Preparation and Characterization of Nano-ZnO Precursor for the Antibacterial Activity against Thielaviopsis paradoxa
2022-02-17YenanWANGDejieYANG
Yenan WANG, Dejie YANG
Coconut Research Institute of Chinese Academy of Tropical Agricultural Sciences, Hainan Innovation Center of Academician Team, Wenchang 571339, China
Abstract [Objectives] The paper was to explore a new method for preparing nano ZnO particles. [Methods] A mixture of 2,6-di (2′,4′-dicarboxylphenyl) pyridine(0.05 mM), Zn(NO3)2·6H2O (0.1 mM) were added to the solution of DMA (3 mL) and H2O (3 mL), and heated at 90 ℃ for 12 h. The PXRD, TGA and fluorescence properties were characterized. [Results] Transparent block crystals of the [Zn2 (2,6-ddpp)(DMA)3(H2O)·H2O]n were obtained (yield 85.4%, based on Zn). The Zn-MOF framework would collapse to form ZnO particles when the temperature exceeded 350 ℃. [Conclusions] A new Zn(II)-MOF is successfully constructed, and the compound exhibits monoclinic P21/c space group. The results will provide a theoretical basis for the preparation of nano ZnO.
Key words Nano ZnO; Zn(II)-MOF; P21/c space group
1 Introduction
Nowadays, bacteria and fungi infection are still the main causes of morbidity and mortality of plants[1]. However, due to the abuse of antibiotics and the emergence of drug-resistant strains, a novel type of bactericidal material is urgently needed. Nanotechnology provides a good platform for adjusting the physical and chemical properties of various materials to produce effective antimicrobials[2]. Nanoparticles have the following advantages over antibiotics. (i) The small size but large specific surface area makes it relatively easy to achieve the effective bacteriostatic activity. (ii) Nanoparticles mainly contact with bacterial cell wall directly, without penetrating cells, which are irrelevant with most of the resistance mechanisms seen with antibiotics[1].
ZnO nanoparticles have a wide range of antimicrobial activity against various microorganisms, which is significantly dependent on the chosen concentration and particle size[3]. At present, the main methods for preparing nano ZnO are hydrothermal synthesis, precipitation and sol-gel method. In this study, we prepared Zn-MOF (Metal Organic Framework) and decomposed it at different temperatures and atmospheres to obtain the nano ZnO. The advantage of this method is that the nano zinc oxide particles with different morphologies and particle sizes can be obtained by changing the treatment temperature. In this paper, we mainly provided a method for preparing nano ZnO precursor Zn-MOF, and characterized its performance, which would be helpful for subsequent antibacterial performance research, especially forThielaviopsisparadoxa, which causes serious disease of coconut.
2 Materials and methods
2.1 MaterialsThe metal salt and organic ligand were commercially available and used without further purification. Thermogravimetric (TG) curves were measured in a NETZSCH 449C thermal analyzer at a heating rate of 10 ℃/min under air atmosphere. Solid fluorescence was measured by Hitachi F-4500 fluorescence spectrophotometer. Power X-ray diffraction (PXRD) was tested by RigakuUltima IV diffractometer (CuKα radiation,λ=1.540 6Å). The simulated powder patterns were calculated using Mercury 2.0. The purity and homogeneity of the bulk products were determined by comparing simulated and experimental X-ray powder diffraction patterns[4].
2.2 Synthesis of Zn-MOFA mixture of 2,6-di(2′,4′-dicarboxylphenyl)pyridine (0.05 mM) and Zn(NO3)2·6H2O (0.1 mM) were added to the solution of DMA (3 mL) and H2O (3 mL), heated at 90 ℃ for 12 h, and then cooled to the room temperature to obtain transparent block crystals of [Zn2(2,6-ddpp)(DMA)3(H2O)·H2O]n (yield 85.4%, based on Zn).
2.3 Structure determinationThe diffraction data for the Zn-MOF were collected by Rigaku XtaLAB mini diffractometer with graphite-monochromated MoKα radiation (λ=0.710 73Å). The collected data were reduced using the programCrystalClear[5], and an empirical absorption correction was applied. The structure was solved by direct methods using SHELXS-97[6], and refined based onF2by full-matrix least-squares methods using SHELXL-97[7]. All non-H atoms were refined anisotropically. The hydrogen atoms of organic ligands were placed in calculated positions and refined using a riding mode on the attached atoms with isotropic thermal parameters 1.2 times of those of their carrier atoms. The positions of hydrogen atoms attached to carbon atoms were generated geometrically. The compound crystallized in the monoclinic system, space groupP21/c. The parameters were as follows: a=15.564(6), b=11.232(4), c=20.569(8), α=90°, β=98.210(4)°, γ=90°, V=3 559(2) Å3, C33H40N4O13Zn2, Mr=831.43, Z=4, Dc=1.552 g/cm3, μ=1.419/mm, F(000)=1 720, the final R=0.051 2(6521), wR2=0.126 0(8175), S=1.069, (Δρ)max=1.069 and (Δρ)min=-0.758e/Å3. The selected bond lengths and bond angles are listed in Table 1.

Table 1 Selected bond lengths (Å) and bond angles (°)
3 Result and analysis
3.1 Structure descriptionSingle-crystal X-ray diffraction analysis indicated that the compound crystallized in monoclinicP21/cspace group and featured a 1D framework, showing a binuclear [Zn2(R-COO)2] structure. Each Zn1 was connected with five O atoms to form a tetragonal cone, four of which were from two pyridine tetraacid ligands and one of which was from DMA molecule. Zn2was connected to four O atoms to form a tetrahedral shape, three of which were from two pyridine tetra acid ligands and one of which was from DMA molecule; each H2L2-coordination group was connected to two Zn atoms through two carboxylic acid groups, and μ2-η1∶η1, forming the basic unit of binuclear zinc [Zn2R-COO)4] with symmetrical structure (Fig.1a) and 1D chain structure (Fig.1b). The asymmetric unit contained two Zn(II) ions, a bipolar deprotonated anion H2L2-, three DMA molecules, one free water molecule and one bound water molecule. One dimensional chain structures formed strong hydrogen bonds between O8—H··O9 (O8—H3A··O9=2.66Å, #1:-x+1,-y, -z), forming a 3D network structure diagram (Fig.1c).

Note: a. Coordination environment; b. 1D chain structure; c. 3D network structure.Fig.1 The structures of the Zn-MOF
3.2 Powder XRDThe phase purity of the Zn-MOF was confirmed by PXRD measurement, and each PXRD pattern of the as-synthesized sample was consistent with the simulated one (Fig.2).
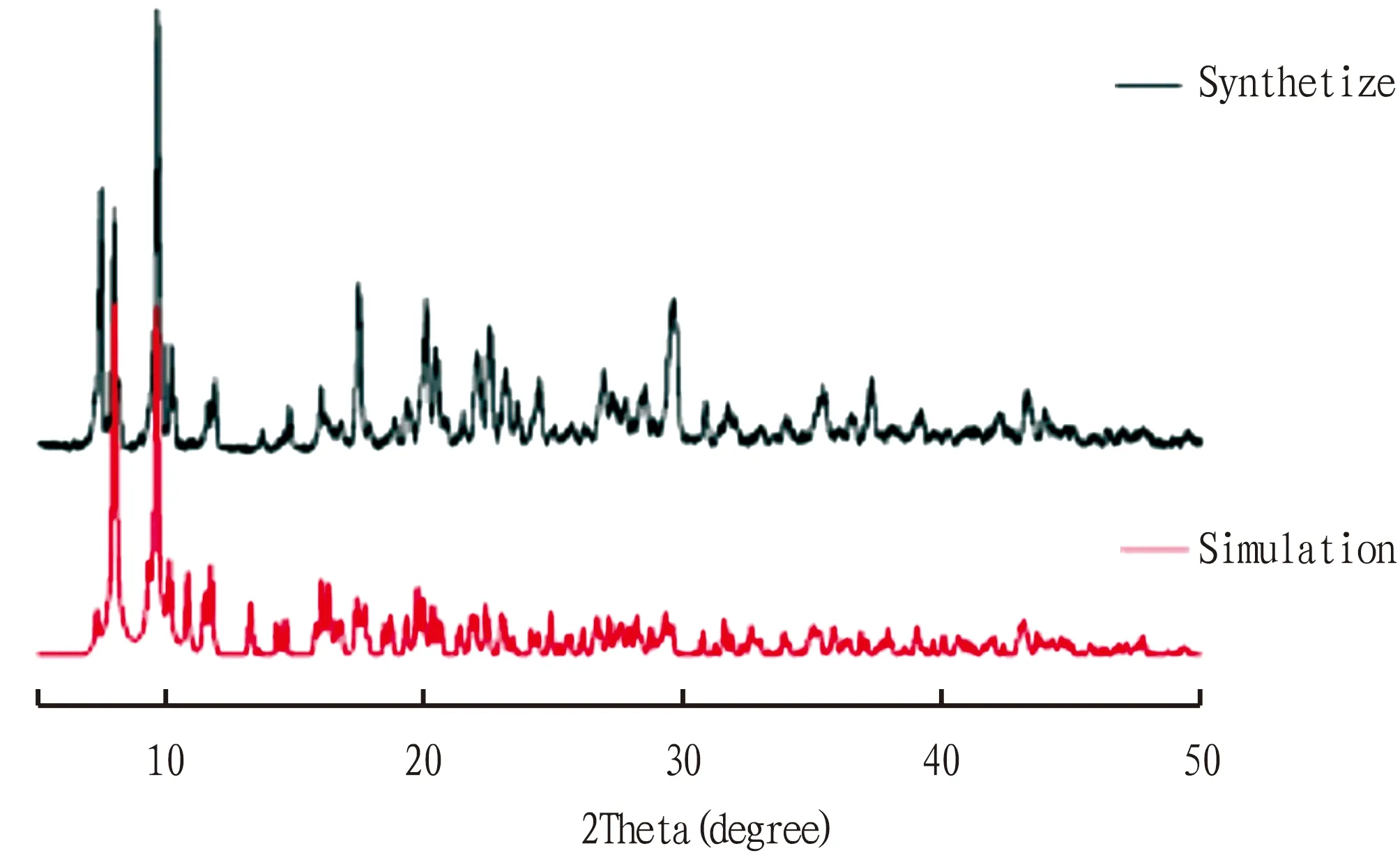
Fig.2 PXRD patterns
3.3 Thermal analysisIn order to investigate the stability of the compound, thermogravimetric analysis (TGA) experiments were carried out under air atmosphere from 30 ℃ to 800 ℃. As shown in Fig.3, the thermogravimetric process was divided into three stages. Firstly, when the temperature raised from 30 ℃ to 150 ℃, the weight loss was 10.2%, which corresponded to the loss of free water. Secondly, when the temperature raised from 150 ℃ to 350 ℃, the weight loss was 30%, and this process corresponded to the loss of DMA molecules in the channel. Finally, when the temperature reached 350 ℃, the weight loss increased sharply, corresponding to the collapse of MOFs material skeleton, and the final residue accounted for 28.1% of the total weight (theoretical value 28.8%). It can be inferred that the final product may be ZnO. Therefore, according to the thermal stability analysis, the temperature should be increased to above 350 ℃ to prepare nano ZnO.
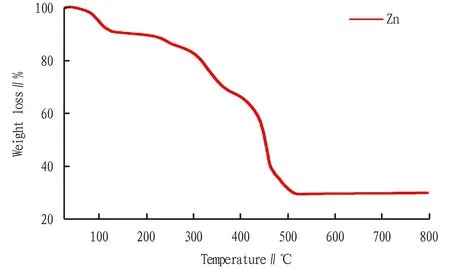
Fig.3 TGA curve
3.4 Solid fluorescenceFig.4a shows the fluorescence emission spectrum of the ligand H4L. According to the analysis, when λEx=367 nm, there was a emission peak at λEm=410 nm. As shown in Fig.4b, when λEx=244 nm, there was a strong emission peak at λEm=394 nm. By comparison, the emission peak of the MOF had a slight blue shift relative to the organic ligand, indicating the interaction of the H4L ligand and metal coordination.

Note: a. H4L ligand; b. Zn-MOF.Fig.4 Solid state fluorescence at room temperature
4 Conclusions
In conclusion, a new Zn(II)-MOF was constructed successfully under solvothermal conditions. The compound exhibits monoclinicP21/cspace group and features a 1D framework based on [Zn2(R-COO)2] group. The TAG investigation shows that the MOF can be stabilized to 350 ℃, and the framework will collapse to form ZnO particles when the temperature exceeds 350 ℃. This study provides a new method for preparing nano ZnO particles. The characterization of its precursor provides the theoretical basis for the preparation conditions of nano ZnO particles. The antibacterial properties of the nano ZnO againstT.paradoxawill be studied in the next step.
