the evaluation results obtained under low and normal N conditions, the genotypes were divided into nine categories.This indicated a wide genotypic diversity in NUE and N tolerance of the population.Under conditions of starvation or normal N levels, the growth of N-efficient genotypes (especially the growth of leaves) was better than the growth of N-inefficent genotypes.Growth status of the leaves (area, biomass and chlorophyll content) was a good indicator of NUE and could be used as the basis for selecting genotypes.Moreover, N utilization characteristics of the plants were stable and the evaluation results reliable.Therefore, based on the results of this study, plants with different NUE should be screened for further study to clarify the different N metabolism strategies inP.deltoides.This could provide a basis for screening and cultivating plants with high NUE.
AcknowledgementsThe authors would like to thank financial support this research and the State Key Laboratory of Tree Genetics and Breeding, Research Institute of Forestry, Chinese Academy of Forestry for the instrument support.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/ licen ses/ by/4.0/.
杂志排行
Journal of Forestry Research的其它文章
- A novel NIRS modelling method with OPLS-SPA and MIX-PLS for timber evaluation
- The dissemination of relevant information on wildlife utilization and its connection with the illegal trade in wildlife
- Endangered lowland oak forest steppe remnants keep unique bird species richness in Central Hungary
- The distribution patterns and temporal dynamics of carabid beetles (Coleoptera: Carabidae) in the forests of Jiaohe, Jilin Province, China
- The disease resistance potential of Trichoderma asperellum T-Pa2 isolated from Phellodendron amurense rhizosphere soil
- Genotype-environment interaction in Cordia trichotoma (Vell.) Arráb.Ex Steud.progenies in two different soil conditions
