磁性阳离子型壳聚糖絮凝剂去除Cr(Ⅵ)
2022-02-25郑怀礼蒋君怡
郑怀礼,钟 政,邹 宏,白 莹,赵 瑞,丁 魏,蒋君怡
磁性阳离子型壳聚糖絮凝剂去除Cr(Ⅵ)
郑怀礼1*,钟 政1,邹 宏2,白 莹3,赵 瑞1,丁 魏1,蒋君怡1
(1.重庆大学,三峡库区生态环境教育部重点实验室,重庆 400045;2.重庆蓝洁广顺净水材料有限公司,重庆 4024652;3.中海油天津化工研究设计院有限公司,天津 300131)
以二氧化硅和硅烷偶联剂(KH-570)包裹的磁性Fe3O4作为磁芯,壳聚糖(CS)、丙烯酰胺(AM)和丙烯酰氧乙基三甲基氯化铵(DAC)作为接枝单体,通过低压紫外光引发合成新型磁性壳聚糖絮凝剂 (FSCAD),研究该材料对Cr(Ⅵ)的去除性能.采用傅立叶变换红外、热重分析、X射线衍射图谱和振动样品磁强计对絮凝剂进行表征,显示材料成功制备并具有良好的磁响应性.系统探究了pH值、絮凝剂投加量、反应时间、干扰离子对絮凝性能的影响并拟合絮凝剂絮动力学模型.结果表明,絮凝剂絮凝动力学符合拟二级动力学方程,在投加量为900mg/L、pH值为3、反应时间为60min时FSCAD对低浓度Cr(Ⅵ)废水的去除效果可达到90.48%.
磁絮凝剂;低压紫外光;壳聚糖;接枝改性;六价铬
重金属污染物中,Cr(VI)具有氧化电位高、易于穿透生物膜的性质,是一种毒性极强的重金属元素[1].Cr(VI)主要来源于铬酸盐制造[2]、电镀、金属抛光[3]、纺织印染[4]、合金和钢铁制造等工业过程[5].含Cr(VI)废水的主要处理方法包括化学还原法[2]、光催化还原法[6-7]、离子交换法[8]、膜过滤法[9]以及絮凝法[10]等.其中絮凝法是水处理中常用的工艺之一,具有工艺简单、适用性广的优点[11].然而传统的絮凝剂对Cr(VI)废水的去除效率低、对pH值的依赖性强.因此需要开发高效的絮凝剂来强化絮凝性能.
磁絮凝技术是一种常用的强化混凝技术,在原混凝体系中加入高比表面积的磁性纳米颗粒MNPs(如纳米氧化铁)作为载体,加强磁性物质、絮凝剂与水体污染物之间的碰撞,形成的絮体密实,混凝去除效率提高[12-13].然而,这类常规磁絮凝也存在磁粉与絮体的结合程度较低、处理效果不稳定等局限性.磁性复合絮凝剂由磁粉与传统絮凝剂复合制成,絮凝后形成的絮体具有磁性,易于分离对水体中重金属离子的去除有良好的效果[14].本文磁性复合絮凝剂中的磁芯采用磁性Fe3O4纳米颗粒,为屏蔽Fe3O4纳米颗粒的团聚作用同时提高其抗氧化性和抗酸性,对其进行无机物包覆.二氧化硅(SiO2)具有良好的化学稳定性、生物相容性和亲水性,同时SiO2能阻止Fe3O4纳米颗粒的聚集[15],为MNPs表面的进一步修饰提供活性位点,本文采用二氧化硅对磁芯进行包裹. C=C通过硅烷偶联剂接枝到磁芯表面,为聚合物提供足够的接口.壳聚糖具有成本低、无毒、可生物降解等优势,已有研究表明壳聚糖对Cr(VI)有良好的去除效果[16-17],因此本文采用壳聚糖作为接枝絮凝材料.
在絮凝剂制备领域中,最广泛应用的光引发方式是以高压紫外灯管作为光源的高压紫外光引发聚合法[18].高压紫外光引发法反应温度高,需加入冷凝管回流装置进行降温,增加了操作的复杂性.近年来研究的低压紫外光引发法具有反应温度低、操作简单、聚合物不易交联、更加的节能环保的优势[19].本研究采用低压紫外光引发接枝聚合反应,以SiO2和硅烷偶联剂(KH-570)包裹的磁性Fe3O4作为磁芯,引入氨基、羟基、季铵盐基团,制备出了Fe3O4@SiO2@C=C@壳聚糖-丙烯酰胺-丙烯酰氧乙基三甲基氯化铵磁性絮凝剂(FSCAD).通过一系列表征,研究磁性絮凝剂的合成与反应机理,将FSCAD应用于Cr(VI)模拟废水的处理,研究絮凝剂对Cr(VI)的最佳去除率.
1 材料和方法
1.1 实验材料
壳聚糖(CS,乙酰化度³95%)、戊二醛(50% wt)、丙烯酰氧乙基三甲基氯化铵(DAC,80% wt)、Fe3O4纳米颗粒均购自成都麦卡西化工有限公司.其他化学品购自成都科龙化学试剂有限公司.超纯水由LZ-PJ-10A净化系统(中国重庆利振科技有限公司)制成.所有试剂均为分析级,使用时无需进一步纯化.
1.2 磁絮凝剂的制备
1.2.1 Fe3O4@SiO2的制备 采用溶胶凝胶法制备Fe3O4@SiO2壳核纳米颗粒.将磁性纳米颗粒Fe3O4分散于酒精和去离子水体积比为2:1的混合溶液中,超声10min.注入质量分数为25%的氨水溶液.在120r/min搅拌下,缓慢连续加入TEOS(正硅酸四乙酯),TEOS与氨水的体积比为3:1,室温搅拌下反应8h.反应完成后先后用酒精和去离子水洗涤Fe3O4@SiO2,去除未反应的TEOS.最后在60℃下真空干燥至恒重.
1.2.2 Fe3O4@SiO2@C=C的制备 采用含有乙烯基的硅烷偶联剂3-(甲基丙烯酰氧基)丙基三甲基氧基硅烷(KH-570)对Fe3O4@SiO2表面进行修饰,引入C=C,提供充分的接枝位点.具体操作方法:将Fe3O4@SiO2纳米颗粒分散于装有酒精的三颈圆底烧瓶中超声处理10min.吹氮气10min,然后加热到78℃.在加热至62℃时,缓慢加入KH-570醇溶液,然后用氮气鼓泡10min.将该气密混合物回流并在78℃下以180r/min搅拌12h.反应完成后,用外部磁体分离表面上被C=C键修饰的Fe3O4@SiO2壳核纳米颗粒,用乙醇纯化6~8次,并在60℃真空干燥至恒重,制得Fe3O4@SiO2@C=C.
1.2.3 FSCAD的制备 FSCAD通过低压紫外光引发的接枝共聚制备.将壳聚糖溶解在100mL 1%乙酸溶液中,加入2g Fe3O4@ SiO2@C=C纳米颗粒和丙烯酰胺(AM),充分振摇并超声处理10min.吹氮气10min,加入1.5mL过硫酸钾溶液(0.1g/mL),继续吹氮气,加入9mL丙烯酰氧乙基三甲基氯化铵(DAC)溶液.摇晃均匀,在低压紫外线灯下照射3h.制备的磁性絮凝剂用乙醇纯化6~8次,然后真空60℃干燥至恒重.
1.3 分析方法
采用傅里叶变换红外光谱(FTIR;Nicolet iS10,美国)表征具有KBr颗粒的FSCAD官能团.利用具有CuKα辐射(=1.54056Å)的X射线衍射图(XRD; DMAX/2C,日本Rigaku),以表征FSCAD的相结构,表面化学结构利用Axis Ultra X射线光电子能谱仪(XPS;Empyrean,PANalytical B.V.,荷兰)检测.
1.4 实验方法
采用模拟Cr(VI)废水评估FACAD的絮凝性能.将重铬酸钾溶于超纯水中制备浓度为500mg/L的Cr(VI)标准水溶液,再用超纯水稀释至预定浓度.分别用0.01mol/L的HCl/NaOH调节pH值.首先将混合溶液在300r/min的高速下搅拌5min,在50r/min的低速下搅拌60min.随后,在磁铁的帮助下分离絮状物.抽吸液体表面以下2cm的上清液以测量金属离子浓度.重金属离子的浓度采用中国标准(GB/ T9723-2007)方法[20]测定.用紫外可见分光光度计(T6Pgeneral Co.,Ltd,中国)在540nm波长下测量上清液的透光率.所有样品浓度结果采用3个平行测量值的平均值表示,并通过计算3个值的标准偏差获得误差限.污染物去除效率计算公式如下:

式中:C和C分别表示的Cr(VI)初始浓度和最终浓度, mg/L.
2 结果与讨论
2.1 磁性混凝剂的特性
2.1.1 FTIR光谱分析 如图1所示,对于Fe3O4@SiO2,559.4cm−1处的吸附峰对应典型的Fe- O拉伸振动[19].在794.1和1064.5cm−1处的吸附峰分别与Si-O-Si的不对称/对称振动和弯曲振动有关.945.7cm−1处的峰值是由Si-O-H振动[21]引起的.这些峰证实了Fe3O4@SiO2中Fe3O4和SiO2的存在.Fe3O4@SiO2@C=C中1661.7cm−1与C=C的振动有关,这表明KH-570的成功接枝.1030.8,1156.6, 1447.8,1614.2和3340.4cm−1分别与C-OH、C-O-C、-CH2、N-H和-OH/-NH2交联CS的振动有关[22-23]. 1730.1cm−1的峰归属于DAC中O-C=O的伸缩振动,955cm−1处的吸附带归属于季铵基[24],表明DAC单体成功接枝.而1536.9和1655.8cm−1的峰分别属于AM中的酰胺II和酰胺I谱带[25].上述特征峰表明,利用AM和DAC对CS进行了成功的改性.

图1 FSCAD的红外光谱
2.1.2 XRD图谱分析 如图2所示,Fe3O4@SiO2的特征峰位于2θ=18.5°,30.3°,35.6°,43.3°,53.6°,57.2°和62.8°,分别归结为(1 1 1),(2 2 0),(3 1 1),(4 0 0),(4 2 2),(5 1 1)和(4 4 0),这表明制备的Fe3O4具有良好的结晶度.对于FSCAD,这些没有变化的峰表明,无论是SiO2还是CS-AM-DAC外壳都不会对Fe3O4磁芯[26]的尺寸和晶相造成任何损害.此外,SiO2和有机共聚物均未见峰,说明其结构为无定形[27].
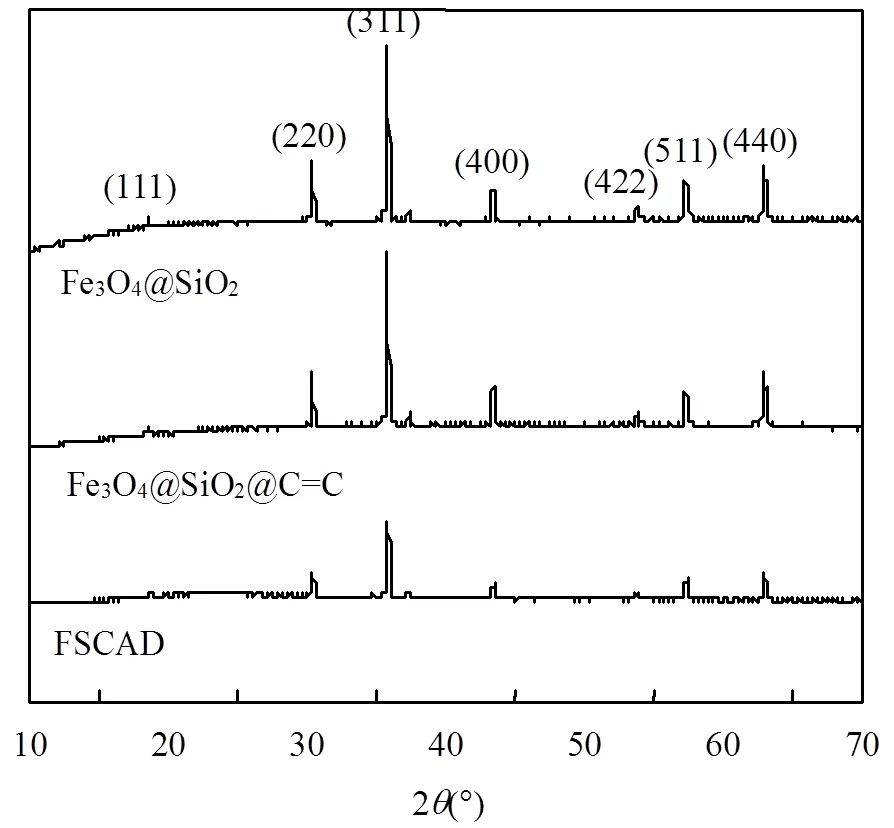
图2 FSCAD X射线衍射表征结果

图3 FSCAD的磁化混线
2.1.3 磁性能分析 图3为±20000Oe磁场下,用VSM分析Fe3O4@SiO2、Fe3O4@SiO2@C=C、FSCAD的磁性能图.3条曲线均呈对称分布,未观察到明显的磁滞现象,说明所有样品均具有超顺磁性特征[28-29].Fe3O4@SiO2的饱和磁化强度为57.9emu/g;接枝C=C后,Fe3O4@SiO2@C=C的饱和磁化强度分别为55.9emu/g,接枝AM与DAC后FSCAD的饱和磁化强度为12.7emu/g.这一现象是因为磁芯引入了非磁性材料MPS和有机共聚物.虽然FSCAD的饱和磁化值远低于Fe3O4@SiO2的饱和磁化值,但在外加磁铁的帮助下,仍能快速有效地从水溶液中分离出来.
2.1.4 热重分析 如图4所示,对于Fe3O4@SiO2来说只有微弱的失重,这部分失重由SiO2造成,证明了SiO2成功包裹在了Fe3O4的表面且Fe3O4@SiO2具有优越的热稳定性;Fe3O4@SiO2@C=C与Fe3O4@SiO2相比也有很小一部分的失重量,这是因为接枝KH-570引入了C=C的原因,证明了C=C双键的成功引入,也证明了Fe3O4@SiO2@C=C良好的热稳定性.KH-570的沸点为255℃,若Fe3O4@SiO2与KH-570为物理吸附,则在255℃前就会发生较大失重,因此可以推断出改性后Fe3O4@SiO2与KH- 570通过共价键相连[30];而对于FSCAD来说有4个明显的失重阶段:其中第1个失重阶段(T1-T2)是亲水性基团吸附水分子蒸发所致[31],失重率为9.3%.第2个失重阶段(T2~T3)与亚胺反应和酰胺基团热分解有关[32-33],失重率为25.6%.第3个失重阶段(T3-T4)与DAC的O=C-O分解有关,失重率为23.4%.第4个失重阶段(T4-800 °C)由共聚物主链的热分解造成[34],失重率为11.2%.
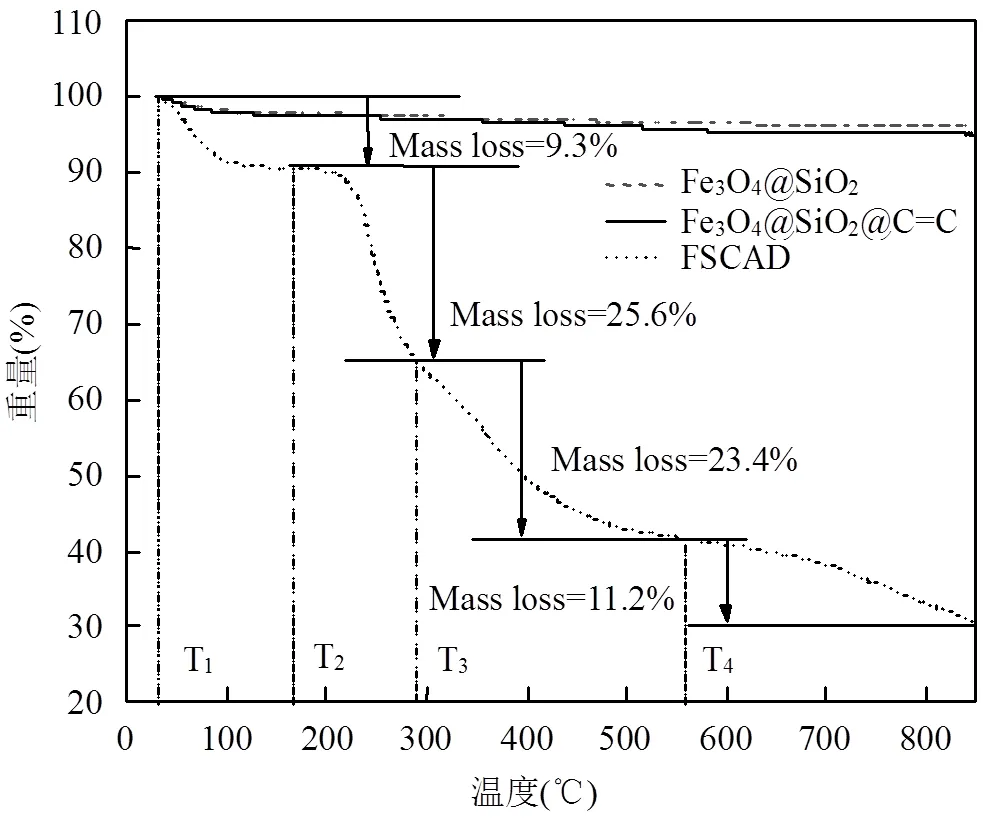
图4 FSCAD热重表征结果
2.2 混凝特性分析

图5 FSCAD投加量对去除Cr(VI)的影响
2.2.1 投加量的影响 如图6所示,絮凝剂的离子去除率(RE(CV))随着投加量的增加而升高,并在投加量为0.9mg/mL后增长速率减缓.从RE(CV)来看, FSCAD对低浓度Cr(VI)的去除效率更高.在絮凝的过程中,各种絮凝剂都有一个最佳的投加量.在低用量的情况下,材料链上没有足够的活性基团与重金属反应,导致Cr(VI)的去除量较低.随着投加量的增加,活性基团数量增加,壳聚糖中极其活泼且相邻的羟基与氨基对Cr(VI)的络合吸附能力增加,阳离子单体DAC引入的季铵盐基团对水溶液中铬酸盐的静电吸附能力不断加强,Cr(VI)浓度不断降低.同时由于絮凝剂的吸附架桥作用,絮体和团聚体迅速形成.当投加量进一步增加时,大分子链中存在的过多的正电荷会增加反应的静电斥力,从而影响絮凝剂对Cr(VI)的进一步去除.本实验结果显示,絮凝剂投加量在1000mg/L以内时去除率有减缓趋势但无明显下降趋势.从经济性的角度出发,选取900mg/L作为后续实验的絮凝剂投加量.
2.2.2 溶液pH值的影响 如图6所示,在酸性条件下,由于质子化氨基的形成和季铵盐基团的存在使得FSCAD的Zeta电位为正值,随着pH值的增大,氨基的去质子化作用加强Zeta电位减小,但由于季铵盐基团的存在FSCAD在整个pH值范围内表面都带正电荷.因此,改性后的絮凝剂具有更大去除水体阴离子污染物的潜力.
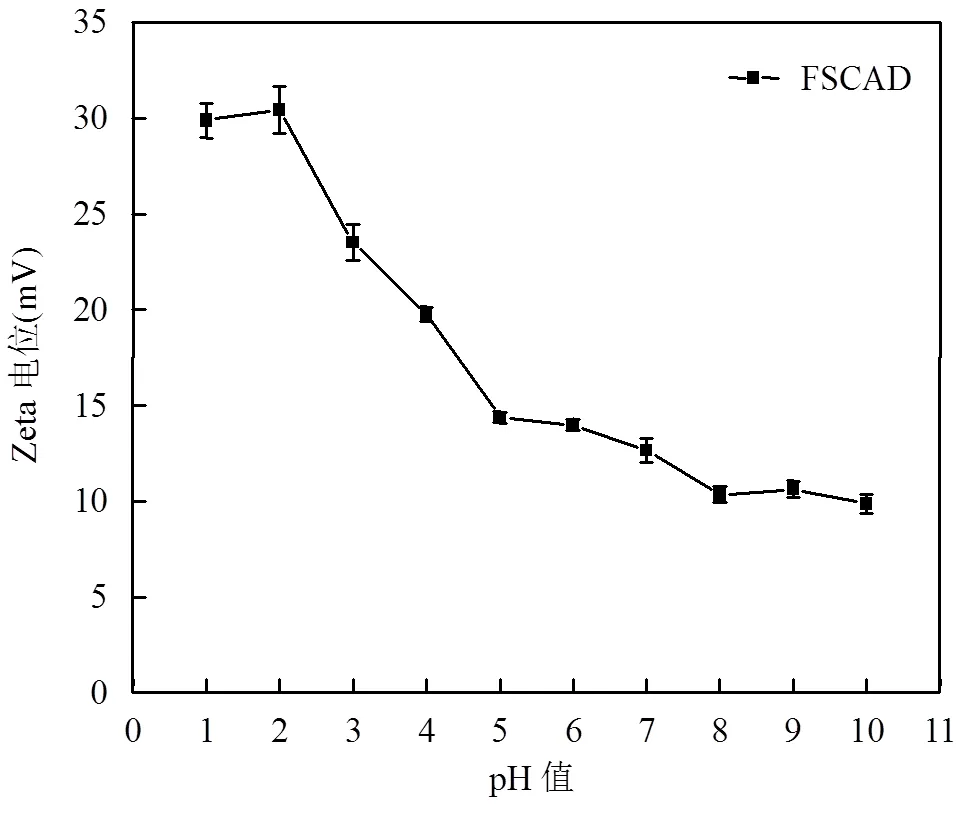
图6 FSCAD的Zeta电位与pH值的关系
Cr(VI)在水中的存在形式与水中氢离子浓度有关.如图7(a)所示,在Cr(VI)浓度为1mmol/L的情况下,当pH<1时,主要以H2CrO4的形式存在;pH= 2~6时,主要为HCrO4-与CrO42-的共同存在;pH=6~ 10时,主要以CrO42-的形式存在, pH>10时几乎只有CrO42-存在.因此本文选取pH值范围为1~10范围内进行研究.如图7(b)所示, pH=1~3时,絮凝剂对Cr(VI)的去除率逐渐增加,并在pH=3时分别对5, 20与50mg/L Cr(VI)获得最高去除率90.48%、84.91%和55.39%.pH=3~10时,去除率逐渐下降. FSCAD中的羟基、氨基、季铵盐基团通过氢键、静电吸附、表面络合与Cr(VI)结合[35-36],从而有效地去除了Cr(VI).pH<3时去除率减小的原因可以从Cr(VI)在该pH值下的存在形式和电性结合起来分析. FSCAD中的氨基在低pH值(pH=3)下Cr(VI)主要以HCrO4-的形式存在,氨基与氢离子结合形成质子化氨基-NH3+,表面带正电(+23.52mV),质子化氨基与季铵盐基团可以与溶液中不同形式的铬酸盐发生静电相互作用,从而形成最大化的络合延伸[16],当pH<3时,水体中存在一定比例的H2CrO4,间接降低了溶液中铬酸盐的含量,H2CrO4存在比例越大,静电相互作用越弱.pH值在3-5时,溶液中Cr(VI)以HCrO4-与CrO42-共同存在,但此时质子化氨基的数量随着pH值增加而减少,正电性减弱,静电相互作用减小.当pH值增加至碱性时,CrO42-为主要存在形式,质子化氨基的数量进一步减少.由于絮凝剂正电性下降、CrO42-所占据活性位点多于HCrO4-[36]、OH-与CrO42-竞争活性位点的现象越来越强烈的原因,导致静电作用不断下降、去除率不断降低.
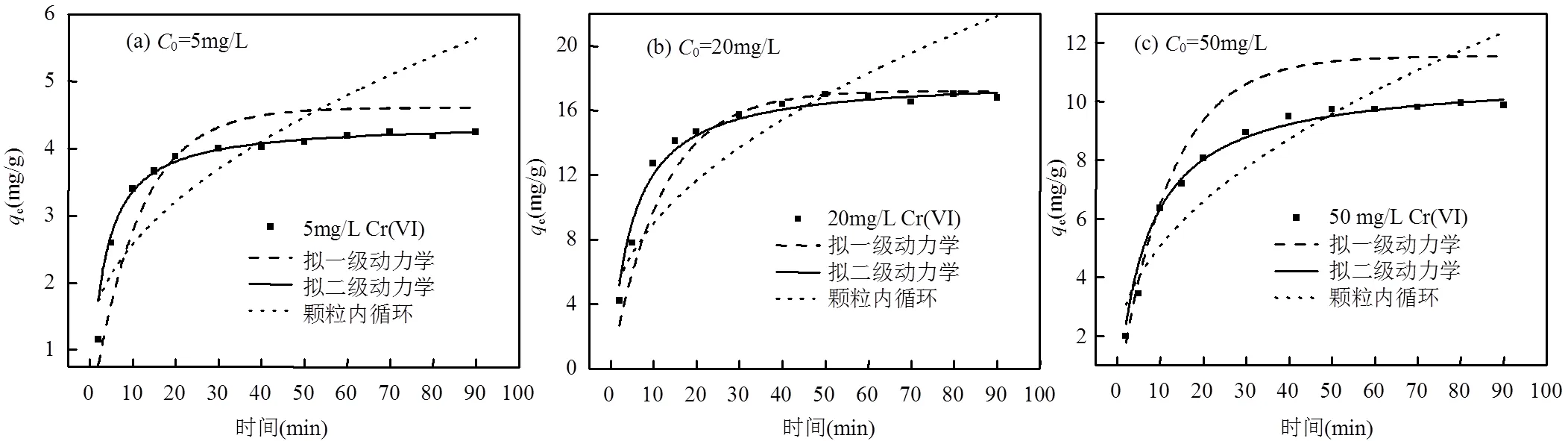
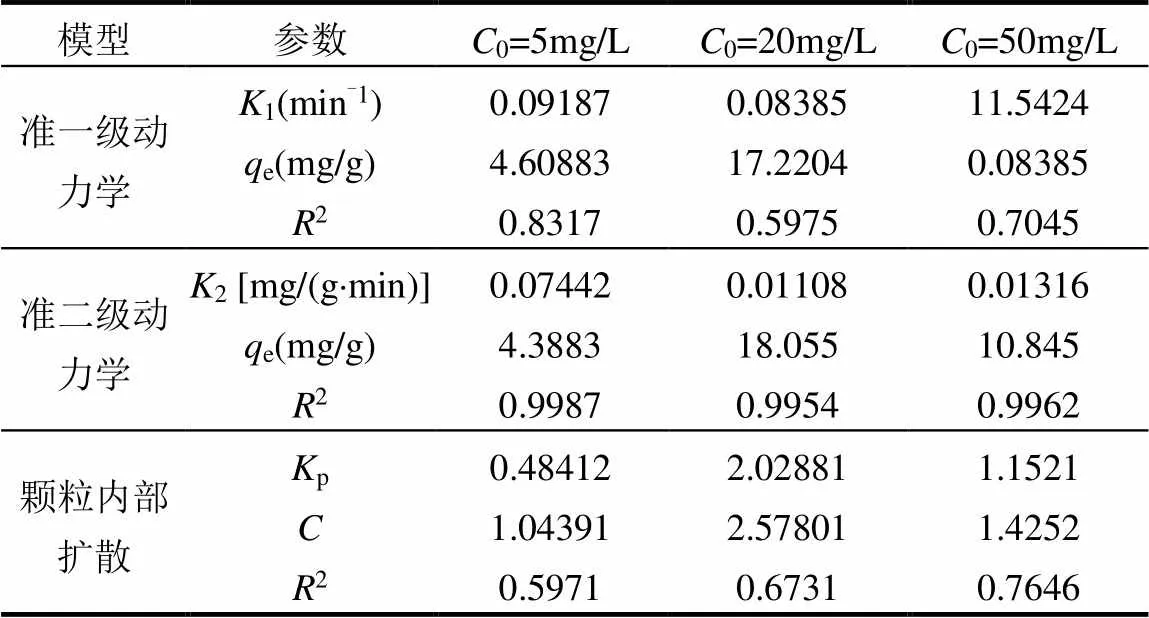
2.2.3 共存离子影响 研究了在水环境中广泛存在的阳离子(Na+、K+、Ca2+)和阴离子(Cl−、NO3−、SO42−)对Cr(VI)去除率的影响[37-38].采用50mmol/L的Na+、K+、Ca2+、Cl−、NO3−、SO42−离子模拟水体中的共存离子, Cr(VI)浓度为20mg/L, pH值调节为7,絮凝剂投加量为900mg/L.如图8(a)所示,共存阴离子对Cr(VI)离子的絮凝去除均表现出不利影响,其影响程度依次为:Cl− 2.2.4 絮凝时间的影响 絮凝速率是评估特定絮凝剂应用潜力的重要因素,通常对于目标污染物的去除速率越快絮凝剂越理想.分别配制5,20mg/L和50mg/L的Cr(VI)离子溶液,在中性条件下进行实验,絮凝剂投加量为900mg/L.如图8(b)所示,絮凝剂对重金属Cr(VI)离子的吸收较快,在反应开始30min后FSCAD对5和20mg/L Cr(VI)离子的去除率超过70%;对50mg/L的Cr(VI)离子的去除率超过40%.随后去除率缓慢增加并达到平衡值,FSCAD对Cr(VI)离子的相对最优去除率分别为76.58%、76.59%、44.76%.最初的去除率快速上升现象是由目标污染物与氨基、羟基和季铵盐基团的相互作用引起,初期活性位点充足,能迅速进行有效反应.但随着反应时间的延长,重金属与FSCAD之间形成絮体,活性位点被占据,线性分子链发生卷曲,造成重金属与活性位点之间的碰撞效率降低.絮凝剂FSCAD的快速初始吸附-絮凝速率表明其在应急水污染净化中具有良好的应用潜力.根据上述动力学数据, 40min后絮凝剂的反应趋近平衡,本文慢速搅拌时间选择60min可以确保磁性絮凝剂去除Cr(VI)达到平衡. 图9 FSCAD在Cr(VI)不同初始浓度条件下的动力学拟合 表1 FSCAD的模型拟合结果 2.2.5 絮凝剂去除Cr(VI)的絮凝动力学 如图9和表1所示,FSCAD的絮凝反应过程与准二级动力学拟合最好,在准二次动力学反应过程中,反应物之间的离子交换是限制反应速率的关键[42-45].因此,离子交换在FSCAD的絮凝反应中起重要作用. 3.1 表征结果表明,絮凝剂成功制备,具有良好的热稳定性与磁响应性. 3.2 絮凝实验证明,在pH=3、絮凝剂投加量为900mg/L、絮凝程序按设计条件操作的条件下, FACAD对5mg/L Cr(VI)的最佳去除率约为90.48%;对20mg/L Cr(VI)的最佳去除率约为84.91%;对50mg/L Cr(VI)的最佳去除率约为55.39%.絮凝剂分子链上的氨基、羟基和季铵盐基团可以快速去除水中的Cr(VI). [1] Marikkani S, Kumar J V, Muthuraj V. Design of novel solar-light driven sponge-like Fe2V4O13photocatalyst: A unique platform for the photoreduction of carcinogenic hexavalent chromium [J]. Solar Energy, 2019,188:849-856. [2] Zi H L, Shu Y X, Guang H X, et al. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron [J]. Journal of Environmental Management, 2019,244:33-39. [3] Chang J J, Wang H, Zhang J,et al. New insight into adsorption and reduction of hexavalent chromium by magnetite: Multi-step reaction mechanism and kinetic model developing [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021,611:125784. [4] Xiao D H, Pei Y L. Surface Water Pollution in the Middle Chinese Loess Plateau with Special Focus on Hexavalent Chromium (Cr6+): Occurrence, Sources and Health Risks [J]. Exposure and Health, 2020:385-401. [5] Park S H, Shin S S, Park C H, et al. Poly(acryloyl hydrazide)-grafted cellulose nanocrystal adsorbents with an excellent Cr(VI) adsorption capacity [J]. Journal of Hazardous Materials, 2020,394:122512. [6] Jiang Z K, Chen K X, Zhang Y C, et al. Dionysiou. Magnetically recoverable MgFe2O4/conjugated polyvinyl chloride derivative nanocomposite with higher visible-light photocatalytic activity for treating Cr(VI)-polluted water [J]. Separation and Purification Technology, 2020,236:116272. [7] Ge T, Jiang Z K, Shen Li, et al. Synthesis and application of Fe3O4/FeWO4composite as an efficient and magnetically recoverable visible light-driven photocatalyst for the reduction of Cr(VI) [J]. Separation and Purification Technology, 2021,263:118401. [8] Ren Y F, Han Y H, Lei X F, et al. A magnetic ion exchange resin with high efficiency of removing Cr (VI) [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020,604:125279. [9] Zhang Y H, Xu X M, Yue C L, et al. Insight into the efficient co-removal of Cr(VI) and Cr(III) by positively charged UiO-66-NH2 decorated ultrafiltration membrane [J]. Chemical Engineering Journal, 2021,404:126546. [10] 王 刚,常 青,杜凤龄,等. pH值对新型高分子螯合絮凝剂捕集Cr(Ⅲ)和Cr(Ⅵ)的影响[C]//中国环境科学学会、四川大学.2014中国环境科学学会学术年会论文集(第五章).中国环境科学学会、四川大学:中国环境科学学会, 2014:336-342. Wang G, Chang Q, Du F L, et al. Effect of pH values on novel polymer chelate flocculants that trap Cr(Ⅲ) and Cr(Ⅵ) [C]//Chinese Society For Environmental Sciences, Sichuan University. Proceedings of the 2014 Annual Meeting of Chinese Society For Environmental Sciences (Chapter V). Chinese Society For Environmental Sciences, Sichuan University: Chinese Society of Environmental Sciences, 2014:336- 342. [11] Zheng H, Ma J, Zhu C, et al. Synthesis of anion polyacrylamide under UV initiation and its application in removing dioctyl phthalate from water through flocculation process [J]. Separation and Purification Technology, 2014,123:35-44. [12] Kyzas G Z, Siafaka P I, Lambropoulou D A, et al. Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake [J]. Langmuir, 2014,30(1):120-131. [13] 刘冰枝.聚羧酸-壳聚糖基新型絮凝剂及应用性能研究 [D]. 重庆:重庆大学, 2018. Liu B Z. Preparation of novel polycarboxylate−chitosan based flocculant and application performance [D]. Chongqing: Chongqing University, 2018. [14] 郑怀礼,蒋君怡,万鑫源,等.磁性纳米颗粒的制备及其复合材料吸附处理工业废水的研究进展 [J]. 中国环境科学, 2021,41(5):1-14. Zheng H L, Jiang J Y, Wan X Y, et al. A research progress on preparation of magnetic nanoparticles and adsorption treatment of their composites in industrial wastewater [J]. China Environmental Science, 2021,41(5):1-14. [15] 吴江渝,许 谦,李 竹,等.磁性二氧化硅纳米粒子的制备及性能 [J]. 武汉工程大学学报, 2014,36(7):43-7. Wu J Y, Xu Q, LI Z, et al. Preparation and properties of magnetic silica nanoparticles [J]. Journal of Wuhan University of Engineering, 2014, 36(7):43-47. [16] Borsagli F G L M, Mansur A A P, Chagas P, et al. O-carboxymethyl functionalization of chitosan: Complexation and adsorption of Cd (II) and Cr (VI) as heavy metal pollutant ions [J]. Reactive & Functional Polymers, 2015,97:37-47. [17] Copello G J, Varela F, Vivot R M, et al. Immobilized chitosan as biosorbent for the removal of Cd(II), Cr(III) and Cr(VI) from aqueous solutions [J]. Bioresource technology, 2008,99(14):6538-6544. [18] 付 坤.低压光引发阳离子聚丙烯酰胺制备及其絮凝性能研究 [D]. 安徽工业大学, 2017. Fu K. Study on preparation and flocculation properties of low pressure lighe induced cationic polyacrylamide [D]. Anhui University of Technology, 2017. [19] Alqadami A A, Naushad M, Abdalla M A, et al. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism [J]. Journal of Cleaner Production, 2017,156:426-436. [20] GB/T 9723-2007 化学试剂火焰原子吸收光谱法通则[S]. GB/T 9723-2007 Chemical flame atomic absorption spectrometry [S]. [21] Yan Z, Yang H, Ouyang J, et al. In situ loading of highly-dispersed CuO nanoparticles on hydroxyl group-rich SiO2-AlOOH composite nanosheets for CO catalytic oxidation [J]. Chemical Engineering Journal, 2017,316:1035-1046. [22] Li K, Wang Y, Huang M, et al. Preparation of chitosan-graft- polyacrylamide magnetic composite microspheres for enhanced selective removal of mercury ions from water [J]. Journal of Colloid and Interface Science, 2015,455:261-270. [23] Li X, Zheng H, Wang Y, et al. Fabricating an enhanced sterilization chitosan-based flocculants: Synthesis, characterization, evaluation of sterilization and flocculation [J]. Chemical Engineering Journal, 2017,319:119-130. [24] Kong Z Y, Wei J F, Li Y H, et al. Rapid removal of Cr(VI) ions using quaternary ammonium fibers functioned by 2-(dimethylamino)ethyl methacrylate and modified with 1-bromoalkanes [J]. Chemical Engineering Journal, 2014,254:365-373. [25] Yuan B, Shang Y, Lu Y, et al. The Flocculating Properties of Chitosan-graft-Polyacrylamide Flocculants (I)-Effect of the Grafting Ratio [J]. Journal of Applied Polymer Science, 2010,117(4):1876- 1882. [26] Zheng X, Zheng H, Zhao R, et al. Polymer-Functionalized Magnetic Nanoparticles: Synthesis, Characterization, and Methylene Blue Adsorption [J]. Materials, 2018,11(8):1312. [27] Ge Y, Li Y, Zu B, et al. AM-DMC-AMPS Multi-Functionalized Magnetic Nanoparticles for Efficient Purification of Complex Multiphase Water System [J]. Nanoscale Research Letters, 2016,11: 217. [28] Li L, Lu W, Ding D, et al. Adsorption properties of pyrene- functionalized nano-Fe3O4mesoporous materials for uranium [J]. Journal of Solid State Chemistry, 2019,270:666-673. [29] Usman T M, Su X, Zhao M, et al. Preparation of hydroxypropyl- cyclodextrin-graphene/Fe3O4and its adsorption properties for heavy metals [J]. Surfaces and Interfaces, 2019,16:43-49. [30] 邵亚辉.新型阳离子聚丙烯酰胺“水包水”乳液的制备及应用 [D]. 广州:华南理工大学, 2020. Shao Y H. Preparation and application of novel cationic polyacrylamide "water-in-water" emulsion [D]. guagnzhou: South China University of Technology, 2020. [31] Yang Z L, Gao B Y, Li C X, et al. Synthesis and characterization of hydrophobically associating cationic polyacrylamide [J]. Chemical Engineering Journal, 2010,161(1/2):27-33. [32] Zhao C, Zheng H, Feng L, et al. Improvement of Sludge Dewaterability by Ultrasound-Initiated Cationic Polyacrylamide with Microblock Structure: The Role of Surface-Active Monomers [J]. Materials, 2017,10(3):282. [33] Xing Y, Zhang J, Chen F, et al. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy [J]. Nanoscale, 2017,9(25):8781-8790. [34] Feng L, Zheng H, Gao B, et al. Fabricating an anionic polyacrylamide (APAM) with an anionic block structure for high turbidity water separation and purification [J]. Rsc Advances, 2017,7(46):28918- 28930. [35] Zhao N, Zhao C F, Tsang D C W, et al. Microscopic mechanism about the selective adsorption of Cr(VI) from salt solution on O-rich and N-rich biochars [J]. Journal of Hazardous Materials, 2021,404(PA): 124162. [36] Zheng C F, Zheng H L, Wang Y J, et al. Synthesis of novel modified magnetic chitosan particles and their adsorption performance toward Cr(VI) [J]. Bioresource Technology, 2018,267:1-8. [37] Sharma R K, Kumar R. Functionalized cellulose with hydroxyethyl methacrylate and glycidyl methacrylate for metal ions and dye adsorption applications [J]. International Journal of Biological Macromolecules, 2019,134:704-721. [38] Bouza-deano R, Ternero-rodriguez M, Fernandez-espinosa A J. Trend study and assessment of surface water quality in the Ebro River (Spain) [J]. Journal of Hydrology, 2008,361(3/4):227-239. [39] Yao Y, Mi N, He C, et al. A novel colloid composited with polyacrylate and nano ferrous sulfide and its efficiency and mechanism of removal of Cr(VI) from Water [J]. Journal of Hazardous Materials, 2020,399:123082. [40] Hu Z L, Cai L M, Jiang J M, et al. Green synthesis of expanded graphite/layered double hydroxides nanocomposites and their application in adsorption removal of Cr(VI) from aqueous solution [J]. Journal of Cleaner Production, 2019,209:1216-1227. [41] Xie Y Q, Lin J, Liang J, et al. Hypercrosslinked mesoporous poly (ionic liquid)s with high density of ion pairs: Efficient adsorbents for Cr(VI) removal via ion-exchange [J]. Chemical Engineering Journal, 2019,378:122107. [42] Khan S, Zhang D, Yang M L, et al. Isotherms, kinetics and thermodynamic studies of adsorption of Ni and Cu by modification of Al2O3nanoparticles with natural organic matter [J]. Taylor & Francis, 2018,26(3):1422490. [43] Sun Y J, Chen A W, Pan S Y, et al. Novel chitosan-based flocculants for chromium and nickle removal in wastewater via integrated chelation and flocculation [J]. Journal of Environmental Management, 2019,248:109241. [44] Sun Y J, Chen A W, Sun W Q, et al. Removal of Cu and Cr ions from aqueous solutions by a chitosan based flocculant [J]. Desalination and Water Treatment, 2019,148:256-269. [45] Sun Y J, Shah K J, Sun W Q, et al. Performance evaluation of chitosan-based flocculants with good pH resistance and high heavy metals removal capacity [J]. Separation and Purification Technology, 2019,215:286-216. Synthesis of magnetic cationic chitosan flocculant by low-pressure UV light for the removal of Cr(Ⅵ). ZHENG Huai-li1*, ZHONG Zheng1, ZOU Hong2, BAI Ying3, ZHAO Rui1, DING Wei1, JIANG Jun-yi1 (1.Key Laboratory of the Three Gorges Reservoir Region's Eco-Environment, StateMinistry of Education, Chongqing University, Chongqing 400045, China;2.Chongqing Lanjie Guangshun Water Treatment Materials Co.Ltd, Chongqing 402465, China;3.CenerTech Tianjin Chemical Research and Design Institute Co. Ltd, Tianjin 300131, China)., 2022,42(2):745~752 A new magnetic chitosan flocculant FSCAD was synthesized byusing magnetic Fe3O4coated with silica and silane coupling agent (KH-570) as magnetic core, chitosan, acrylamide and acryloyloxyethyl trimethylammonium chloride as graft monomers and low-pressure ultraviolet light as initiator,the Cr(Ⅵ) removal properties of the material were studied. The flocculants were characterized by fourier transform infrared spectroscopy, thermogravimetric analysis, X-ray diffractometer and vibration sample magnetometer, showing the materials were successfully prepared and have good magnetic response. The effects of pH, dosage, reaction time and interference ions on flocculation properties were explored and fitted to the flocculation dynamics model. The flocculation kinetics accorded with the pseudo second order kinetic equation and the removal effect of FSCAD on low concentration Cr(Ⅵ) wastewater reached 90.48% at the added amount of 900mg/L, pH of 3 and the reaction time of 60min. magnetic flocculant;low-pressure UV;chitosan;grafting modification;Cr(Ⅵ) X703.1 A 1000-6923(2022)02-0745-08 郑怀礼(1957-),男,重庆人,教授,博士,主要从事水处理及水处理剂研究.发表论文400多篇. 2021-07-10 国家自然科学基金资助项目(21677020);重庆市技术创新与应用示范专项重点研发项目(cstc2018jszx-zdyfxmX0002) * 责任作者, 教授, zhl@cqu.edu.com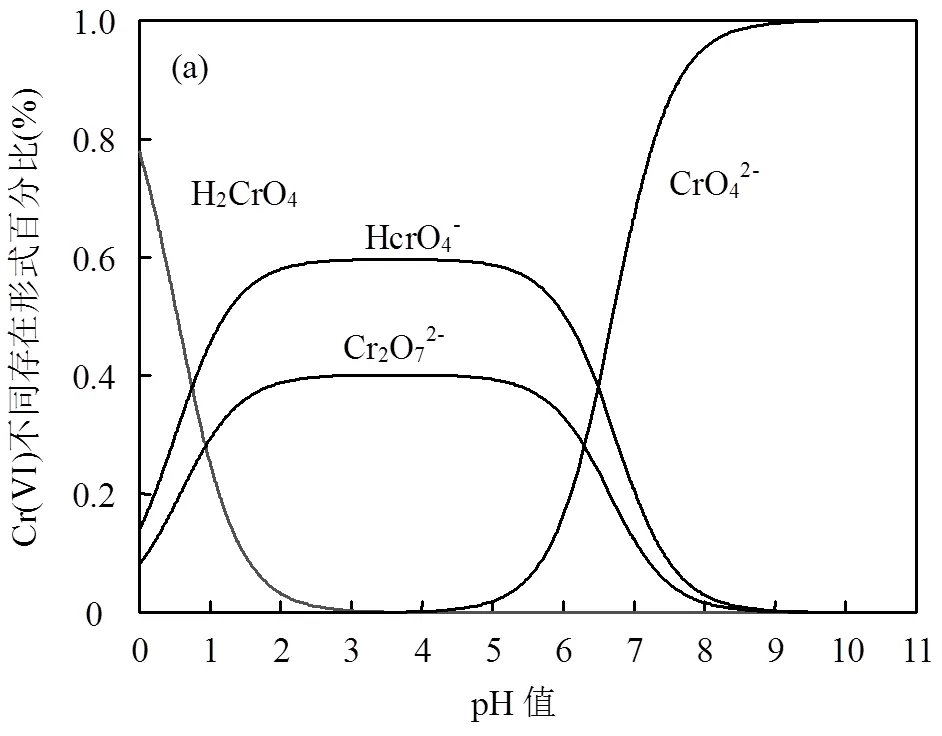

3 结论
