河流水沙运动对微塑料运移过程影响研究进展
2022-02-25王薪杰王一宁刘晟东夏星辉
王薪杰,王一宁,赵 俭,刘晟东,夏星辉,李 阳
河流水沙运动对微塑料运移过程影响研究进展
王薪杰,王一宁,赵 俭,刘晟东,夏星辉,李 阳*
(北京师范大学环境学院,教育部水沙科学重点实验室,水环境模拟国家重点实验室,北京 100875)
对河流中微塑料的分布、微塑料在河流中发生的聚沉、重悬、水平运输和潜流交换等迁移过程进行了归纳总结.由于河流中广泛存在的泥沙会影响微塑料的迁移.本研究剖析了含沙河流中微塑料迁移,包括微塑料与泥沙的聚集和共沉降,沉积物对微塑料的再悬浮和渗透过程影响,探究了微塑料与泥沙的相互作用机制和影响因素.提出未来含沙河流中微塑料的运移研究应重视开发可靠模型预测微塑料迁移和通量,实验室模拟应考虑生物和水体扰动对老化微塑料传输的影响.
微塑料;纳米塑料;河流;迁移;泥沙;沉积物;影响因素
微塑料(MPs)是指尺寸<5mm的一种人工合成的高分子制品[1].在尺寸上至少一个维度上小于<100nm的塑料颗粒称为纳米塑料(NPs)[1-2].通过个人护理品、光电子产品和靶向药物等产品的使用,MPs可以直接排放到天然水体中[3].环境中的塑料也会因生物降解、机械磨损、水解、紫外辐射降解和热降解等作用破碎成MPs和NPs,很容易富集在水生生物体内影响其生长发育[4-5].此外,MPs能够吸附有机污染物(如多环芳烃和有机氯农药等)和重金属(如铅和铬等),以及释放邻苯二甲酸盐、有机锡、双酚A等有毒物质,进而加剧其对生态系统的危害[6-10].
泥沙是河流中重要的组成部分,负载量约为5~ 20000mg/L[11-13].全球气候变化导致大暴雨的发生频率增加,加剧水土流失和沉积物再悬浮,致使河流的高泥沙含量成为一个全球性问题[14].水中的泥沙运动显著影响MPs的聚沉、再悬浮和渗透等重要迁移行为,进而影响环境中MPs的稳定性、流动性和生物可利用性[15-16].MPs与悬浮泥沙发生聚集会导致MPs聚集体密度增加,从而影响MPs在水中的垂直分布及长期迁移[16-17].沉积物-水界面水流的剪切应力和紊流会导致沉积物颗粒的再悬浮,进而引起沉积物中的MPs向上覆水体释放[18].MPs自身特性(组成、形状和大小等)、水体理化性质(pH值、盐度、离子种类和强度等)、泥沙/沉积物性质(粒径分布、组成和有机质含量等)、扰动时间与扰动强度等是影响河流中MPs迁移的关键影响因素[19].因此,准确描述MPs的归趋需要考虑泥沙运动和环境因素耦合作用的影响.目前针对含沙河流中MPs的迁移行为研究仍处于探索阶段.
河流被视为从陆地向海洋生态系统输送MPs的主要途径[20].MPs在河流中的分布和迁移行为,决定了河流系统中MPs的入海通量.全球从河流进入海洋的塑料碎片数量为0.41~4×106t/a[21].一旦MPs进入海洋,将很难进行收集和清理.有效的解决方案是控制MPs在内陆和水体(如河流)中的运输过程.因此,研究河流中MPs浓度的时空变化、迁移转化过程以及驱动其运输的关键作用力已成为国际上环境科学领域的热点问题[9,22],对阐明MPs的环境归趋和生态风险具有重要意义.含沙水体中MPs的聚集、沉降和再悬浮受控于自身理化性质和多种环境因素耦合的影响,这使得MPs的迁移行为复杂多样、难以预测.因此,本文总结了全球范围内河流的上覆水和沉积物中MPs的分布情况,探讨了河流中MPs的迁移转化过程,总结了泥沙影响上覆水体和沉积物中MPs迁移的影响机制,并展望了未来含沙河流中MPs迁移转化的研究方向,为更全面了解MPs在水环境中的分布规律、建立评估方法和控制MPs污染提供了科学支撑.
1 河流中微塑料的分布及赋存
研究表明,MPs在淡水、海洋环境中均广泛分布[20,23].MPs可以通过城市污水处理厂排放、雨水径流、轮胎和道路磨损、生活农业垃圾不合理倾倒进入河流,或经地表径流汇入河流、湖泊、地下水中,并最终进入海洋[22,24].影响河流中微塑料丰度的因素包括人口密度、人类活动程度、水流流速、流量、河流类型、集水区大小、废弃物管理方案和污水处理方法等[9,22].根据模型预测,每年大约有1533t MPs通过多瑙河流入黑海[25].欧盟环境基金会预测每年有20~30t塑料垃圾通过河流汇入北海.在意大利,每年大约有120t塑料垃圾汇入地中海[26].除了汇入海洋之外,MPs也会通过聚集、沉降等运动沉积在河床中,使得MPs在沉积物中积蓄,因此沉积物成为MPs一个重要的“汇”[16-17].
根据表1,实地测量的结果表明在河流上覆水中MPs的浓度范围在0.1~104particles/m3数量级之间,而在沉积物中MPs的浓度范围在10~ 104particles/ kg数量级之间[27-33].值得注意的是,在中国多个地点的河流沉积物中(海河、珠江和长江),发现了浓度达103~104particles/kg的MPs,比国外河流沉积物(葡萄牙Antuã River和加拿大Ottawa River)中的MPs浓度高1个数量级.不同研究报道的河流中MPs浓度存在巨大差异,可相差2~5个数量级[22],这主要是由研究地点、样品选择、采集方法以及样品处理方法之间的差异所导致[34].由于目前没有标准的MPs采样方法,采样深度,采样装置(滤网的孔径)和度量单位等均会影响到河流中MPs浓度的测量结果.因此,目前急需开发统一和标准化的MPs采样与分析方法.
就河流中MPs的分布情况而言,大多数研究发现MPs在沉积物中的浓度一般高于上覆水[27-33].在河流上覆水和沉积物中,最常检出的MPs种类为聚乙烯(PE)、聚丙烯(PP)、聚氯乙烯(PVC)、聚苯乙烯(PS)和聚对苯二甲酸乙二酯(PET)[35].其中,在上覆水中低密度PE(0.91~0.93g/cm3)占主导,而在沉积物中,高密度PE(0.93~0.97g/cm3)占比更高[36].为了进一步探究上覆水与沉积物中MPs赋存的分布关系,同时开展同一地点的上覆水和沉积物中MPs垂直分布的研究至关重要.综上,目前MPs在水环境中的广泛分布,尤其是MPs在河流的上覆水和河床的沉积物中浓度较高.沉积物中的MPs是否可能受到外界作用而重新释放到水环境中,由“汇”转变为“源”,威胁生态安全和人体健康.

表1 河流的上覆水和沉积物中微塑料的分布情况

续表1
注:a为水体表面以下;b为沉积物表面以下;PES,聚酯纤维; PAN, polyacrylonitrile,聚丙烯腈;-,未提及.
2 微塑料在河流中的典型运移行为
常见污染物(有机污染物、重金属和磷氮等营养物质)进入河流后,形成均质溶液,一定时间内会在水相和悬浮颗粒物相达到分配平衡,常用平衡分配系数p和辛醇-水分配系数ow界定其传输[37].而MPs进入河流后,会形成高分散和多相的悬浮液,是热力学不稳定的体系[18].由于MPs在河流中各相间无法处于热力学平衡状态[18],因此MPs传输需使用胶体理论来描述.MPs进入水体后会发生聚集、沉降、水平传输、再悬浮、埋藏和河床交换等过程(图1).这些过程可以循环往复地发生,也可能自发终止[3,24,38].水介质特性以及MPs自身特性会影响MPs在河流中的传输过程[18,39].对于水环境中MPs的聚集行为,首先要考虑MPs自身理化性质(如疏水性、比表面积和表面电荷)、水环境条件(如光照、离子强度、组成和pH值)以及水体中共存颗粒等因素的影响[39-40].而MPs的沉降速率主要受控于MPs与水体间的密度差异[41-42].
2.1 聚集
聚集是河流中MPs的常见传输过程.聚集行为可分为同质聚集(在相同类型颗粒之间发生的聚集)和异质聚集(在不同类型颗粒之间发生的聚集)[24].由于自然水环境中其他悬浮颗粒物的浓度远远高于MPs的浓度,MPs异质聚集发生的概率往往远高于同质聚集发生的概率[39].塑料表面常含有疏水官能团,易吸附藻类和细菌等生物体,在塑料表面形成生物膜或发生微生物定殖[17,43].MPs/NPs与生物相关的聚集已被广泛报道[17,44].此外,有机胶体(海藻酸盐)、黏土矿物(悬浮泥沙、Fe2O3)和纳米颗粒(纳米银)也可与MPs聚集[45-46].和其他工程纳米材料相似,MPs在天然水环境中的聚集行为是由布朗运动、不等速沉降和流体运动引起的[47].由于NPs及天然和人工纳米颗粒具有相似的胶体性质及高扩散系数[18,39-40],布朗运动主导这些小颗粒(£300nm)的聚集[47].小颗粒布朗运动又受颗粒自身性质和环境因素影响.具体而言,小颗粒受到液体分子碰撞后,动量传递与颗粒质量成反比,颗粒越轻,碰撞后的运动速度越大.颗粒大小与运动速度成反比,即小颗粒运动更快[48-49].因此颗粒尺寸和密度越大,布朗运动越不明显,对聚集影响程度会降低.而温度越高,液体分子运动越剧烈、流体的黏度越低,液体内流动阻力越小,均会加剧布朗运动,导致布朗运动更易影响小颗粒的聚集[48-49].

图1 河流中微塑料的典型迁移过程和影响因素
根据Derjaguin-Landau-Verwey-Overbeek (DLVO)理论,颗粒组成、结构、尺寸、表面电性和水环境中离子强度均可影响MPs的聚集速率[39].非DLVO力,包括空间位阻、水合作用、聚合物桥联作用以及磁性作用等也会影响MPs的稳定性[47].关于水环境条件、共存的固体介质以及MPs自身性质(如疏水性、比表面积和表面电荷)等因素对MPs在水环境中聚集行为的影响,已有综述论文进行阐述[19,24,39],本文不再展开讨论.MPs聚集将影响其迁移、降解及其在环境中的持久性和生物有效性[3,39].
2.2 沉降
沉降是水环境中MPs的重要环境行为之一,会影响MPs在水中的垂直分布[50].通过布朗扩散、浮力和重力沉降等作用,MPs在河流中能够迁移到沉积物中[51].对于NPs,布朗运动是其沉降的主要机制[16].浮力和重力沉降则主要控制微米尺寸MPs和团聚体的沉积[47].沉降过程可分为3个阶段:(1)初始阶段,此时聚集过程仍在进行,沉降缓慢;(2)快速沉降阶段;(3)减速沉积阶段,此时大部分聚集体已经沉降完全,上覆水中残留悬浮颗粒的数量较少[52].由于MPs颗粒的物理性质(密度和形状)与泥沙颗粒存在很大差异,需要对传统泥沙沉降(斯托克斯定律)和输移模型进行改进才能应用于MPs沉降输移的模拟,沉降相关方程中MPs的沉降速度和临界剪切应力等关键参数,需要通过合理的理论分析和沉降柱实验观测获得.目前, Waldschlager等[53]基于斯托克斯公式建立了无泥沙的静止淡水环境中球形和颗粒状MPs沉降速度的修正公式(1)~式(5),可较好地预测淡水中MPs的垂直迁移过程.


(3)

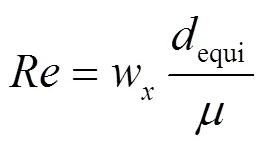
式中:w为沉降速度, m/s;equi为颗粒等效粒径, m;、、分别为单颗粒最长,中间和最短的长度, m;D为无量纲的阻力系数; CSF为Corey形状因子;为无量纲的颗粒雷诺数;为流体的黏度, m2/s;为重力加速度, 9.8N/kg;为流体密度, kg/m3;s为颗粒密度, kg/m3.
此外,也可使用一阶动力学沉降模型对MPs/ NPs的沉降速率进行拟合(式(6))[54].

式中:C为取样时MPs的浓度, mg/L;0为溶液中MPs初始浓度, mg/L;res为某一时间后溶液中MPs的残余浓度, mg/L;s为沉降速率, m/s;为采样点的深度, m;diss为颗粒溶解常数,对MPs/NPs通常为0.
MPs的形状会影响其沉降速率.与相同密度和体积的球形颗粒相比,具有理想光滑表面的球形颗粒比不规则形状的颗粒受到的阻力作用小,因此光滑的颗粒具有较大的下沉速度[55-56].纤维和薄膜形态的MPs颗粒比球状或其他不规则颗粒状的MPs具有更高的浮力和更低的沉降速度[55-56].不同长度但直径相同的纤维状MPs在水中具有近似的沉降速度.当纤维状MPs的直径增加时,沉降速度也会随之增加[53].该现象的原因尚不明确.也有报道称,具有较高暴露面积的纤维和薄膜形态的MPs更易形成生物膜,从而密度增加,沉降更快[57-58].藻类等微生物定殖会导致MPs聚集体密度增加,使MPs以更高的速率沉降.密度为0.89~0.91g/cm3的PP MPs与藻类聚集后密度增加到1.19g/cm3[59].水生生物的活动也会影响MPs在水中的沉降.鱼类和蝌蚪等动物可吞食摄入MPs,使MPs与排泄物或生物碎屑一起迁移到沉积物中[19].电解质浓度对PET MPs、PS NPs的沉降影响较大,沉降速率及沉降程度均随电解质浓度的增大而增强[16,60].二价阳离子比一价阳离子更能够显著促进水环境中PET MPs的沉降[60].
2.3 上浮
由于部分MPs的密度比水更小或者接近水的密度,有些MPs也会在水体中发生上浮[53,61].水与MPs之间的密度差,MPs的粒度和形状均可影响MPs在水中的上浮过程.针对上浮过程的研究多集中在海洋环境,这是由于海洋环境密度更高,更易使MPs发生上浮[62-63].为研究河流中MPs的上浮行为,Waldschläger等[53]使用沉降柱确定了商业MPs在非扰动的蒸馏水中的上升速度.MPs的形状包括球形、碎片和纤维状,成分包括PE、PP、PS、发泡聚苯乙烯(EPS)、PVC、PET和聚酰胺(PA).该研究构建的用于描述颗粒和球形商业MPs的上升速度w(m/s)的公式与公式(1)相同,式中仅D计算方法不同,如公式(7)所示.该研究的到MPs上升速度为0.65cm/s(直径3mm碎片状PE MPs)~31.4cm/s(碎片状EPS MPs).

式中:为MPs颗粒的圆度(0~6,颗粒越圆数值越大),由不同研究人员主观评测,最终取平均值获得.
Waldschlagera等[61]随后使用同样的装置实验和公式,测定并计算了河流中自然风化MPs(EPS, PE, PP)的上升速度为0.18cm/s(PE MPs)~19.85cm/s (EPS MPs).其中碎片、颗粒、泡沫状颗粒的相对平均偏差在50%及以下,但薄膜状MPs平均偏差较高(425%).这是由于薄膜状MPs易发生变形而使上升行为更为复杂.相比较商业MPs而言,河流风化后的MPs上升速度较低,这是由于风化后MPs颗粒表面和裂缝内部可形成生物膜或掺入了悬浮泥沙或藻类,从而降低上升速度[58].此外,风化后颗粒表面粗糙度和表面积较大也导致上升减慢[61].淡水环境中MPs的上升速度与其在海水中相差不大(0.5~5.0mm 碎片MPs和纤维状MPs上升速度分别为(0.9± 0.4)~(1.9±0.6)cm/s和(0.6±0.1)~(0.8±0.2)cm/s)[62].
2.4 推移质移动和重悬
在水流流速较高和湍流作用下(如洪水事件),沉积物中的MPs可发生重悬浮返回到上覆水体,并构成上覆水体中MPs的重要来源[64-65].MPs在沉积层的传输方式随流体动力学条件变化而变化,当水底流速较小时,MPs颗粒主要以滑动或滚动为主,即发生推移质移动;随着水底的流速增大,MPs颗粒将发生跳跃并悬浮.风和水温也是影响MPs再悬浮的重要因素.风驱动的水体振荡混合可以促进沉积物中MPs发生再悬浮[66].降雨导致水体流量增加、水体扰动增强,也利于MPs发生重悬[67].对于沉降在小河和河口地区的MPs颗粒,底流作用对其传输的影响大于气象条件.研究发现,大尺寸的MPs上浮速度较大,因此有些地区沉积物中较小尺寸的MPs含量较多[53].沉积物中MPs也可能发生表面生物膜脱落,密度降低,再次浮到上覆水体[68].
河流水体扰动会造成水体发生紊流扩散以及MPs再悬浮[69-70].沉积物中MPs的丰度与水体流量或流速之间呈负相关[71].弱的水动力扰动有利于MPs沉积[71];当湍流存在时,再悬浮的MPs含量增加[70].雨季排水、潮汐和洪水事件等强水动力扰动均会加剧沉积物中MPs的重悬[70,72].据Hurley等[69]的估算,英格兰河床上的MPs中约70%是通过洪水作用重新进入水体.在潮间带地区,表层沉积物中MPs的含量往往高于深层沉积物[73].造成这种现象的原因为,部分MPs在退潮时沉积在沉积物表面,但在涨潮时重新悬浮,因此MPs无法永久保留在较深的沉积物中[70].由于河口处水体密度和水深增加,此区域的临界切应力和河床切应力更有利于MPs的重悬,MPs运输距离相对较长[38].
2.5 水平运输
水体中部分MPs会随河流水平流动输送到下游,并最终进入海洋[24,74].MPs初始密度可能高于或低于水体(海洋密度为1.02~1.04g/cm3,淡水密度为1.00g/cm3)的密度[30,36].若MPs密度低于水体密度,如PE和PP,则可能在浮力作用的主导下漂浮或悬浮在水中,随水流运输.若MPs密度大于水体,如PMMA、PVC和PET,则一方面会在水体扰动较小的水体中直接沉积到河床,另一方面在水流强度大和流体紊乱的水体中,也可在浮力作用下发生水平运输[75].在静态或非扰动状态下,未团聚的MPs沉降非常缓慢,它们通常发生水平传输或在水体中停留数周至数年.河流中MPs的水平运输受到风、河流形态、水体流速和植被等多种因素的影响[76].
风对水面漂浮MPs的水平输送影响最大,尤其是对聚苯乙烯泡沫EPS MPs[75].漂浮的MPs在受到风的影响后,水平移动速度加快,移动距离更远,更有可能被输送停留在海滩和河岸上或输送到海洋.针对河口处漂浮MPs运输的建模研究表明,波浪和地表径流是影响漂浮MPs空间分布的关键因素[77-78].河流形态会通过影响水体流速或通过弯道拦截来影响MPs的运输.高流速会导致城市河流水体中MPs丰度降低[79].相比较弯曲河道而言,笔直河道内MPs更易发生水平迁移[80].水体中的植被可通过降低水流流速,拦截作用,吸附MPs,固定沉积物从而降低沉积物中MPs的重悬等多个方式降低MPs的水平迁移[70].
目前,实测河道内MPs水平运输过程的研究较少.模型运算可一定程度上弥补此研究不足,Atwood等[77]利用2种模型(水动力模型和遥感模型)预测了1.5a内意大利波河河口中直径为1mm的球形MPs(密度为0.91g/cm3)沿海岸线的积累情况.2种模型预测的MPs累积结果近乎一致,并与9个河口样品的测量浓度数据相符.水动力模型模拟结果表明,波河释放的MPs中有80%以上进入海洋.遥感模型则能更好地捕捉MPs沿海岸线积累模式,具有更高的空间分辨率.但目前通过模型估算的河道内MPs的水平输运通量也存在较大差异[19].Mai等[74]使用基于人类发展指数的模型预测出2010~2050年全球河流塑料的外流通量值,仅是Jambeck等[81]使用基于塑料废物排放模型预测数值的2%.
2.6 沉积、潜流交换和渗透
潜流带是指河床界面以下的区域[82].MPs进入潜流带后,将存在5种不同形态的颗粒:可自由移动的未发生团聚的MPs、可自由移动的MPs团聚体、吸附到沉积物上的MPs、吸附到沉积物上的MPs团聚体和河床沉积物[83].MPs在此处的迁移涉及(1)沉积(过滤)、(2)渗透和(3)潜流交换.
(1)沉积(过滤)是指胶体和沉积物间在静电作用力和范德华力等化学作用力下发生相互作用,使胶体沉积在沉积物上[84].无论MPs在潜流带中有没有发生迁移,都受到一定的过滤作用.水化学条件(如离子强度、阳离子类型和pH值)主要通过改变MPs和介质之间的静电吸引和排斥作用而影响其迁移[85-86].土壤和NPs的Zeta电位通常与pH值成反比,低pH值条件下,NPs和土壤之间的排斥力减小,将更容易保留在固相中[85].当增大孔隙水中离子浓度时,会压缩双电层厚度和降低表面电位,这使得MPs更易于被沉积物截留[87].此外,在相同的离子强度下,二价的Ca2+在抑制PS NPs的迁移方面比一价的Na+表现出更大的抑制作用,归因于Ca2+比Na+具有更有效的电荷中和作用[87].Ca2+还会通过桥连作用促进MPs聚集[88].当胶体在沉积物的孔隙中运动距离后,过滤作用对胶体浓度的影响可由下式求得[83]:

式中:为河流沉积物中胶体的浓度, mg/L;为胶体在任一运动轨迹中的运动距离, m;f(m-1)为河流沉积物对胶体的过滤系数,其可通过下式计算得出:


式中:c为沉积物颗粒的平均直径, µm;f为胶体颗粒与沉积物颗粒间的碰撞效率,无量纲;0为接触效率,无量纲;s为孔隙度参数,s=(2(1–5))/(2–3+35– 26),=1–,无量纲;R为颗粒粒径比沉积物粒径,无量纲;Pe为佩克莱数,pe=c/D,无量纲;vdw为范德华数,vdw=/b,无量纲;gr为重力数,gr=πp4(p–f)/(3b),无量纲;为沉积物孔隙度,无量纲;为多孔介质中流体速度,m/s;D为扩散系数, m2/s;为哈梅克常数,J;b为玻尔兹曼常数, 1.3805×10-23J/K;为绝对温度,K;p为颗粒半径,µm;p为颗粒密度, kg/m3;f为流体密度, kg/m3.
(2)渗透指在重力、湍流、潮汐和渗流作用下MPs在沉积物中发生的向下迁移[89-90].对于孔隙水中尺寸较大的胶体颗粒或聚集体,受到重力作用后在河床介质中发生沉降并吸附或沉积到沉积物颗粒表面,则也称为沉降作用.沉降作用是胶体在沉积物中沉积的另一重要机制[91].研究表明,可使用斯托克斯定律对球形颗粒的沉降速度进行求解,并通过以下公式计算胶体移动路径[83]:
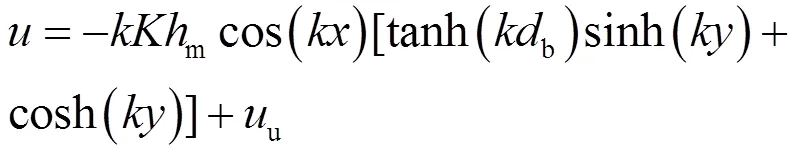
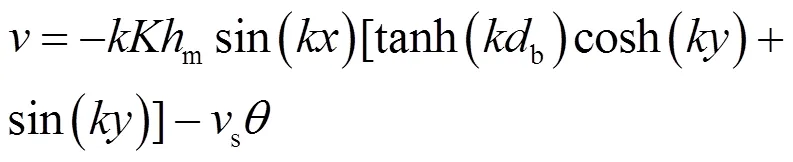
式中:和分别为水平和垂直方向上的孔隙水速度, m/s;为床面波数, 1/m;为沿河床的纵向坐标,m;为垂直方向坐标, m;为河床表面动压力,无量纲;m为沙丘上方水头变幅的半幅值,m;为导水率,cm/ min;u为底流速度(u=·), m/s,是河床坡度,无量纲;b是河床深度,m;s为斯托克斯沉降速度,m/s;为沉积物孔隙度,无量纲.
沉积物中MPs的渗透行为可能导致MPs迁移到含水层和地下水中,从而增加沉积物中MPs对生物的暴露时间,并对饮用水安全造成威胁[83,89-90].研究表明,MPs的平均浓度随沉积物深度(0~50cm)的增加而增加,深层沉积物中的小尺寸(<2mm)MPs占比逐渐升高,最终可能迁移到地下水中[92].研究发现,美国伊利诺伊州16个地下水样品中均能检测到微量MPs的存在[93].其形态均为纤维,中值浓度为6.4particle/L,最高浓度为15.2particle/L[93].沉积物中MPs的沉积(过滤)、沉降和渗透同时发生.这些过程与多孔介质表面粗糙度、流体动力学和MPs尺寸等都有关系.
(3)潜流交换是指由于近河床区的湍流和河床表面的压力变化,致使水和悬浮物颗粒(如MPs、小粒径沉积物和微生物等)进出沉积物[94-95].潜流交换是调节天然水系统中胶体和污染物运输的重要过程[83,96].河床形态能够影响河流中颗粒的潜流交换,主要归因于两种交换机理:平流泵吸作用/泵吸交换和冲淤交换[84].平流泵吸作用是指由于河床形状不平整,引起河床表面压力变化,带动河床中孔隙水的运动,导致胶体进入河床内并在其中发生迁移.冲淤交换多发生在河床冲刷区.由于河床受到水流冲刷,上游泥沙被冲走,释放孔隙水.被冲走的泥沙在下游发生沉降形成新河床并蓄积孔隙水,在此过程中孔隙水的释放和截留会引起河床与河流间的胶体交换.
在实地取样勘测中,发现不同取样地点的沉积物中MPs种类和数量分布不均,说明可能受到了潜流交换的作用[82].另一项实测研究发现,23% MPs的潜流交换率高于它们的沉降率[94].对于低密度MPs(如PS和聚氨酯塑料),这一比例高达42%.无论聚合物类型如何,潜流交换对于直径小于100μm的粒子输运和归宿都很重要.潜流交换延长了约57% MPs的停留时间,并且这部分MPs受风暴活动才可被清除.因此,不包括潜流交换的数学模型可能大大低估了MPs在河床沉积物中的沉积时间.与沉积物中MPs的过滤和渗透保留机制相比,潜流交换对沉积物中MPs迁移过程的影响还未被广泛研究[82,94].
总之,当MPs沉积到河流底部,可能通过潜流交换进入沉积物,穿过和离开河床.在运输过程中,MPs可能与其他胶体发生碰撞并产生大的聚集体,发生沉降沉积到沉积物表面.同时,由于过滤作用,它们也可能被沉积物截留.当形成的聚集体足够大时,它们可能会被固定在沉积物中;然而,如果是NPs或所形成的聚集体相对于沉积物的孔径足够小,则它们仍可以穿透空隙发生渗透或在沉积物中发生水平迁移.
3 水沙运动对微塑料动态传输过程的影响
3.1 上覆水体中泥沙与微塑料相互作用对微塑料运移的影响
一般来说,河流中的MPs作为单分散颗粒被观察到的可能性极低.相反,MPs会吸附水体中溶解性有机质(DOM)和表面活性剂等物质,并可能与其他颗粒物聚集或缠绕,从而以团聚体的形式存在.如图2a所示,通常存在于河流上覆水体的悬浮泥沙会改变MPs的稳定性.非黏性沙粒(砂粒,50>64µm)的冲击和拖曳会携带MPs进入沉积层,而黏性泥沙则可能会与MPs形成不稳定的聚集体影响MPs的悬浮和沉积[97].由于MPs的尺寸在1nm~5mm之间,而黏性泥沙颗粒的尺寸一般为<62µm,中位径(50)<4µm.因此MPs与黏性泥沙聚集可能存在2种情况:(1)小尺寸的MPs可以黏附在尺寸大于MPs颗粒的大泥沙颗粒表面形成聚集体;(2)小泥沙颗粒可能黏附于大尺寸的MPs表面形成聚集体[18].MPs与悬浮泥沙之间的相互作用受水环境条件(如水动力学特征、离子的浓度和种类、溶解或颗粒状的有机/无机胶体、微生物和浮游植物等)和MPs/泥沙自身理化特性(如颗粒Zeta电势大小、电荷分布和表面极性官能团等)的影响[45-46].
由于河流盐度低于海水,PS NPs的流体力学直径在天然河水中比在天然海水中几乎减小了一倍[45].悬浮泥沙由于表面上存在有机质,含有羧酸和酚等官能团,所以在天然水中会携带负电荷[98].一方面,带负电的悬浮泥沙会排斥携带负电荷的MPs,增强MPs在溶液中的稳定性.在低离子强度下(<0.01mol/L NaCl),携带净负电荷的黏土(蒙脱土)与带负电荷PS NPs之间存在强静电排斥力,从而增强了PS NPs在溶液中的稳定性[45].但高离子强度条件下,颗粒表面负电荷被中和、双电层被压缩,两者之间的静电斥力降低,异质聚集增强[45].并且二者表面吸附的阳离子(如Ca2+和Fe3+)可以通过桥连作用,增加聚集[24].另一方面,带相反电荷的颗粒由于静电吸引而发生聚集.带负电的氧化铁(III)矿物可吸附带正电的PS NPs形成异质聚集体[46].携带净负电荷悬浮泥沙的边缘等部位可能存在局部携带正电荷的区域.因此,在黏土矿物边缘带正电荷的部位能够吸附带负电荷的PS NPs[3].天然水体中悬浮泥沙组成、形状和大小各异,这为MPs-悬浮泥沙相互作用提供了充足的可能,通常会导致异质聚集.实验测得近似球形的PS MPs在天然淡水中与悬浮泥沙(高岭土与膨润土)异质聚集的附着效率在0.004~0.2之间,此值随MPs大小和悬浮泥沙特性变化而变化[99].
与悬浮泥沙的异质聚集作用可能影响塑料颗粒的沉降行为[99-100].Besseling等[99]考虑了对流传输、均聚、异聚、沉降-再悬浮、聚合物降解、生物膜的存在和埋藏对MPs的作用,使用模型预测得出40km河流上覆水中的近球形MPs的浓度在5d达到稳定状态.异质聚集主导了MPs在河流中的沉降率和沉降位置[100].对于小于5µm的MPs在下游14km处的沉降区达到最高浓度,而较大颗粒MPs(³5µm)则在上游1km处发生沉降.MPs颗粒大小对其在沉积物中的保留率有显著影响.直径约5µm的中等尺寸颗粒,保留率最低(18%~25%).直径100nm~ 1µm的MPs,保留率约50%;直径大于50µm的MPs保留率可高达100%(大于200 µm).导致该现象的原因在于,100nm~1 µm MPs异质聚集体的沉降主要由悬浮泥沙密度和数量决定.但随MPs粒径增加到几微米,异质聚集体沉降逐渐由MPs密度决定,异质聚集沉降速率降低,因此导致低保留率.这意味着大部分NPs和毫米大小颗粒很可能保留在河流中,而微米大小的MPs则由河流运输,进入沿海地区和海洋.
通常河口处的浊度高,悬浮泥沙组分以黏性泥沙为主,并且水体盐度较高,MPs易发生絮凝过程.实验室模拟河口处MPs聚沉实验的研究发现,悬浮泥沙的存在会降低PS NPs和PVC MPs的稳定性[16,50].其中PS NPs(100nm)与大泥沙(150~500µm)的聚沉受盐度和DOM的影响,当NaCl浓度从50mmol/L增加到200mmol/L时,高离子强度降低了PS NPs与大泥沙颗粒之间的静电斥力,提高了两者之间的聚集速率,形成了PS NPs和大泥沙的异质聚集体,提高了PS NPs沉降速率[16].在200和500mmol/L NaCl溶液中,由于异质聚集进入扩散控制区,沉降速率没有明显的差异.腐殖酸(HA)能够吸附到PS NPs或大泥沙颗粒的表面,提高了两者之间的静电斥力和空间阻力,降低了两者之间的异质聚集速率和沉降速率.碎片和细丝状PVC MPs (63~125µm)与天然细颗粒泥沙(8µm)在2h内发生絮凝,絮凝后PVC MPs的平均沉降速度为(0.74±0.30) mm/s,比分散MPs的平均沉降速度增加约8倍[50].但其絮凝和沉降行为不受水环境因素、MPs形状和PVC与悬浮泥沙比例的影响.相反,PE MPs(1mm)即使在高浓度(500mg/L)小粒径的泥沙(<10µm)存在下,仍然能够长时间(90d)漂浮在水表面[16].这是由于PE MPs在水中的沉降行为主要受浮力的作用,PEMPs和悬浮泥沙的异质聚集对PEMPs的沉降影响很小.
总体而言,悬浮泥沙既可能增强,也可能降低水柱中MPs的稳定性[45,50].但现有的模拟实验多是在静水中进行,没有考虑湍流的作用[16].实际环境中,悬浮泥沙与MPs之间的聚集和沉降往往是在湍流作用下进行,并且形成的絮体也可能由于湍流作用而发生物理破坏[100].流动水体中悬浮泥沙对MPs的聚集行为的影响,以及二者形成的团聚体在水平迁移的传输过程尚未开展研究.非球形颗粒和非黏性泥沙之间的作用对MPs传输过程的影响也缺少定论.对于纤维和非黏性泥沙,二者缠绕现象可能比附着聚集更易发生,从而提高水相中MPs的去除率.
3.2 沉积物对微塑料再悬浮过程的影响
水体沉积物中分布和埋藏着大量的MPs,其在河床上的运动主要由拖曳力、浮力和重力决定[101].沉积物再悬浮过程在天然河流中普遍发生,可由潮汐、风浪、暴风雪和洪涝事件等自然条件引起,还可以通过船只运行、清淤和拖网捕鱼等人为条件引起[102].泥沙颗粒由静止状态变为运动状态的临界水流条件,常用起动流速或临界剪切应力衡量[64].起动流速和临界起动剪切应力是密度、粒径和雷诺数相关的函数.河流沉积物发生再悬浮时临界剪切应力范围是0.2~75N/m2[103].沉积物中的MPs由于密度低(0.8~1.5kg/m3),粒径小,在受到水流运动过程的剪切力作用时,其分散性更容易被影响,即发生再悬浮或随沉积物的再悬浮过程重新进入水相[53].
微塑料在沉积物的重悬受其特性影响.研究学者利用大型水槽装置模拟实验,研究了14种不同粒径(0.5~8mm)、材质和形状(球形、纤维和碎片)的MPs在非黏性沉积物中的推移质运动,发现直径为4.8mm的PS MPs在光滑无泥沙沉积层的临界剪切应力最小(0.002N/m2),直径为2.7mm的PET MPs在尺寸为0.3~4mm的无黏性大沙粒沉积层的临界剪切应力最大(0.233N/m2)[64].粒径和形状与MPs的临界剪切应力无显著相关性.但MPs临界剪切应力与其密度成正相关,剪切应力符合如下顺序:PVC和PET>聚酰胺(PA)>PS.利用数值模拟方法(TUFLOW Finite Volume)研究MPs在河流沉积物中的运输过程结果也证实,密度较低的MPs具有较高的迁移率[38].因此,沉积物中低密度的PE MPs和PP MPs更易重悬进而随河流运输.而高密度的PA MPs和PET MPs会倾向于沉积在靠近其释放源点的位置,并可能停留数年.
沉积物的粒径结构、粗糙度和有机质组成等可影响MPs的迁移和分配[33,64,80].泥沙粗糙度与MPs的临界剪切应力显著正相关(<0.001).在光滑的沉积面上,MPs更易发生滑行,而在沉积物床上,MPs运动主要受控于阻力,MPs更易发生跳跃.不同大小的MPs在沉积物上具有隐藏暴露效应.依据隐藏暴露效应建立了沉积物中MPs的临界剪切应力的计算公式.公式可适用于各种形状、密度和大小的天然沉积物(均匀和不均匀)和MP颗粒.粒径较小沉积物处积累的MPs比粒径较大的沉积物处更多,这可能归因于隐藏-暴露效应(图2b),沉积在粗颗粒沉积物的MPs更易悬浮[64,80].沉积在松散沙质沉积物的MPs更易于重悬返回上覆水中,而黏性较高且富含有机物的沉积物被视为塑料碎片的储存库,此处的MPs较少发生重悬,会在此积累[66,80].然而,也有研究发现,渥太华河沉积物中MPs的含量与沉积物的粒径或有机质含量没有显著相关性[33].
3.3 沉积物对微塑料渗透过程的影响
沉积物主要由砾石(2~64mm)、砂(0.0625~ 2mm)、粉砂(0.0039~0.0625mm)和黏土(<0.0039mm)组成[104].MPs能够在河流沉积物立体多孔隙结构中迁移(自上向下、水平或自下向上)[89].在天然沉积物中MPs的渗透迁移过程首先受到自身理化性质[105]、环境因素和其他胶体颗粒等的影响[106],当前研究主要针对MPs在沉积物中自上向下的渗透过程展开研究.
如图2c所示,小尺寸的MPs可相对自由在多孔介质中渗透迁移,较大粒径的MPs或者MPs聚集体的渗透迁移则会受到显著抑制.根据胶体过滤理论,随着MPs粒径与多孔介质粒径中值之比的增加, MPs的截留率也会增加[107].Fan等[108]发现,在中国珠江流域,MPs多分布在表层沉积物,但随着沉积物深度增加,小颗粒MPs(<0.45mm)占比逐渐提高,在42cm处可达到100%,表明小粒径MPs更易向下渗透.同样,在城市河流(秦淮河)采集的沉积物越深,小尺寸MPs占比越高[92].在浅层(0~10cm)沉积物中,大颗粒MPs(>4mm)的占比为40.5%,在深层沉积物(41~50cm)中小颗粒(<2mm)MPs为主要成分(63.5%)[92].据估计,深层沉积物中埋藏的MPs总量是表层沉积物中的5倍[109].沉积物中MPs和聚集体的保留积累,会逐渐堵塞沉积物颗粒间的孔隙,抑制甚至完全阻碍沉积物中MPs的渗透迁移[110].
通常使用DLVO理论来描述微塑料与多孔介质间的相互作用.MPs和多孔介质的表面电性会影响MPs的迁移过程[111-112].如表面带正电荷的MPs将被表面带负电荷的砂子等吸附截留[111-112].相反,表面带负电荷的MPs与带负电荷的砂子之间的静电斥力作用能够增强MPs的稳定性和迁移量(图2c).天然有机质通过疏水作用、配体交换和静电作用吸附在MPs或多孔介质表面[113].在无金属离子或存在一价阳离子电解液的情况下,吸附在表面的天然有机质会在颗粒之间提供负电荷与空间位阻作用,从而提高颗粒的稳定性[114-115].当二价或三价金属阳离子存在时,天然有机质通过与金属离子络合的桥联作用影响颗粒聚集并进一步影响MPs的迁移[116].有机质构象和表面性质、MPs的粒径、表面官能团都会影响沉积物中MPs的稳定性[117].研究表明,在干净的浅层砂中,较大的颗粒态有机质可以提高NPs的稳定性和迁移率,而较小的溶解态有机质则会促进团聚[111].HA显著增加了PS MPs在氧化锰覆膜砂中的渗透,随HA浓度从0增加到10mg/L,渗透率也相应增加[118].
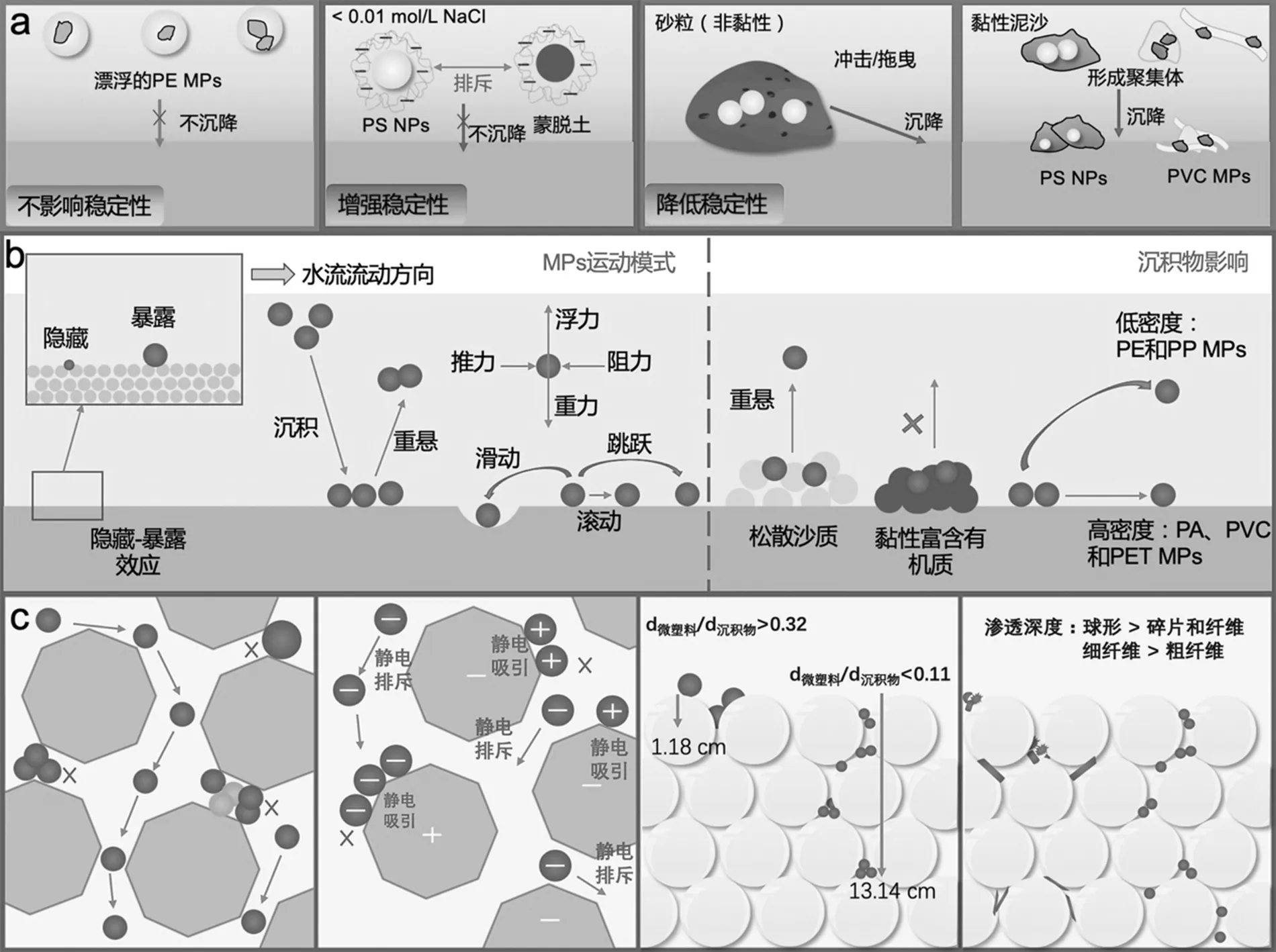
图2 泥沙/沉积物对微塑料(a)聚集沉降、(b)再悬浮和(c)渗透过程的影响
沉积物的矿物组成和孔隙度在MPs迁移中起着重要作用.在石英砂(300~425μm)柱中添加铁氧化物(针铁矿和赤铁矿)后,尺寸为0.2μm的MPs穿透率从73%降低至27%,尺寸为2μm的MPs穿透率从83%降低至2%[119].PS NPs在土壤中保留量与铁/铝氧化物含量呈正相关,这是由于铁/铝氧化物表面在中性pH值下产生正电荷,从而通过静电吸引促进带负电的MPs吸附在饱和土壤表面[85].Waldschläger等[89]使用玻璃珠模拟天然沉积物进行MPs渗透实验,发现MPs的渗透深度随玻璃球直径的增加而增加.当MPs与玻璃球直径比大于0.32时,几乎观察不到任何渗透,MPs仅停留在沉积物表面(1.2cm);当MPs与玻璃球直径比<0.11,MPs可以进入沉积物的深度为13.1cm.形状不规则的MPs易在孔中缠结,因此球形MPs颗粒的渗透要比碎片和纤维深,直径较细的纤维渗透得较深(图2c).
4 研究趋势与展望
微塑料作为一种新污染物,当进入水环境后,由于物理、化学和生物等作用的耦合影响,其运移过程十分复杂,可能对水生生物、淡水和海洋生态系统甚至人类健康产生不利影响.目前,研究学者对于河流中,尤其是含沙河流中MPs的聚集、沉降和再悬浮等迁移过程和微观机理研究仍不够透彻,研究方法不足,模型建立尚不准确.在今后的研究中,应该着重加强以下4个方面的研究:
4.1 加强不同MPs在含沙河流中迁移行为的研究工作.不同单体聚合而成的MPs的吸光性、密度和刚性等性质不尽相同,因此MPs的理化性质能够影响其在水中和沉积物中的分散性和运输过程.实际环境中的MPs受到温度、紫外线辐射、生物降解和湍流等作用的影响,易发生老化和微生物定殖.微塑料表面上生物膜的生长、降解会改变其密度和表面性质,强烈影响MPs的聚集沉降等过程.可降解塑料产量预计每年将以超过30%的速度增长.未来的工作应探究不同材质的MPs颗粒,尤其是可降解MPs和老化MPs在水中的迁移过程,以更全面和真实地描述自然水域中MPs的迁移行为.
4.2 深入开展泥沙运动、水动力和水化学等多因素耦合作用下MPs迁移过程的实验室模拟研究. MPs在水体中的迁移是泥沙/沉积物特性、河床面特征、水动力学特征、水化学特征和MPs自身特性多因素共同作用的结果.然而,目前绝大部分的研究均以单因素为主考察MPs的迁移过程,多因素系统性研究还相对欠缺.此外,考虑到真实环境中复杂的泥沙运动和水文条件,并且泥沙/沉积物等介质具有非均质性,未来研究中需加强多因素耦合作用对河流中MPs迁移过程影响的研究,确定关键过程,完善微观机理.
4.3 进行含沙河流中微塑料水平运输和河床交换过程模拟研究.目前关于水中MPs聚集和沉降的研究已经广泛开展,MPs的水平运输和河床交换过程尚未进行系统性的研究.实验室中通常以玻璃微珠和石英砂作为填充物的柱体模拟研究塑料颗粒在沉积物中的迁移和沉积行为,很难代表复杂的自然环境.泵吸交换、冲淤交换作用和过滤作用均未开展研究.未来的研究可考虑使用大型水槽装置、利用荧光MPs开展定量研究,以完善水环境中MPs水平运输和沉积物中迁移的基本过程和阐明作用机制.
4.4 开展河流中微塑料迁移的数值模拟和实地勘测研究,计算MPs在水体中的传输通量.由于室内模拟实验与天然环境仍存在较大差异,室内模拟实验不能准确说明真实含沙河流中MPs的输运方式.数学模型是了解MPs迁移过程的重要研究手段.现有的模型往往仅针对球形的MPs颗粒在上覆水体和沉积物表面的迁移过程.未来的研究需要结合水动力模型、遥感模型、MPs自身形貌、降解和老化等过程,以及实验室模拟所获得的附着效率和过滤系数等参数,尝试开发模拟真实河流和深层沉积物中MPs的迁移和交换模型,对MPs输移和传输通量进行精确描述和科学评估.实地勘测可准确获得MPs在水体各层的分布特征,推测其传输过程,检验模型的准确性.
[1] Cole M, Galloway T S. Ingestion of nanoplastics and microplastics by Pacific oyster larvae [J]. Environmental Science & Technology,2015, 49(24):14625-14632.
[2] Ter Halle A, Ladirat L, Gendre X, et al. Understanding the fragmentation pattern of marine plastic debris [J]. Environmental Science & Technology,2016,50(11):5668-5675.
[3] Brewer A, Dror I, Berkowitz B. The mobility of plastic nanoparticles in aqueous and soil environments: A critical review [J]. ACS ES&T Water,2020,1(1):48-57.
[4] Liu J, Ma Y, Zhu D, et al. Polystyrene nanoplastics-enhanced contaminant transport: Role of irreversible adsorption in glassy polymeric domain [J]. Environmental Science & Technology,2018,52(5):2677-2685.
[5] Wagner S, Reemtsma T. Things we know and don't know about nanoplastic in the environment [J]. Nature Nanotechnology,2019, 14(4):300-301.
[6] Godoy V, Blazquez G, Calero M, et al. The potential of microplastics as carriers of metals [J]. Environmental Pollution,2019,255(3): 113363.
[7] Guo B, Meng J, Wang X, et al. Quantification of pesticide residues on plastic mulching films in typical farmlands of the North China [J]. Frontiers of Environmental Science Engineering,2020,14(1):1-10.
[8] 马思睿,李舒行,郭学涛.微塑料的老化特性、机制及其对污染物吸附影响的研究进展[J]. 中国环境科学,2020,40(9):3992-4003.
Ma S, Li S, Guo X. A review on aging characteristics, mechanism of microplastics and their effects on the adsorption behaviors of pollutants [J]. China Environmental Science, 2020,40(9):3992-4003.
[9] Alam F C, Sembiring E, Muntalif B S, et al. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia) [J]. Chemosphere,2019,224:637-645.
[10] Castelvetro V, Corti A, Bianchi S, et al. Quantification of poly (ethylene terephthalate) micro- and nanoparticle contaminants in marine sediments and other environmental matrices [J]. Journal of Hazardous Materials,2020,385:121517.
[11] Bilotta G S, Brazier R E. Understanding the influence of suspended solids on water quality and aquatic biota [J]. Water Research,2008, 42(12):2849-2861.
[12] Hu P, Guo C, Zhang Y, et al. Occurrence, distribution and risk assessment of abused drugs and their metabolites in a typical urban river in north China [J]. Frontiers of Environmental Science Engineering,2019,13(4):1-11.
[13] Tang W, Sun L, Shu L, et al. Evaluating heavy metal contamination of riverine sediment cores in different land-use areas [J]. Frontiers of Environmental Science Engineering,2020,14(6):1-11.
[14] Wang S, Fu B, Piao S, et al. Reduced sediment transport in the Yellow River due to anthropogenic changes [J]. Nature Geoscience,2016,9(1): 38-41.
[15] Yang X, Flynn R, Von Der Kammer F, et al. Influence of ionic strength and pH on the limitation of latex microsphere deposition sites on iron-oxide coated sand by humic acid [J]. Environmental Pollution,2011,159(7):1896-1904.
[16] Li Y, Wang X, Fu W, et al. Interactions between nano/micro plastics and suspended sediment in water: implications on aggregation and settling [J]. Water Research,2019,161:486-495.
[17] Long M, Paul-Pont I, Hégaret H, et al. Interactions between polystyrene microplastics and marine phytoplankton lead to species- specific hetero-aggregation [J]. Environmental Pollution,2017,228: 454-463.
[18] Huffer T, Praetorius A, Wagner S, et al. Microplastic exposure assessment in aquatic environments: learning from similarities and differences to engineered nanoparticles [J]. Environmental Science & Technology,2017,51(5):2499-2507.
[19] Yan M, Wang L, Dai Y, et al. Behavior of microplastics in inland waters: aggregation, settlement, and transport [J]. Bulletin of Environmental Contamination and Toxicology,2021:700-709.
[20] Horton A A, Walton A, Spurgeon D J, et al. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities [J]. Science of the Total Environment,2017,586:127-141.
[21] Schmidt C, Krauth T, Wagner S. Export of plastic debris by rivers into the sea [J]. Environmental Science & Technology,2017,51(21):12246- 12253.
[22] Yang L, Zhang Y, Kang S, et al. Microplastics in freshwater sediment: A review on methods, occurrence, and sources [J]. Science of the Total Environment,2020:141948.
[23] Shahul Hamid F, Bhatti M S, Anuar N, et al. Worldwide distribution and abundance of microplastic: how dire is the situation? [J]. Waste Management & Research,2018,36(10):873-897.
[24] Alimi O S, Farner Budarz J, Hernandez L M, et al. Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport [J]. Environmental Science & Technology,2018,52(4):1704-1724.
[25] Lechner A, Keckeis H, Lumesberger-Loisl F, et al. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe's second largest river [J]. Environmental Pollution,2014,188: 177-181.
[26] Bellasi A, Binda G, Pozzi A, et al. Microplastic contamination in freshwater environments: a review, focusing on interactions with sediments and benthic organisms [J]. Environments,2020,7(4):30.
[27] Lin L, Zuo L Z, Peng J P, et al. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China [J]. Science of the Total Environment,2018, 644:375-381.
[28] Hu L, Chernick M, Hinton D E, et al. Microplastics in small waterbodies and tadpoles from Yangtze River Delta, China [J]. Environmental Science & Technology,2018,52(15):8885-8893.
[29] Zhang L, Liu J, Xie Y, et al. Distribution of microplastics in surface water and sediments of Qin river in Beibu Gulf, China [J]. Science of the Total Environment,2020,708:135176.
[30] Wu N, Zhang Y, Zhang X, et al. Occurrence and distribution of microplastics in the surface water and sediment of two typical estuaries in Bohai Bay, China [J]. Environmental Science: Processes & Impacts,2019,21(7):1143-1152.
[31] Lenaker P L, Baldwin A K, Corsi S R, et al. Vertical distribution of microplastics in the water column and surficial sediment from the Milwaukee River Basin to Lake Michigan [J]. Environmental Science & Technology,2019,53(21):12227-12237.
[32] Rodrigues M O, Abrantes N, Goncalves F J M, et al. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antua River, Portugal) [J]. Science of the Total Environment,2018,633:1549-1559.
[33] Vermaire J C, Pomeroy C, Herczegh S M, et al. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries [J]. Facets,2017,2:301-314.
[34] Adomat Y, Grischek T. Sampling and processing methods of microplastics in river sediments - A review [J]. Science of the Total Environment,2021,758:143691.
[35] Aljaibachi R, Callaghan A. Impact of polystyrene microplastics onmortality and reproduction in relation to food availability [J]. PeerJ,2018,6:e4601.
[36] Liu Y, You J, Li Y, et al. Insights into the horizontal and vertical profiles of microplastics in a river emptying into the sea affected by intensive anthropogenic activities in Northern China [J]. Science of the Total Environment,2021,779:146589.
[37] Yin H, Liu Q, Deng X, et al. Organophosphate esters in water, suspended particulate matter (SPM) and sediments of the Minjiang River, China [J]. Chinese Chemical Letters,2021.
[38] He B, Smith M, Egodawatta P, et al. Dispersal and transport of microplastics in river sediments [J]. Environmental Pollution,2021, 279:116884.
[39] Wang X, Bolan N, Tsang D C W, et al. A review of microplastics aggregation in aquatic environment: influence factors, analytical methods, and environmental implications [J]. Journal of Hazardous Materials,2021,402:123496.
[40] Wang X, Li Y, Zhao J, et al. UV-induced aggregation of polystyrene nanoplastics: effects of radicals, surface functional groups and electrolyte [J]. Environmental Science: Nano,2020,7(12):3914-3926.
[41] Kowalski N, Reichardt A M, Waniek J J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors [J]. Marine Pollution Bulletin,2016,109(1): 310-319.
[42] 张晓栋,刘志飞,张艳伟,等.海洋微塑料源汇搬运过程的研究进展[J]. 地球科学进展, 2019,34(9):936-949.
Zhang X D, Liu Z F, Zhang Y W, et al. Research progress on source- to-sink transport processes of marine microplastics [J]. Advances in Earth Science, 2019,34(9):936-949.
[43] Long M, Moriceau B, Gallinari M, et al. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates [J]. Marine Chemistry,2015,175:39-46.
[44] Zhang Z, Chen Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: a review [J]. Chemical Engineering Journal,2019,382:122955.
[45] Singh N, Tiwari E, Khandelwal N, et al. Understanding the stability of nanoplastics in aqueous environments: effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals [J]. Environmental Science: Nano,2019,6(10):2968-2976.
[46] Oriekhova O, Stoll S. Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter [J]. Environmental Science: Nano,2018,5(3):792-799.
[47] Abbas Q, Yousaf B, Amina, et al. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: a review [J]. Environment International,2020,138: 105646.
[48] Jia D, Hamilton J, Zaman L M, et al. The time, size, viscosity, and temperature dependence of the Brownian motion of polystyrene microspheres [J]. American Journal of Physics,2007,75(2):111-115.
[49] Sun H, Jiao R, Wang D. The difference of aggregation mechanism between microplastics and nanoplastics: Role of Brownian motion and structural layer force [J]. Environmental Pollution,2021,268:115942.
[50] Andersen T J, Rominikan S, Olsen I S, et al. Flocculation of PVC microplastic and fine-grained cohesive sediment at environmentally realistic concentrations [J]. The Biological Bulletin,2021,240(1): 42-51.
[51] Wong J K H, Lee K K, Tang K H D, et al. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions [J]. Science of the Total Environment,2020, 719:137512.
[52] Phenrat T, Saleh N, Sirk K, et al. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions [J]. Environmental Science & Technology,2007,41(1):284-290.
[53] Waldschlager K, Schuttrumpf H. Effects of particle properties on the settling and rise velocities of microplastics in freshwater under laboratory conditions [J]. Environmental Science & Technology,2019, 53(4):1958-1966.
[54] Velzeboer I, Quik J T, Van De Meent D, et al. Rapid settling of nanoparticles due to heteroaggregation with suspended sediment [J]. Environmental Toxicology and Chemistry,2014,33(8):1766-1773.
[55] Filella M. Questions of size and numbers in environmental research on microplastics: methodological and conceptual aspects [J]. Environmental Chemistry,2015,12(5):527-538.
[56] Khatmullina L, Isachenko I. Settling velocity of microplastic particles of regular shapes [J]. Marine Pollution Bulletin,2017,114(2):871-880.
[57] Fazey F M, Ryan P G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity [J]. Environmental Pollution,2016,210:354-360.
[58] Chubarenko I, Bagaev A, Zobkov M, et al. On some physical and dynamical properties of microplastic particles in marine environment [J]. Marine Pollution Bulletin,2016,108(1/2):105-112.
[59] Lagarde F, Olivier O, Zanella M, et al. Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type [J]. Environmental Pollution,2016,215:331-339.
[60] 董姝楠,夏继红,王为木,等.典型水环境因素对聚酯微塑料沉降的影响机制研究[J]. 中国环境科学,2021,41(2):735-742.
Dong S, Xia J, Wang W, et al. Effect mechanism of aquatic environmental factor on the sedimentation of polyethylene terephthalate microplastic [J]. China Environmental Science, 2021, 41(2):735-742.
[61] Waldschlaeger K, Born M, Cowger W, et al. Settling and rising velocities of environmentally weathered micro-and macroplastic particles [J]. Environmental Research,2020,191:110192.
[62] Kooi M, Reisser J, Slat B, et al. The effect of particle properties on the depth profile of buoyant plastics in the ocean [J]. Scientific Reports,2016,6(1):1-10.
[63] Kooi M, Nes E H V, Scheffer M, et al. Ups and downs in the ocean: Effects of biofouling on vertical transport of microplastics [J]. Environmental Science & Technology,2017,51(14):7963-7971.
[64] Waldschlager K, Schuttrumpf H. Erosion behavior of different microplastic particles in comparison to natural sediments [J]. Environmental Science & Technology,2019,53(22):13219-13227.
[65] Eo S, Hong S H, Song Y K, et al. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea [J]. Water Research,2019,160:228-237.
[66] Fischer E K, Paglialonga L, Czech E, et al. Microplastic pollution in lakes and lake shoreline sediments-a case study on Lake Bolsena and Lake Chiusi (central Italy) [J]. Environmental Pollution,2016,213: 648-657.
[67] Flynn K F, Chudyk W, Watson V, et al. Influence of biomass and water velocity on light attenuation ofL.(Kuetzing) in rivers [J]. Aquatic Botany,2018,151:62-70.
[68] Ye S, Andrady A L. Fouling of floating plastic debris under Biscayne Bay exposure conditions [J]. Marine Pollution Bulletin,1991,22(12): 608-613.
[69] Hurley R, Woodward J, Rothwell J J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding [J]. Nature Geoscience,2018,11(4):251-257.
[70] Wu F, Pennings S C, Tong C, et al. Variation in microplastics composition at small spatial and temporal scales in a tidal flat of the Yangtze Estuary, China [J]. Science of the Total Environment,2020, 699:134252.
[71] Zhang K, Xiong X, Hu H, et al. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China [J]. Environmental Science & Technology,2017,51(7):3794- 3801.
[72] Ockelford A, Cundy A, Ebdon J E. Storm response of fluvial sedimentary microplastics [J]. Scientific Reports,2020,10(1):1-10.
[73] Willis K A, Eriksen R, Wilcox C, et al. Microplastic distribution at different sediment depths in an urban estuary [J]. Frontiers in Marine Science,2017,4:419.
[74] Mai L, Sun X F, Xia L L, et al. Global riverine plastic outflows [J]. Environmental Science & Technology,2020,54(16):10049-10056.
[75] Schwarz A E, Ligthart T N, Boukris E, et al. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: a review study [J]. Marine Pollution Bulletin,2019,143:92-100.
[76] Mani T, Burkhardt-Holm P. Seasonal microplastics variation in nival and pluvial stretches of the Rhine River-From the Swiss catchment towards the North Sea [J]. Science of the Total Environment,2020, 707:135579.
[77] Atwood E C, Falcieri F M, Piehl S, et al. Coastal accumulation of microplastic particles emitted from the Po River, Northern Italy: comparing remote sensing and hydrodynamic modelling with in situ sample collections [J]. Marine pollution bulletin,2019,138:561-574.
[78] Van Sebille E, Wilcox C, Lebreton L, et al. A global inventory of small floating plastic debris [J]. Environmental Research Letters,2015, 10(12):124006.
[79] Tibbetts J, Krause S, Lynch I, et al. Abundance, distribution, and drivers of microplastic contamination in urban river environments [J]. Water Research,2018,10(11):1597.
[80] Corcoran P L, Belontz S L, Ryan K, et al. Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada [J]. Environmental Science & Technology,2019,54(2):818-825.
[81] Jambeck J R, Geyer R, Wilcox C, et al. Plastic waste inputs from land into the ocean [J]. Science,2015,347(6223):768-771.
[82] Frei S, Piehl S, Gilfedder B, et al. Occurence of microplastics in the hyporheic zone of rivers [J]. Scientific Reports,2019,9(1):1-11.
[83] Areepitak T, Ren J. Model simulations of particle aggregation effect on colloid exchange between streams and streambeds [J]. Environmental Science & Technology,2011,45(13):5614-5621.
[84] 杨小全,金光球,李 凌,等.河流潜流带中胶体迁移的研究进展[J]. 水利水电科技进展,2010,30(6):78-83.
Yang X Q, Jin G Q, Li L, et al. Advances in researches on colloid transport in hyporheic zone [J]. Advances in Science and Technology of Water Resources, 2010,30(6):78-83.
[85] Wu X, Lyu X, Li Z, et al. Transport of polystyrene nanoplastics in natural soils: effect of soil properties, ionic strength and cation type [J]. Science of the Total Environment,2020,707:136065.
[86] Dong Z, Zhang W, Qiu Y, et al. Cotransport of nanoplastics (NPs) with fullerene (C60) in saturated sand: Effect of NPs/C60ratio and seawater salinity [J]. Water Research,2019,148:469-478.
[87] Li S, Liu H, Gao R, et al. Aggregation kinetics of microplastics in aquatic environment: Complex roles of electrolytes, pH, and natural organic matter [J]. Environmental Pollution,2018,237:126-132.
[88] Xia T, Qi Y, Liu J, et al. Cation-inhibited transport of graphene oxide nanomaterials in saturated porous media: The Hofmeister effects [J]. Environmental Science & Technology,2017,51(2):828-837.
[89] WaldschläGer K, SchüTtrumpf H. Infiltration behavior of microplastic particles with different densities, sizes, and shapes-from glass spheres to natural sediments [J]. Environmental Science & Technology,2020,54(15):9366-9373.
[90] Li M, Zhang M, Rong H, et al. Transport and deposition of plastic particles in porous media during seawater intrusion and groundwater- seawater displacement processes [J]. Science of the Total Environment,2021:146752.
[91] Tufenkji N, Elimelech M. Correlation equation for predicting single- collector efficiency in physicochemical filtration in saturated porous media [J]. Environmental Science & Technology,2004,38(2):529-536.
[92] Niu L, Li Y, Li Y, et al. New insights into the vertical distribution and microbial degradation of microplastics in urban river sediments [J]. Water Research,2021,188:116449.
[93] Panno S V, Kelly W R, Scott J, et al. Microplastic contamination in karst groundwater systems [J]. Groundwater,2019,57(2):189-196.
[94] Drummond J D, Nel H A, Packman A I, et al. Significance of hyporheic exchange for predicting microplastic fate in rivers [J]. Environmental Science & Technology Letters,2020,7(10):727-732.
[95] 张中天,金光球,陈鹤翔,等.胶体颗粒在潜流带中沉积过程及机制[J]. 水科学进展, 2021,32(5):738-750.
Zhang Z T, Jin G Q, Chen H X, et al. Deposition process and mechanism of colloidal particles in hyporheic zone [J] Advances in Water Science, 2021,32(5):738-750.
[96] Boano F, Harvey J W, Marion A, et al. Hyporheic flow and transport processes: Mechanisms, models, and biogeochemical implications [J]. Reviews of Geophysics,2014,52(4):603-679.
[97] Pohl F, Eggenhuisen J T, Kane I A, et al. Transport and burial of microplastics in deep-marine sediments by turbidity currents [J]. Environmental Science & Technology,2020,54(7):4180-4189.
[98] Zhu B, Xia X, Zhang S, et al. Attenuation of bacterial cytotoxicity of carbon nanotubes by riverine suspended solids in water [J]. Environmental Pollution,2018,234:581-589.
[99] Besseling E, Quik J T K, Sun M, et al. Fate of nano- and microplastic in freshwater systems: a modeling study [J]. Environmental Pollution,2017,220(Pt A):540-548.
[100] Quik J T, De Klein J J, Koelmans A A. Spatially explicit fate modelling of nanomaterials in natural waters [J]. Water Research,2015,80:200-208.
[101] Nakki P, Setala O, Lehtiniemi M. Seafloor sediments as microplastic sinks in the northern Baltic Sea - Negligible upward transport of buried microplastics by bioturbation [J]. Environmental Pollution,2019,249:74-81.
[102] Xie M, Alsina M A, Yuen J, et al. Effects of resuspension on the mobility and chemical speciation of zinc in contaminated sediments [J]. Journal of Hazardous Materials,2019,364:300-308.
[103] Dong J, Xia X, Wang M, et al. Effect of recurrent sediment resuspension-deposition events on bioavailability of polycyclic aromatic hydrocarbons in aquatic environments [J]. Journal of Hydrology,2016,540:934-946.
[104] Bartram J, Ballance R. Water quality monitoring: A practical guide to the design and implementation of freshwater quality studies and monitoring programmes [M]. Taylor & Francis Group, London and New York, 1996,332.
[105] Jiang Y, Yin X, Xi X, et al. Effect of surfactants on the transport of polyethylene and polypropylene microplastics in porous media [J]. Water Research,2021,196:117016.
[106] 李宵慧,徐红霞,孙媛媛,等.多孔介质中微塑料的环境行为研究进展[J]. 中国环境科学, 2021,41(6):2798-2811.
Li X H, Xue H X, Sun Y Y, et al. Review on the environmental behaviors of microplastics in porous media [J]. China Environmental Science, 2021,41(6):2798-2811.
[107] Bradford S A, Simunek J, Bettahar M, et al. Modeling colloid attachment, straining, and exclusion in saturated porous media [J]. Environmental Science & Technology,2003,37(10):2242-2250.
[108] Fan Y, Zheng K, Zhu Z, et al. Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China [J]. Environmental Pollution,2019,251:862-870.
[109] Xue B, Zhang L, Li R, et al. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment [J]. Environmental Science & Technology,2020,54(4):2210-2217.
[110] Dong Z, Qiu Y, Zhang W, et al. Size-dependent transport and retention of micron-sized plastic spheres in natural sand saturated with seawater [J]. Water Research,2018,143:518-526.
[111] Song Z, Yang X, Chen F, et al. Fate and transport of nanoplastics in complex natural aquifer media: Effect of particle size and surface functionalization [J]. Science of the Total Environment,2019,669: 120-128.
[112] Shaniv D, Dror I, Berkowitz B. Effects of particle size and surface chemistry on plastic nanoparticle transport in saturated natural porous media [J]. Chemosphere,2021,262:127854.
[113] Baalousha M, Manciulea A, Cumberland S, et al. Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter [J]. Environmental Toxicology and Chemistry,2008,27(9):1875-1882.
[114] Wu J, Jiang R, Lin W, et al. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges [J]. Environmental Pollution,2019, 245:836-843.
[115] Yu S, Shen M, Li S. Aggregation kinetics of different surface- modified polystyrene nanoparticles in monovalent and divalent electrolytes [J]. Environmental Pollution,2019,255:113302.
[116] Wegner A, Besseling E, Foekema E M, et al. Effects of nanopolystyrene on the feeding behavior of the blue mussel () [J]. Environmental Toxicology and Chemistry,2012,31(11): 2490-2497.
[117] Louie S M, Tilton R D, Lowry G V. Critical review: impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials [J]. Environmental Science: Nano,2016,3(2):283-310.
[118] Tan M, Liu L, Zhang M, et al. Effects of solution chemistry and humic acid on the transport of polystyrene microplastics in manganese oxides coated sand [J]. Journal of Hazardous Materials,2021,413:125410.
[119] Li M, He L, Zhang M, et al. Cotransport and deposition of iron oxides with different-sized plastic particles in saturated quartz sand [J]. Environmental Science & Technology,2019,53(7):3547-3557.
Recent progress of the effect of suspended sediment movement on the transport of microplastics in rivers.
WANG Xin-jie, WANG Yi-ning, ZHAO Jian, LIU Sheng-dong, XIA Xing-hui, LI Yang*
(Key Laboratory of Water and Sediment Sciences of Ministry of Education, State Key Laboratory of Water Environment Simulation, School of Environment, Beijing Normal University, Beijing 100875, China)., 2022,42(2):863~877
This review summarized the occurrence and abundance of microplastics (MPs) firstly and then the main transformation processes of MPs in rivers, including aggregation, settling, rising, resuspension, horizontal migration, and hyporheic exchange. In this paper, we critically evaluated the transportation of MPs in sediment-laden river, such as the heteroaggregation and co-settling of MPs with suspended sediments, influence of sediments on the resuspension and infiltration of MPs. The interaction mechanisms between suspended sediments and MPs concerning influencing factors of MP transportation have been discussed. Finally, recommendations for future research were discussed: (1) reliable models for predicting the migration process and flux of MPs in rivers are needed to develop; (2) researchers are suggested to use aged-MPs to conduct experiments, and consider the effect of turbulent water and organisms on the transportation behavior of MPs during laboratory work.
microplastics;nanoplastics;river;transport;suspended sediment;sediment;influence factors
X522
A
1000-6923(2022)02-0863-15
王薪杰(1994-),女,山东烟台人,北京师范大学博士研究生,主要从事水环境中微塑料迁移转化方面研究.发表论文11篇.
2021-06-28
国家重点研发计划项目课题(2021YFC3200401);国家自然科学基金资助项目(52170024,21677015)
* 责任作者, 副教授, liyang_bnu@bnu.edu.cn
