Ultrathin Nitrogenated Carbon Nanosheets with Single-Atom Nickel as an Efficient Catalyst for Electrochemical CO2 Reduction
2022-02-26XiaoxiongHuangYingjieMaLinjieZhi
Xiaoxiong Huang,Yingjie Ma,Linjie Zhi,*
1 CAS Key Laboratory of Nanosystem and Hierarchical Fabrication,CAS Center for Excellence in Nanoscience,National Center for Nanoscience and Technology,Beijing 100190,China.
2 University of Chinese Academy of Sciences,Beijing 100049,China.
Abstract:The gradual increase of CO2 concentration in the atmosphere is believed to have a profound impact on the global climate and environment.To address this issue,strategies toward effective CO2 conversion have been developed.As one of the most available strategies,the CO2 electrochemical reduction approach is particularly attractive because the required energy can be supplied from renewable sources such as solar energy.Electrochemical reduction of CO2 to chemical feedstocks offers a promising strategy for mitigating CO2 emissions from anthropogenic activities;however,a critical challenge for this approach is to develop effective electrocatalysts with ultrahigh activity and selectivity.Herein,we report the facile synthesis of a highly efficient and stable atomically isolated nickel catalyst supported by ultrathin nitrogenated carbon nanosheets (Ni-N-C)for CO2 reduction through pyrolysis of Ni-doped metal-organic frameworks (MOFs)and dicyandiamide (DCDA).MOFs are crystalline and assembled by metalcontaining nodes and organic linkers,which have a large specific surface area,tunable pore size and porosity,and highly dispersed unsaturated metal centers.Thus,Ni-doped MOFs were chosen as the precursors to endow Ni-N-C with a porous carbon structure and nickel ions.The nitrogen in Ni-N-C came from DCDA,which acts as the active site to anchor nickel ions.Because of the porous structure and numerous nitrogen sites,the Ni content of Ni-N-C was as high as 7.77% (w).There were two types of nickel ion-containing structures,including Ni+-N-C and Ni2+-N-C.The structure transformation of the Ni+-N-C species from the initial Ni2+ (Ni-MOF)was achieved by pyrolysis,and the ratio of Ni+ and Ni2+ varied with the pyrolysis temperature.Compared to other Ni-N-C prepared at other temperatures,Ni-N-C-800 contained more Ni+-N-C species that possessed the optimum *CO binding energy and thus boosted the CO desorption and evolution rate,thereby exhibiting higher CO Faradaic efficiency (FE)up to 94.6% at -0.9 V (vs.the reversible hydrogen electrode,RHE)in 0.1 mol·L-1 KHCO3.In addition,it has been found that the rate of CO formation on the Ni-N-C-800 electrode relies on the electrolyte concentration.With the optimal electrolyte concentration,the Ni-N-C-800 electrode achieved a superior Faraday efficiency of > 90% for CO over a wide potential range of -0.77 to -1.07 V (vs.RHE)and displayed a CO FE as high as 97.9% with a current density of 12.6 mA·cm-2 at -0.77 V (vs.RHE)in 0.5 mol·L-1 KHCO3.After testing at -0.77 V for 12 h,the Ni-N-C-800 electrode maintained a CO FE of approximately 95%,indicating superior long-term stability.We believe that this study will contribute to the design and synthesis of highly catalytically active atomically dispersed monovalent metal sites for metal-N-C catalysts.
Key Words:Single-atom nickel;Nitrogenated two-dimensional carbon matrix;Ni-N-C catalyst;Pyrolysis;CO2 reduction;Electrocatalysis;Carbon monoxide
1 Introduction
Excessive carbon dioxide (CO2)emissions from the utilization of traditional fossil energy sources have raised public awareness on numerous environment-related issues,such as global warming,ocean acidification,and the extinction of species,etc.1,2.As clean and renewable energy technologies,electrochemically converting CO2into valuable chemical products (ranging from C1 products including CO3and formic acid or formate4to multicarbon (C2+)products such as ethylene5and ethanol6)represents a viable alternative to help close the anthropogenic carbon cycle and convert intermittent electricity from renewable energy sources (e.g.,solar energy,wind and tidal power)to chemical energy7,8.However,due to the intrinsic inertness of CO2molecules,the CO2electroreduction reaction(CO2RR)is an uphill process that requires overcoming a high energy barrier and together with the competition of hydrogen evolution reaction (HER)9,10.Thus,to boost CO2RR,developing novel electrocatalysts and exploring their working mechanisms are crucial and yet challenging.
Since the groundbreaking work by Horiet al.in the 1980s,Cu11,Au12,Ag13,Sn14and Pb15among others,have been widely investigated for electrocatalysis of CO2reduction.Noble metals,such as Au and Ag,are catalysts for selective reduction of CO2to CO,while Sn and Pb are for HCOOH/HCOO-production.Among these metals,Cu and Cu-based catalysts16-18enable CO2to convert into hydrocarbons and oxygenates,such as ethylene (C2H4),ethanol (C2H5OH).Nevertheless,the drawbacks of prohibitive cost,limited reserves and low catalytic activity hinder their large-scale implementation.Additionally,it has also been reported that the energy efficiencies of C1products should be higher than that of C2+products because of its mechanistic simplicity19.In particular,the direct generation of CO from CO2is beneficial as CO is an important feedstock for Fischer-Tropsch and alcohol synthesis reactions20,21.Hence,a superior catalyst with high activity and selectivity toward electrochemical reduction CO2to CO is much desired.To date,a kind of promising efficient catalysts for CO2-to-CO conversion are single-atom catalysts (SACs)with atomically dispersed active metal species on support22-24.Benefiting from the maximum atom efficiency and tunable well-defined metal active centers,SACs show outstanding catalytic performance and unprecedented selectivity,particularly in the electroreduction of CO2-to-CO.A general consensus on the active site of SACs for CO2is the atomically dispersed metal center with unsaturated coordination environment25-27,especially the metal-Nx-C species.For instance,Fe3+ions anchored by pyrrolic nitrogen atoms have been reported to be efficient for selective electrocatalytic CO2RR to CO28.In addition,Fe-N5-C sites has been demonstrated to exhibit higher CO2RR (i.e.CO2-to-CO)efficiency than Fe-N4-C sites29.Very recently,a well-defined active sites of nickel phthalocyanine molecules (Ni-N4-C)supported on carbon nanotubes has been reported to exhibit excellent catalytic performance for CO2RR30.In contrast,it is reported that monovalent Ni(I)atomic center with ad9electronic configuration also exhibits higher CO2-to-CO selectivity than Ni-N4-C sites31.Although several studies have been expended in exploring the efficient catalysts with M-Nx-C active sites towards CO2-to-CO and significant improvement has been achieved,developing catalysts possessing M-Nx-C active sites with ultrahigh activity for CO2is still demanded to meet the requirement of practical applications.
Herein,we report the facile synthesis of an ultrathin nitrogenated carbon nanosheets with single-atom nickel dopants(Ni-N-C),as a robust electrocatalyst for CO2-to-CO conversion,by pyrolysis of Ni-MOF and DCDA.It is found that the formation of Ni2+(or Ni+)-N-C sites from initial Ni2+(Ni-MOF)depends on the pyrolysis temperature.The physical,chemical and surface properties of these catalysts prepared under different pyrolysis temperatures were explored to illustrate structureproperty correlations of these Ni-N-C catalysts by several techniques,including transmission and scanning electron microscopy (TEM and SEM),physisorption (the BET method),X-ray diffraction (XRD),X-ray photoelectron spectroscopy(XPS),and Raman spectroscopy.Over a wide potential range of-0.77 to -1.07 (vs.RHE),the Ni-N-C-800 catalyst achieves a maximum Faraday efficiency of > 90% for CO with high current density.This work should benefit designing high-efficiency electrocatalytic composites with M-Nx-C active sites for CO2conversion.
2 Experimental
2.1 Chemicals
All chemicals including Nickel (II)nitrate hexahydrate(Ni(NO3)2·6H2O), dicyandiamide (DCDA, 99%),pbenzenedicarboxylic acid (PTA,99%),N,N-dimethylformamide(DMF)and NaOH were purchased from Shanghai Sinopharm Chemical Reagent Co.KHCO3(99.7%)and Nafion (R)perfluorinated resin solution (~5% in a mixture of lower aliphatic alcohols and water)were purchased from Sigma-Aldrich and used without further purification.All aqueous solutions were freshly prepared with high purity water (18 MΏ·cm).
2.2 Materials synthesis
2.2.1 Synthesis of Ni-MOF
Ni(NO3)2·6H2O (0.332 g)and PTA (0.192 g)were completely dissolved in DMF (60 mL).Subsequently,NaOH aqueous solution (6 mL,0.2 mol·L-1)was dropped into the solution and the mixture was then continuously sonicated for 1 h.After that,the mixture was added into a 100 mL autoclave and kept at 100 °C for 10 h.After cooling down to room temperature,the resulting precipitate was separated by filtration,washed with DMF and ethanol in sequence,and then dried at 40 °C in the vacuum oven,giving Ni-MOF.
2.2.2 Synthesis of Ni-N-C-x catalysts
Ni-MOF (10 mg)was mixed with DCDA (100 mg)by grinding in a mortar and then carbonized at different temperatures (700,800,900 and 1000 °C)for 2 h under Ar atmosphere at a heating rate of 5 °C·min-1.After cooling down to room temperature,these materials were leached in 3 mol·L-1H2SO4for 24 h to remove metal particles and unstable species.Finally,four kinds of Ni-N-C-x(xis the pyrolysis temperature,°C)catalysts were obtained as black powders,which were named as Ni-N-C-700,Ni-N-C-800,Ni-N-C-900 and Ni-N-C-1000,respectively.
For comparison,Ni-MOF (10 mg)was annealed without DCDA under the same conditions (800 °C for 2 h under Ar atmosphere at a heating rate of 5 °C·min-1)and then leached with 3 mol·L-1H2SO4for 24 h to remove metal particles and unstable species,denoted as Ni-C-800.PTA (10 mg)and dicyandiamide(100 mg)were mixed by grinding and annealed under the same conditions,denoted as N-C-800.
2.3 Characterization
Powder XRD measurements were recorded with a Rigaku D/MAX-TTRIII (CBO)X-ray power diffractometer with CuKαradiation (λ= 0.15418 nm)(Japan).Scanning electron microscopy (SEM)was conducted on a Hitachi SU8220 field emission scanning electron microscope operated at 10 kV(Japan).The detailed microstructure of samples was analyzed by a transmission electron microscope (TEM,FEI Tecnai G2F20 U-TWIN,USA).Raman spectra were collected using a Renishaw inVia Raman microscope with a laser wavelength of 514 nm (UK).The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM)characterization was performed on a JEOL JEM-ARM 300F TEM operating at 300 kV (Japan).XPS was performed by a Thermo Fisher Scientific ESCALAB 250Xi using an AlKα(1486.6 eV)X-ray source (UK).Inductively coupled plasmaatomic emission spectrometry (ICP-AES)was performed with an Agilent 725 (USA).The N2adsorption-desorption isotherms of catalysts were recorded at 77 K on a Micrometritics TriStar II 3020 analyzer (USA),and the specific surface area and pore size distribution were determined by the Brunauer-Emmett-Teller(BET)method and Barrett-Joyner-Halenda (BJH)model,respectively.
2.4 Electrocatalytic measurements
2.4.1 Electrode preparation
5 mg catalyst was mixed with 25 μL of Nafion solution and 975 μL of ethanol.The mixture was then placed in an ultrasonic bath for at least 30 min to achieve a homogeneous ink.50 μL of the catalyst ink was pipetted onto a carbon paper electrode (0.5 cm2).The loading mass of catalysts was 0.5 mg·cm-2.
2.4.2 CO2 electrolysis
All the electrochemical measurements were carried out in a home-made gas-tight two compartment electrochemical cell with a proton exchange membrane (Nafion 117,Dupont)as the separator,equipped with Ag/AgCl reference electrode and platinum counter electrode.Each compartment contained 100 mL electrolyte (0.1 mol·L-1KHCO3aqueous solution).Before measurement,the electrolyte was pre-saturated with CO2(99.999%,the pH value of the saturated solution was measured to be 6.8)by bubbling for 30 min.During measurement,CO2was continuously bubbled into the electrolyte at a flow rate of 20 mL·min-1.The electrochemical tests were performed in a CHI660D electrochemical workstation (Shanghai Chenhua Instrument Co.,China)at room temperature.All of the applied potentials were recorded against an Ag/AgCl (saturated KCl)reference electrode and then converted to those versus reversible hydrogen electrode (RHE)usingE(vs.RHE)=E(vs.Ag/AgCl)+0.197 V + 0.0591pH.All data are presented withoutiRcompensation.
2.4.3 Product analysis
Gas products of electrocatalysis were analyzed by an online gas chromatograph (Shimadzu GC 2014)equipped with several column,including molecular sieves C13X,Al2O3column (50 m,0.53 mm,10 μm),Rt-Q-BOND PLOT column (30 m,0.32 mm ID,10 μm),and two flame ionization detectors (FIDs)as well as a thermal conductivity detector (TCD).A GC run was initiated every 15 min.High purity Ar (99.999%)was used as the carrier gas.Liquid product was quantified using1H NMR (Bruker Advance 400 spectrometer,400 MHz)viawater suppression using a prestarvation method.Dimethyl sulfoxide and phenol were chosen as the reference for quantitative1H NMR analysis.The gaseous products were sampled and analyzed on line every 15 min during the reaction,and the averaged result was used for discussion.The liquid products were collected and analyzed after the operation for 1 h.
In present work,the Faradaic efficiencies (FE)of gas products were calculated from the concentration determined by GC using the following equitation:
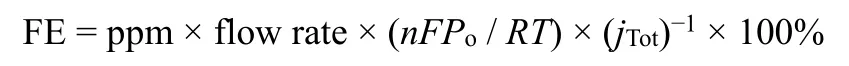
where ppm (1 ppm = 1 × 10-6(volume fraction))is the concentration of gas (CO or H2etc.)determined by GC,nis the electron transfer number,Fis the Faradaic constant,Pois the pressure,T= 298 K andjTotis the total current density.
The FE of liquid products were calculated as follows:FE = (n×C×V×e×NA× 10-3)× 100%/Q
wherenis the electron transfer number,C(mol·L-1)is the concentration of liquid products in the electrolyte,V(mL)is the volume of the electrolyte,Fwas the Faradaic constant,e(C mol-1)= 1.6 × 10-19,NA(Avogadro Number)= 6.02 × 1023andQ(C)was the total amount of charge passed through the system.
3 Results and discussion
3.1 Synthesis and characterization of Ni-N-C-x
The Ni-N-C-xcatalysts were prepared by pyrolyzing a mixture of a nitrogen-free Ni-MOF ([Ni3(OH)2(C8H4O4)2(H2O)4]·2H2O)(Fig.S1,Supporting Information)32and DCDA at different temperatures under argon atmosphere followed by acid etching(Scheme 1).Ni-MOF was chosen as the precursor to provide Ni and carbon for the Ni-N-C-xsingle atom catalysts as MOFs are composed of nickel ions and organic linkers.While,the previous M-Nx-C single atom catalysts prepared by pyrolysis of MOFs that suffer from low metal loading because of the limited content of N in MOFs33.In contrast,in this work,DCDA was used as nitrogen source to endow Ni-N-C-xcatalysts with high nitrogen content,which is crucial to fabricate the highly stable single atomic metal anchored by nitrogen-doped carbons.During the heat treatment process,DCDA can react with the carboxylic acid groups in PTA linkers and form an ultrathin two-dimensional(2D)sheet structure with smooth surfaces (Fig.1a,b,the HRTEM images of Ni-N-C-800).The high-angle annular dark field scanning transmission electron microscope (HAADFSTEM)image forNi-N-C-800 (Fig.1c)shows isolated bright spots corresponding to Ni atoms are homogeneously distributed across the entire carbon framework,demonstrating formation of the single-atom Ni in Ni-N-C-800.Energy-dispersive X-ray spectroscopy (EDS)elemental mappings (Fig.1d)also confirms the homogeneous distribution of Ni atoms across N-doped C substrates.The ultrathin structure of Ni-N-C-800 is beneficial for exposing more active sites for catalytic reactions.
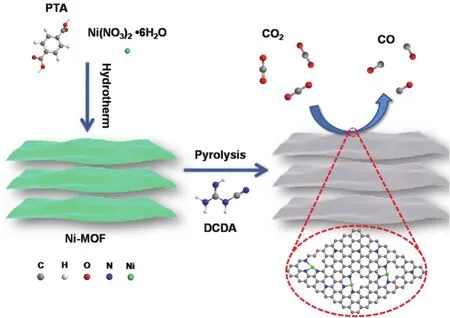
Scheme 1 The fabrication of ultrathin Ni-N-C catalysts for electrocatalytic CO2 reduction.

Fig.1 HRTEM (a,b),HAADF-STEM (c)and EDS mappings (d)images of Ni-N-C-800.
The structures of Ni-N-C-xcatalysts were fully characterized by XRD.All the Ni-N-C-xsamples only possess broad diffraction peaks owing to graphitic carbon and no characteristic peaks of Ni nanoparticles can be observed,indicating that there is only atomic nickel in agreement with HRTEM results (Fig.2a).The nitrogen doped carbon structures of all these Ni-N-C-xcatalysts were analyzed by the Raman spectroscopy.As shown in Fig.2b,theGband (around 1580 cm-1)becomes strong and sharp when increasing the pyrolysis temperature,while theDband (around 1360 cm-1)become weaker gradually,illustrating a higher graphitization degree.The degree of structural disorder of Ni-N-C-xcatalysts were estimated by the intensity ratios ofD-band toG-band (ID/IG).As increasing pyrolysis temperature,the ratio ofID/IG(0.99,0.98,0.97,0.97 for 700,800,900 and 1000)gradually decreases,suggesting that high annealing temperature gives rise to high graphitization degree.Thus,higher pyrolysis temperature gives rise to Ni-N-C-xsamples with higher degree of graphitization,although all the Ni-N-C-xcatalysts (700,800,900,1000,N-C-800 and Ni-C-800)are ultrathin sheets (Fig.S2,Supporting Information).
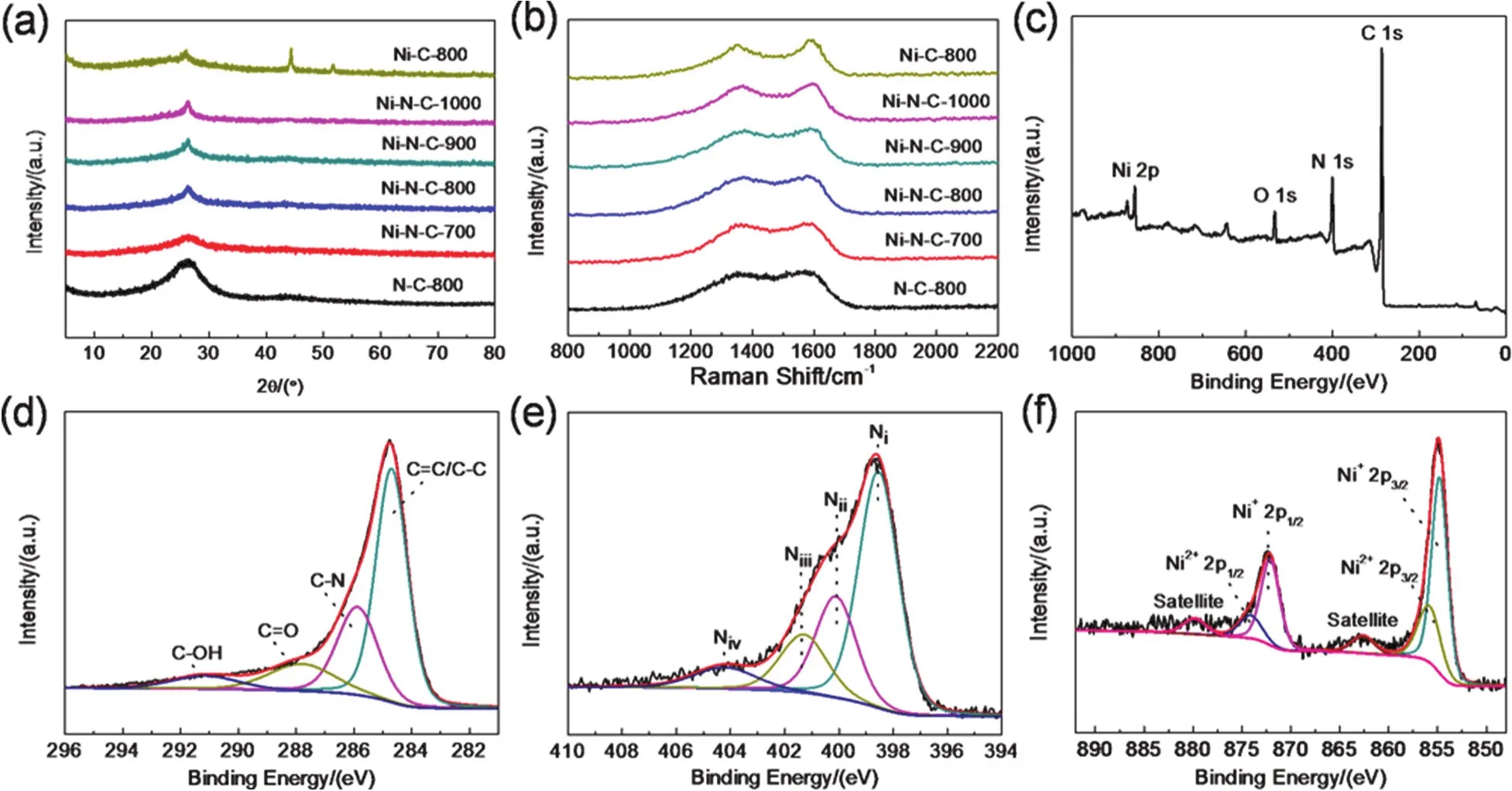
Fig.2 XRD patterns (a)and Raman spectra (b)of N-C-800,Ni-N-C-700,Ni-N-C-800,Ni-N-C-900,Ni-N-C-1000 and Ni-C-800;XPS survey spectrum (c)and high-resolution XPS spectra of C 1s (d),N 1s (e)and Ni 2p (f)of Ni-N-C-800.
To further investigate the structure features of these Ni-N-C-xcatalysts,their surface and pore-size characteristics were measured by high-resolution nitrogen adsorption/desorption measurements at 77 K (Fig.S3,Table S1,Supporting Information).All the Ni-N-C-x catalysts feature a representative type-IV isotherm,suggesting mesoporous pore structures.It is found that the specific surface area and pore volume of these Ni-N-C-xcatalysts decrease as the pyrolysis temperature increases.It is supposed that higher temperatures,such as 800,900 or 1000 °C,would enable Ni-N-C-xformed under low temperatures to undergo further crosslinking and eliminate some pores,consequently decreasing the specific area and pore volume.While,Ni-N-C-700 and Ni-N-C-800 possess the similar the specific surface area (224 and 201 m2·g-1)and largest pore volume (0.29 and 0.29 cm3·g-1),which are higher than other Ni-N-C-xcatalysts.The superior specific surface area and unique mesoporous structure would facilitate adsorption of CO2and mass transport to achieve an excellent electro-catalytic performance.
Furthermore,the chemical environment of Ni-N-C-xactive sites was analyzed in detail by X-ray photoelectron spectroscopy(XPS).As shown in Fig.2c,the XPS spectrum of Ni-N-C-800 confirms that there are C,N,O and Ni in Ni-N-C-800.The highresolution C 1sanalysis shows that there are four main carbon species in Ni-N-C-80034,includingsp2C (C=C/C―C)at 284.4 eV,sp3C (C―O and C―N)at 285.7 eV,―C=O at 287.9 eV and ―COOH at 290.1 eV (Figs.2d and S4,Supporting Information).The percentage of these carbon species in Ni-N-C-xsamples is determined by pyrolysis temperature.As the pyrolysis temperature increases from 700 to 1000 °C,the content of C=C/C―C group significant increases from 42.9% (700 °C)to 57.4% (900 °C),and then decreases to 46.3% (1000 °C),showing that the degree of graphitization can be turned by pyrolysis temperature and Ni-N-C-800 possesses higher degree of graphitization compared to Ni-N-C-700,which might endow Ni-N-C-800 with higher conductivity and thus benefit its electro-catalytic performance in CO2RR (Fig.S5a,Supporting Information).The high-resolution N 1sanalysis displays that all the four Ni-N-C-xcatalysts (x= 700,800,900 and 1000)contain four kinds of nitrogen35,as the corresponding peaks at 398.7,399.4,400.7 and 402.0 eV can be observed in their N 1shigh resolution XPS spectra,respectively (Figs.2e,S5b and S6,Supporting Information).Besides,relative content of N decreased along with increasing the pyrolysis temperature (700-1000 °C).While Ni-N-C-800 contains considerable pyrrolic and pyridinic N (total content > 10%),which can anchor nickel atoms.
The valence state and content of Ni in all these Ni-N-C-xcatalysts (700,800,900,and 1000)were also analyzed by XPS.The results of XPS show that all the Ni-N-C-xcatalysts contain Ni+(~854.7 eV)36,37and Ni2+(~855.8 eV)38,indicating multivalent Ni in these Ni-N-C-xsamples (Figs.S5c and S7,Supporting Information).Inductively coupled plasma-atomic emission spectrometry (ICP-AES)analysis shows that the weight fractions of Ni in Ni-N-C-700,Ni-N-C-800,Ni-N-C-900 and Ni-N-C-1000 are 9.32%,7.77%,7.32% and 0.44% (w),respectively (Table S2,Supporting Information).Apparently,the weight fraction of Ni decreases gradually with the increase of pyrolysis temperature.We propose that high temperature,especially 1000 °C,might damage the nitrogen-carbon structures(N-C)that can anchor Ni atoms.As a result,under 1000 °C,most of the nitrogen-carbon structures (N-C)are destroyed and consequently most Ni atoms from the Ni-MOF precursors cannot be anchored and stabilized,which thus should be removed in the following washing procedure.Therefore,the weight fraction of Ni in Ni-N-C-1000 decreases to 0.44% (w).Moreover,Ni+dominates in Ni-N-C-800 (Fig.2f).The contents of Ni+in Ni-N-C-xcatalysts were evaluated by their relative peak areas in high resolution XPS.The relative peak area of Ni+increases with the increase of pyrolysis temperature from 700 to 800 °C,while decreases from 800 to 1000 °C (Fig.S5c,Supporting Information),showing a volcano-like behavior.This result reveals that Ni-N-C-800 has the highest percentage of Ni+(about 43%),indicating that almost half of Ni2+was reduced to Ni+during pyrolysis at 800 °C.These results further indicated that Ni-N-C-800 possesses appropriate N contents,forming abundant Ni+active sites and then regulating the surface electronic structures of Ni species.Notably,monovalent Ni+atomic centers have been identified as the efficient catalytically active sites for the reduction of CO2to CO because they can lower the barrier in CO2RR through forming the *COOH intermediate while keeping the CO coverage low39.
3.2 Electrocatalytic performance for the reduction of CO2
To investigate the electrocatalytic activities of Ni-N-C-xcatalysts (700,800,900 and 1000)for CO2reduction,CO2RR was performed in CO2-saturated 0.1mol·L-1KHCO3solutionviaa gas-tight H-type cell.The products of CO2RR were analyzed by gas chromatography (GC)and1H nuclear magnetic resonance(NMR),showing that gaseous products of H2and CO were formed but no liquid products during CO2RR catalyzed by these Ni-N-C-xcatalysts.As revealed by the linear sweep voltammetry(LSV)curves,Ni-N-C-800 exhibits significantly higher current response in CO2stream than other Ni-N-C-x(700,900,1000,NC-800 and Ni-C-800)catalysts,indicating the significantly superior catalytic capability toward the reduction of CO2to CO(Fig.3a).It is found that different Ni-N-C-xcatalysts (700,800,900 and 1000)give rise to different FE and current density of CO at -0.6 to -1.1 V (Fig.3b,c),revealing that the pyrolysis temperature is crucial to the electrocatalytic activities of Ni-NC-xcatalysts.The current density of CO is found as follows: Ni-N-C-800 > Ni-N-C-900 > Ni-N-C-1000 > Ni-N-700 (Fig.3c),which correlates with the trend of the overall relative peak area of Ni+(Fig.S6c),suggesting that the percentage of Ni+in Ni-NC-xsamples determines the current density of CO in CO2RR.Particularly,Ni-N-C-800 shows the highest FE for CO production (94.6%)at -0.9 V with an extraordinary current density of 10.5 mA·cm-2,which is 3-fold,1.2-fold and 1.5-fold higher than Ni-N-C-700,Ni-N-C-900 and Ni-N-C-1000,respectively.Additionally,Ni-N-C-800 shows the smallest Tafel slope (136 mV·dec-1)among all the Ni-N-C-xcatalysts,suggesting that Ni-N-C-800 is an excellent CO2RR catalyst with superior kinetics.To identify the role of Ni and N in carbon materials,N-C-800 and Ni-C-800 were prepared and used as the reference.As shown in Fig.3b,c,both N-C-800 and Ni-C-800 show negligible current response and CO FE compared Ni-N-C-xcatalysts,consequently highlighting the critical role of Ni-N-C sites in CO2RR catalytic activity.

Fig.3 Electrocatalytic performances of the CO2RR over the catalysts.LSV curves in the CO2-saturated 0.1 mol·L-1 KHCO3 (a);Applied potential dependence of CO Faradaic efficiency (b);Geometrical current density (c);Tafel plots (d).
Electrolyte concentration also affects the electro-catalytic performance of Ni-N-C-800.The investigation of FE and current density (insert)of CO at different potentials and different concentrations of KHCO3(0.1,0.3,0.5 and 1 mol·L-1)shows that both FE and current density of CO display a volcano trend(Fig.4a).We observed that the onset potential for current shifted to less negative values as the concentration of KHCO3was increased,indicating the pH value of the electrolyte have a direct impact on the energy barrier of the CO2RR product formation40.The high pH electrolyte is kinetically beneficial to the CO2RR41,which can effectively suppress the HER to achieve higher selectivity.However,the high pH value will inevitably lead to a decrease in the concentration of CO2and an increase in byproducts production (formateetc.)—the high pH would reduce the ability of a temporary hydronium molecule that assists in the first protonation step on the CO reaction pathway42.Therefore,an intermediate concentration of KHCO3(0.5 mol·L-1)gives rise to the optimal FE and current density of CO production.Notably,over a wide potential range of -0.77 to -1.07 V (versusRHE),Ni-N-C-800 exhibits the CO FE of > 90% in 0.5 mol·L-1KHCO3solution.Especially,Ni-N-C-800 presents a FE of 97.9% with a current density of 12.6 mA·cm-2at -0.77 V,which is comparable to state-of-the-art highly efficient Ni-based catalysts reported in literature (Table S3,Supporting Information).Additionally,to illustrate the change in the valence state of Ni after CO2RR,we analyzed Ni-N-C-800 after CO2RR in 0.5 mol·L-1KHCO3,namely Ni-N-C-800-used,by XPS.The XPS analysis shows that the relative peak area of Ni+increased slightly after CO2RR (Fig.S8).In consequence,it can be concluded that some Ni2+ions should be reduced into Ni+ions during CO2RR.
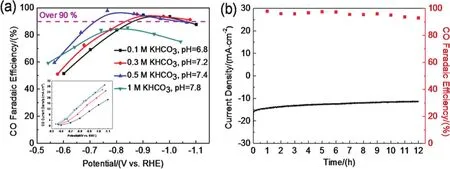
Fig.4 Electrocatalytic performances of the CO2RR at different electrolyte concentrations.CO FE of Ni-N-C-800 and CO partial current density (insert)dependence on applied potentials (a);Durability test of Ni-N-C-800 at a constant potential of -0.8 V vs. RHE in CO2-saturated 0.5 mol·L-1 KHCO3 solution (b).
Finally,the stability of the Ni-N-C-800 electrode for CO2RR was evaluated in 0.5 mol·L-1KHCO3at -0.77 V,as presented in Fig.4b.After 12 h of continuous operation,the current density does not decrease and FE maintains 95%,exhibiting high activity and robust stability during CO2RR (Fig.4b).
4 Conclusions
In summary,atomically isolated nickel catalyst supported by ultrathin nitrogenated carbon nanosheets (Ni-N-C),which enables efficient reduction of CO2to CO,has been prepared by pyrolysis of Ni-doped MOFs and DCDA.Ni-N-C-800 exhibits superior electrocatalytic activity,achieving high CO FE of above 90% over a wide potential range of -0.77 to -1.07 V (vs.RHE),and giving a maximum CO FE of 97.9% with a current density of 12.6 mA·cm-2at -0.77 V in 0.5 mol·L-1KHCO3along with superior long-term stability.The excellent catalytic performance of Ni-N-C-800 is attributed to its high percentage of Ni+-N-C,superior specific area and pore volume as well as sufficient degree of graphitization.The content of Ni in Ni-N-C-800 is characterized as high as7.77% (w).It has been found that the pyrolysis temperature leads to the structural transformation of initial Ni2+(Ni-MOF)into Ni+-N-C,which is confirmed to the catalytic active sites for conversion of CO2to CO.In addition,the electro-catalytic performance of Ni-N-C-xcatalysts can be improved by optimizing the electrolyte concentration.This work should contribute to the electrochemical reduction of CO2and it can be expected that these Ni-N-C-xnanosheets would also be of value for other electro-catalytic reactions,such as oxygen reduction reaction (ORR)and oxygen evolution reaction (OER).
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
