Protective effect of high-oleic acid peanut oil and extra-virgin olive oil in rats with diet-induced metabolic syndrome by regulatingbranched-chain amino acids metabolism
2022-02-15ZHAOZhihaoSHlAiminGUORuiLlUHongzhiHUHuiWANGQiang
ZHAO Zhi-hao,SHl Ai-min,,GUO Rui,LlU Hong-zhi,,HU Hui,WANG Qiang,
1 Institute of Food Science and Technology,Chinese Academy of Agricultural Sciences/Key Laboratory of Agro-Products Processing,Ministry of Agriculture and Rural Affairs,Beijing 100193,P.R.China
2 Collaborative Innovation Center for Modern Grain Circulation and Safety,College of Food Science and Engineering,Nanjing University of Finance and Economics,Nanjing 210023,P.R.China
Abstract High-oleic acid peanut oil (HOPO) and extra-virgin olive oil (EVOO) have been reported previously to have an attenuating effect on metabolic syndrome (MS). This study aimed to evaluate the metabolic effect of HOPO and EVOO supplementation in attenuating MS and the role of gut microbiota in regulating the metabolic profile. Sprague-Dawley rats were continuously fed with a normal diet,high-fructose and high-fat (HFHF) diet,HFHF diet containing HOPO,or a HFHF diet containing EVOO for 12 weeks. The metabolomics profiles of feces and serum samples were compared using untargeted metabolomics based on UPLC-Q/TOF-MS. Partial Least Squares Discriminant Analysis (PLS-DA) was used to identify the potential fecal and serum biomarkers from different groups. Correlation between gut microbiota and biomarkers was assessed,and pathway analysis of serum biomarkers was conducted. Differences in metabolic patterns in feces and serum were observed among different groups. There were 8 and 12 potential biomarkers in feces and 15 and 6 potential biomarkers in serum of HOPO group and EVOO group,respectively,suggesting that HOPO and EVOO supplementation mainly altered amino acids,peptides,and their analogs in feces and serum. The branched-chain amino acids (BCAAs)biosynthesis pathway was identified as a major pathway regulated by HOPO or EVOO. This study suggests that HOPO and EVOO supplementation ameliorate diet-induced MS,mainly via modulation of the BCAAs biosynthesis pathway.
Keywords:metabolic syndrome,high-oleic acid peanut oil,extra-virgin olive oil,metabolomics,UPLC-Q/TOF-MS
1.lntroduction
Metabolic syndrome (MS) is characterized by the cooccurrence of at least three of five clinical disorders:abdominal obesity,dyslipidemia,hypertension,hyperglycemia,and insulin resistance. It is widely acknowledged that MS could raise the risk of many chronic metabolic diseases,such as cardiovascular disease (CVD) and type 2 diabetes (T2DM)(Albertiet al.2006). Patients suffering from MS have twice the death risk and three times the heart attack or stroke risk of those without MS. The incidence rate of MS has been growing;it is estimated that a quarter of people worldwide suffer from MS (Saklayen 2018). Moreover,developing countries face a rapid rise in MS cases,which can be accounted for by dietary habits and lifestyles changes. The current MS prevalence among adults in China is 24.2% and has experienced a huge rise from one decade ago (9.8%) using the same diagnostic criteria(Guet al.2005;Liet al.2018). Accordingly,the global epidemic of MS has already become a huge threat,and it is urgent to find effective intervention strategies.
Virgin olive oil is widely used as the main dietary oil in Mediterranean countries. Importantly,an association has been demonstrated between regular olive oil consumption and a decreased risk of MS (Martínez-González and Martín-Calvo 2013). The benefits of virgin olive oil have been attributed to the high monounsaturated fatty acid (MUFA)content and the bioactive components (Cristinaet al.2013).Similar to olive oil,high-oleic acid peanut oil (HOPO) is also a dietary vegetable oil with a high MUFA content,while the fatty acid profile,composition and content of active ingredients of these two kinds of oil are different. The protective effects of HOPO and extra-virgin olive oil (EVOO) on MS rats have been described in our previous study (Zhaoet al.2019). HOPO and EVOO supplementation significantly suppressed body weight gain,HDL/LDL decrease and insulin resistance process. HOPO and EVOO group showed a 59.83 and 81.08 g less body weight gain,2.21 and 2.45 less HOMA-IR,and 0.55 and 0.25 higher HDL/LDL ratio than M group,respectively. Additionally,16S rDNA sequencing confirmed that HOPO and EVOO supplementation prevented HFHFD-induced gut microbiota disturbance and promoted the proliferation of probiotics (Zhaoet al.2019). However,the metabolic effects of HOPO and EVOO supplementation in alleviating diet-induced MS remain unknown.
Metabolomics is the qualitative or quantitative analysis of all small molecule metabolites in tissues or biological fluids through high-throughput detection technology(Wuet al.2018). It has become a promising analytical technique,widely used in disease diagnosis,toxicity screening,and drug efficacy assessments (Kirbaevaet al.2014;Segerset al.2019). Hence,a comparison of metabolic effects on MS rats that received HOPO or EVOO supplementation was conducted in the present study. The metabolomics profiles of feces and serum samples were compared using untargeted metabolomics based on ultra performance liquid chromatography quadruple/time of flight-mass spectrometry (UPLC-Q/TOF-MS). The aim of the present study was to determine the changes in the feces and plasma metabolome of rats fed with a HOPO and EVOO diet. This study has a significance for understanding the protective mechanism of HOPO and EVOO on MS.
2.Materials and methods
2.1.Materials
HOPO was kindly supplied by Luhua Group Co.,Ltd.(Laiyang,China),while EVOO was purchased from a local supermarket (Mueloliva Co.,Ltd.,Córdoba,Spain).Fatty acid compositions of HOPO and EVOO are shown in Table 1. Fructose was obtained in edible grade from SIWANG SUGAR Co.,Ltd.(Binzhou,China). Chromatographic grade solvents acetonitrile and methanol were obtained from Thermo Fisher Scientific(Beijing,China). Chromatographic grade formic acid was obtained from Sigma-Aldrich (Beijing,China).
2.2.Animals and treatments
Six-week-old male Sprague-Dawley rats were provided by Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China). After acclimation for one week,48 rats,randomly divided into four groups (n=12),were fed withdesigned experimental dietsadlibitumfor 12 weeks:(A)NC group (normal control,normal chow diet and normal water);(B) M group (model,high-fat diet and 10% fructose drinking water);(C) HOPO group (high-oleic acid peanut oil diet,the high-fat diet contained 10% HOPO and 10%fructose drinking water);and (D) EVOO group (extra-virgin olive oil diet,the high-fat diet contained 10% EVOO and 10% fructose drinking water). The diets were supplied by the Laboratory Animal Diet Platform of Trophic Animal Feed High-tech Co.,Ltd.(Nantong,China). The diets’ingredients are shown in Table 2. The rats were kept in a well-ventilated room with a diurnal 12 h light cycle. The room temperature was maintained at (25±2)°C.
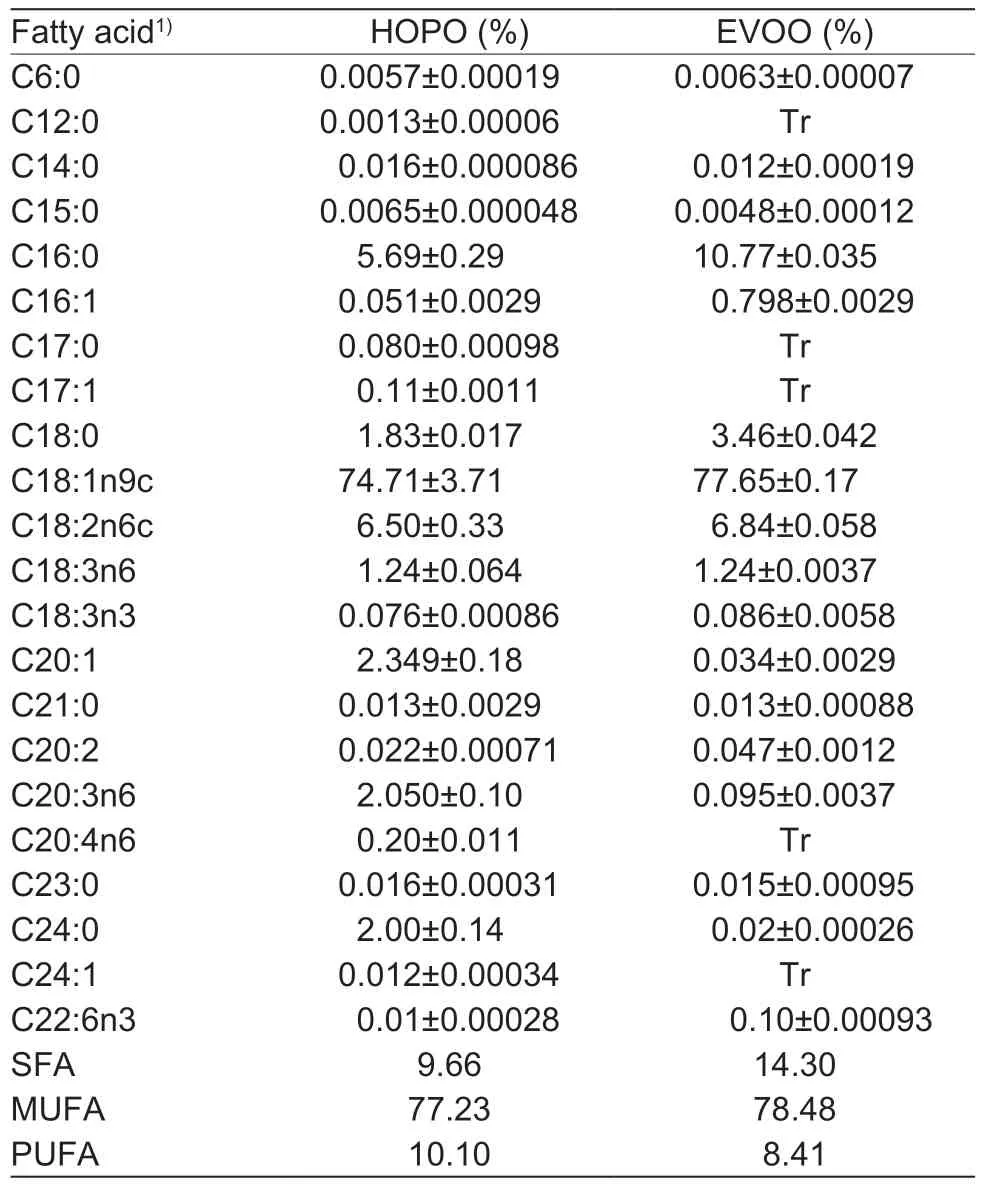
Table 1 Fatty acid profiles of high-oleic acid peanut oil (HOPO)and extra-virgin olive oil (EVOO)
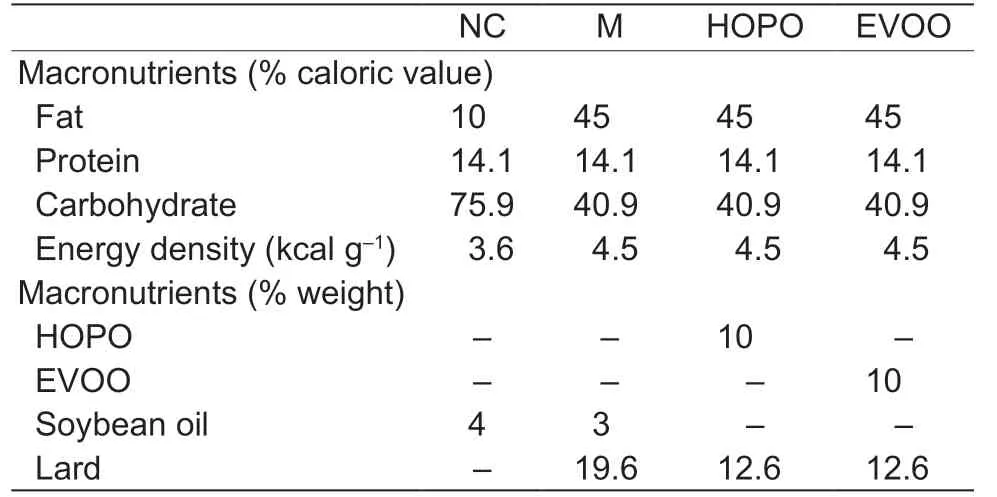
Table 2 Nutritional compositions of experimental diets1)
2.3.Sample preparation
Forty rats (n=10 for each group) were selected randomly for fecal and serum sample preparation. Fresh fecal samples were collected during the final three days.Each fecal sample (100 mg) was mixed with 1.0 mL cold methanol in a 2.0-mL microcentrifuge tube,and ultrasound-assisted extracts were obtained in an ice bath for 60 min. The mixtures were centrifuged for 10 min (4°C,12 000 r min–1). The 800 μL supernatant was transferred and concentrated by vacuum evaporation. Each extract was redissolved in 200 μL methanol and used for HPLCMS. After 12 weeks,rats were anesthetized with sodium pentobarbital (2%,50 mg kg–1) and blood samples were obtained from carotid artery. Then,blood samples were centrifuged at 2 000×g and 4°C for 15 min to collect the serum samples. Each serum sample (200 μL) was mixed with 600 μL cold methanol in a 1.5-mL microcentrifuge tube,then ultrasound-assisted extracted for 30 min. The mixtures were centrifuged for 10 min (4°C,12 000 r min–1).The supernatant was used for HPLC-MS analysis.
2.4.Gut microbiota analysis
Bacterial genomic DNA was extracted from fecal samplesusing a QIAamp DNA Stool Mini Kit from Qiagen (Hilden,Germany) following the manufacturer’s instructions.The V3–V4 variable region was amplified and then assessed by agarose electrophoresis and a nanodrop spectrophotometer for integrity and quantity inspection,respectively.The 16S rDNA was paired-end sequenced and analyzed with the Illumina MiSeq 2500 System by Biomarker Technologies Co.,Ltd.(Beijing,China). The details of gut microbiota sequencing and analysis were described in Zhaoet al.(2019).
2.5.UPLC-Q/TOF-MS analysis
The untargeted metabolomics technique was based on UPLC-Q/TOF-MS. Separation of metabolites was carried out by ultrahigh performance liquid chromatography(UHPLC;Nexera UHPLC LC-30A System,Shimadzu Technologies,Japan) and screened with ESI-MS. The LC system includes a C18 column (Agilent ZORBAXRRHD Eclipse Plus,100 mm×3 mm,1.8 mm). The mobile phase was a mixture of solvent A (0.1% CH3COOH-H2O solution)and solvent B (pure acetonitrile) with gradient elution (0–0.5 min,5% A and 95% B;0.5–30 min,5–95% A and 95–5% B;30–33 min,95% A and 5% B;33–35 min,95–5%A and 5–95% B). The flow rate of the mobile phase was 0.5 mL min–1. The temperature of the column and sample manager was set at 45 and 4°C,respectively. The quadrupole time of flight mass spectrometer (Q/TOF-MS,G6540B,Agilent Technologies Inc.,USA) equipped with a Dual Agilent Jet Stream ESI source was used for mass spectrometry. The scanning range of mass-to-charge (m/z)was from 50 to 1 500. The operating parameters were as follows:capillary voltage in positive mode,4 000 V;capillary voltage in negative mode,3 500 V;fragmentor voltage,175 V;nebulizer pressure,35 psi;drying gas temperature,325°C;drying gas flow rate,5 L min–1. The instrument mode was set to an extended dynamic range.The quality control (QC) samples consisted of mixed fecal samples and mixed serum samples. One QC sample was injected after every five individual sample runs.
2.6.Statistical analysis
The Mass Hunter Workstation (Agilent Technologies,USA) was used to obtain and align raw data based on them/zvalue and the retention time of ion signals.Raw data was imported into MS-DIAL Software,and data preprocessing of peak extraction,noise reduction,deconvolution,and peak alignment was conducted by MS-DIAL (Tsugawaet al.2015). Then,the detected peak information was compared with three databases including MassBank,Respect and GNPS to identify metabolites. There were 339 and 205 metabolites identified in feces and serum,respectively. The data were normalized using R 3.5.2 Software and Metnormalizer package based on the QC samples (Shenet al.2016).The partial least squares discriminant analysis (PLSDA) was used to identify potential biomarkers. The screening criteria for biomarkers consisted of Student’st-test (P<0.05) and variable importance in projection(VIP) values (>1.0). Then,enrichment and pathway analyses based on the KEGG database were performed.Identification of potential biomarkers and enrichment and pathway analyses were conducted using online software MetaboAnalyst 4.0 (http:www.metaboanalyst.ca) (Chonget al.2018). Finally,the biomarkers data of 40 samples in four groups and their corresponding gut microbiota data (in phylum level and top 10 genera) were used in Spearman correlation analysis.
3.Results
3.1.Metabolic profiling of feces samples
PLS-DA is a multivariate data analysis method with a supervised mode,which can reduce the matrix’s dimension and provide a preliminary understanding of the main patterns and trends of the data. The PLS-DA analysis of metabolites profile in feces samples is shown in Fig.1,and Mvs.NC,HOPOvs.M and EVOOvs.M were compared,respectively.All groups were significantly separated,as shown in the PLS-DA scores plot. These results suggested that different diets induced different fecal metabolic profiles. HFHFD with different dietary fat proportions also induced different fecal metabolic profiles,while the nutritional compositions of the three HFHFDs were the same.
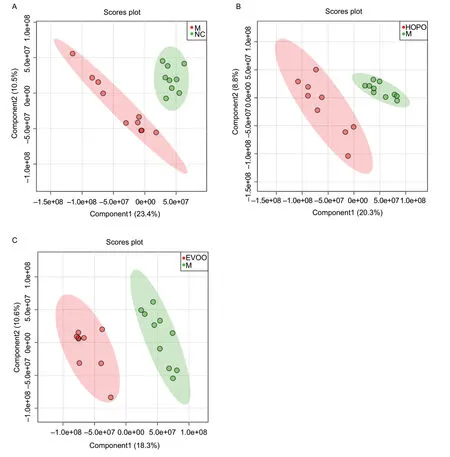
Fig.1 The partial least squares discriminant analysis(PLS-DA) score scatter plots based on UPLC-Q/TOF-MS spectra of feces distinguishing between M vs.NC,HOPO vs.M and EVOO vs.M. M,model group;NC,normal control group;HOPO,high-oleic acid peanut oil group;EVOO,extra-virgin olive oil group. The cross-validation of PLS-DA model indicate high accuracy of Q2=0.76,0.74 and 0.65,respectively.
3.2.Metabolic profiling of serum samples
The PLS-DA analysis of metabolites profile in serum samples is shown in Fig.2,and Mvs.NC,HOPOvs.M and EVOOvs.M were compared,respectively. All groups were significantly separated,as shown in the PLS-DA scores plot. These results suggested that different diets induced different serum metabolic profiles. Similar to metabolic profiling of feces mentioned above,HFHFD with different dietary fat proportions induced different serum metabolic profiles,although the nutritional compositions of the three HFHFDs were the same.
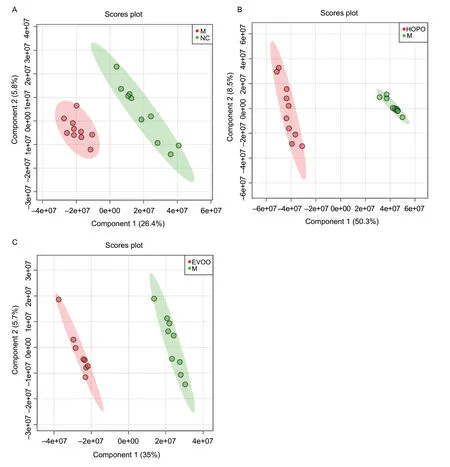
Fig.2 The partial least squares discriminant analysis(PLS-DA) score scatter plots based on UPLC-Q/TOFMS spectra of serums distinguishing between M vs.NC,HOPO vs.M and EVOO vs.M. M,model group;NC,normal control group;HOPO,high-oleic acid peanut oil group;EVOO,extra-virgin olive oil group. The crossvalidation of PLS-DA model indicate high accuracy of Q2=0.62,0.98 and 0.94,respectively.
3.3.ldentification of potential biomarkers in feces
A total of 32 metabolites were selected as potential biomarkers,of which 12,8 and 12 metabolites were between NCvs.M,HOPOvs.M and EVOOvs.M,respectively (Table 3).Glycerophospholipids (LPC 18:2,phosphatidylethanolamine alkenyl 16,phosphatidylethanolamine alkenyl 18,phosphatidylethanolamine 16 and phosphatidylcholine 15),steroids and derivatives (deoxycholic acid,dehydrocholic acid and cholic acid),and amino acids,peptides,and analogues (glutamic acid and glycyl-L-proline)were the major compound categories between NCvs.M (5,3 and 2 of 12 metabolites,respectively).Glycerophospholipids (phosphatidylethanolamine alkenyl 16,phosphatidylethanolamine alkenyl 18,phosphatidylethanolamine 16,phosphatidylcholine 15 and phosphatidylcholine alkyl 16),and amino acids,peptides,and analogues (valine and glycyl-Lproline) were the major compound categories between HOPOvs.M (5 and 2 of 8 metabolites,respectively).Glycerophospholipids (phosphatidylethanolamine alkenyl 16,phosphatidylethanolamine alkenyl 18,phosphatidylethanolamine 16 and phosphatidylcholine 15),amino acids,peptides,and analogues (proline,valine,citrulline and glutathione (reduced)),and fatty acids and derivatives (oleic acid,FA 18:4+1O and FA 18:0+2O+SO4) were the major compound categories between EVOOvs.M (4,4 and 3 of 12 metabolites,respectively).
The relative abundance of glycyl-L-proline,phosphatidylethanolamine alkenyl 16,phosphatidylethanolamine alkenyl 18 and phosphatidylcholine 15 significantly increased (P<0.05) in M compared to NC. The supplement of HOPO reversed this in cremental change.Similarchangeswere observed for phosphatidylethanolamine alkenyl 16,phosphatidylethanolamine alkenyl 18 and phosphatidylcholine 15 in EVOO compared to M. Valine,one amino acid of branched-chain amino acids (BCAAs) (leucine,isoleucine and valine),was a potential biomarker in both HOPOvs.M and EVOOvs.M. The relative level of valine in HOPO or EVOO increased significantly compared with that in M. However,oleic acid was identified as potential biomarker only between EVOOvs.M,although most HOPO-diets contain some level of oleic acid.
3.4.ldentification of potential biomarkers in serum
A total of 33 metabolites were selected as potential biomarkers,of which 12,15 and 6 metabolites were between NCvs.M,HOPOvs.M and EVOOvs.M,respectively (Table 4). Amino acids,peptides,and analogues (valine,threonine,leucine,glutamic acid and gamma-Glu-Tyr) and glycerophospholipids(LPC 18:2,phosphatidylethanolamine lyso 20,phosphatidylcholine lyso 18 and phosphatidylcholine 15) were the major compound categories between NCvs.M (5 and 4 of 12 metabolites,respectively). Amino acids,peptides,and analogues (proline,isoleucine,leucine,lysine,methionine,phenylalanine,arginine,glutamine and Ala-Ile),glycerophospholipids (LPE 16:0,phosphatidylinositol lyso 18 and phosphatidylinositol lyso 20) and pyrimidine nucleosides (2´-deoxycytidine and 2´-o-methylcytidine) were the major compound categories between HOPOvs.M (9,3 and 2 of 15 metabolites,respectively). Amino acids,peptides,and analogues (leucine,Ala-Ile and folic acid) were the major compound categories between EVOOvs.M (3 of 6 metabolites). Amino acids,peptides,and analogues altered in all of three comparisons.
It is worth noting that BCAAs and oleic acid were identified as potential biomarkers among NCvs.M,HOPOvs.M and EVOOvs.M. The relative level of BCAAs potential biomarkers in HOPO or EVOO increased significantly compared with that in M,while HFHFD induced a significantly lower relative abundance of BCAAs potential biomarkers (leucine and valine). Among three BCAAs,leucine was the only one potential biomarker in NCvs.M,HOPOvs.M and EVOOvs.M. Compared with HOPOvs.M,the potential biomarker among EVOOvs.M was very small (15vs.6),which might indicate that the metabolic profiling of serum was more similar between EVOO and M than between HOPO and M.
3.5.Spearman correlation between gut microbiota and potential biomarkers in feces
A total of 13 correlations were detected at the phylum level between gut microbiota and potential fecal biomarkers (Table 5). These correlations included 5 phyla and 11 potential biomarkers. The top 10 genera were selected to analyze the correlation between gut microbiota and potential biomarkers in feces at the genus level. A total of 22 correlations were detected between bacterial genera and potential fecal biomarkers(Table 5). These correlations included 7 bacterial genera and 13 potential biomarkers. These biomarkers mainly included glycerophospholipids,amino acids and their derivatives in correlations between potential fecal biomarkers and gut microbiota (both at phylum level and genus level).
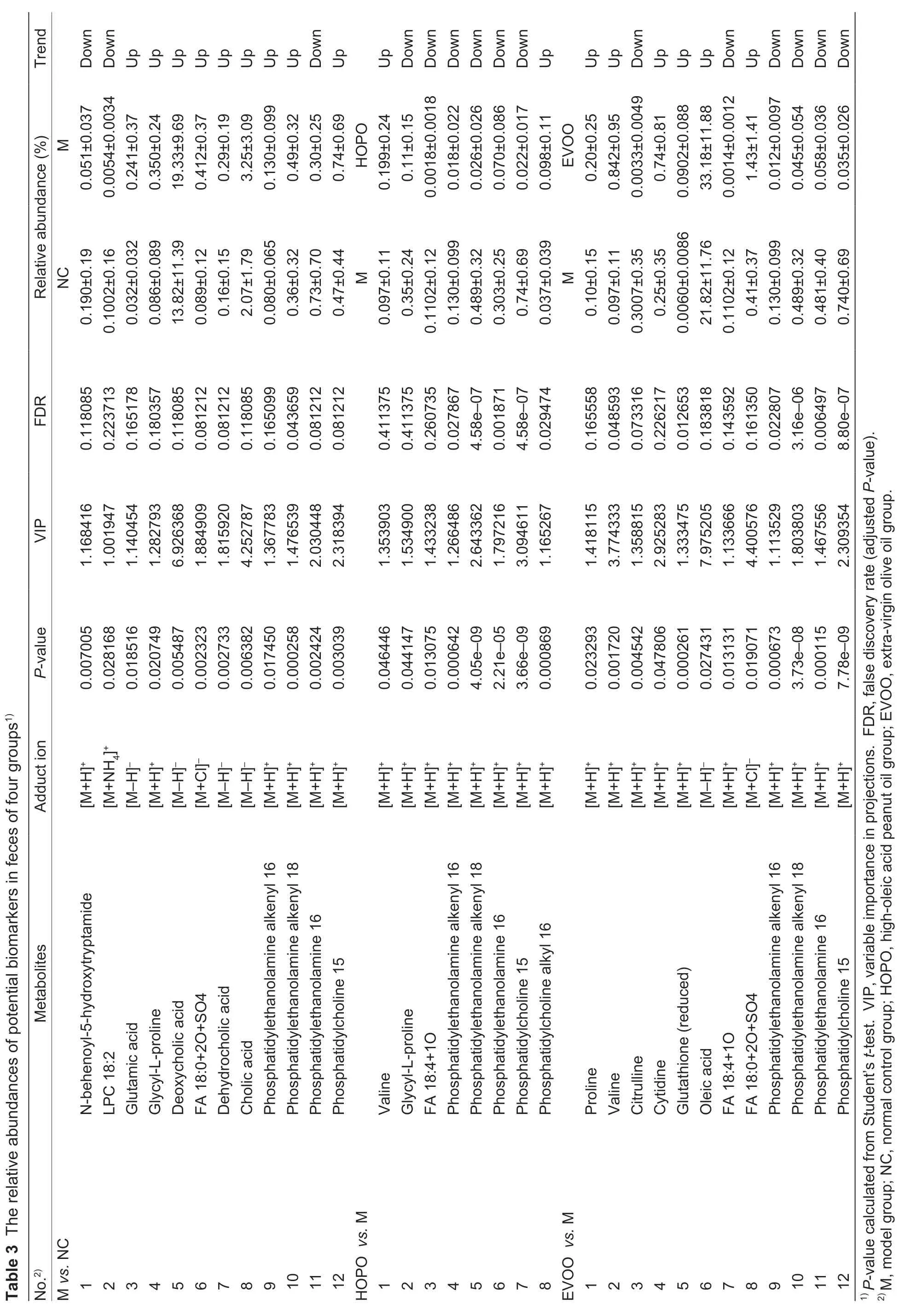
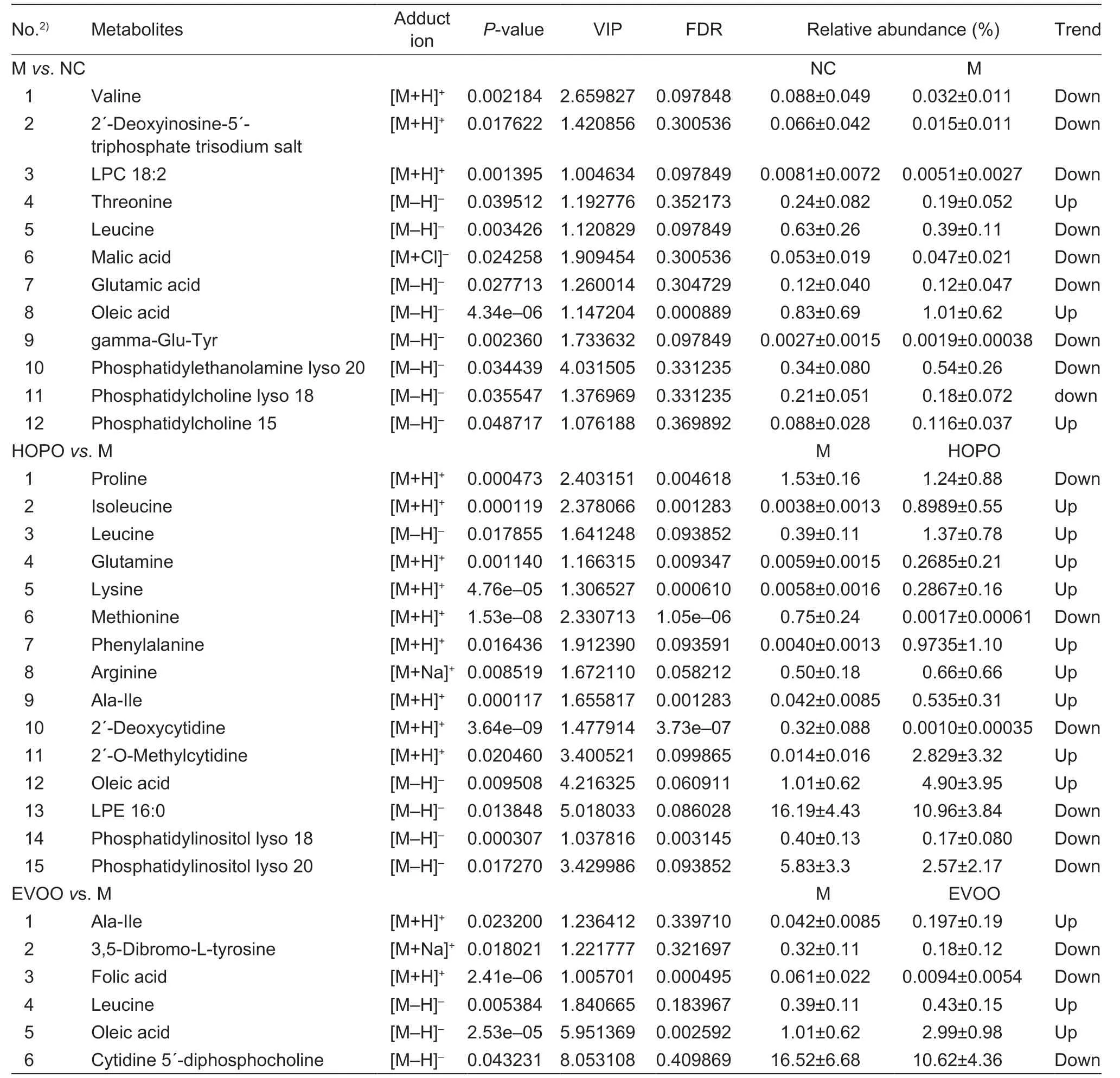
Table 4 The relative abundances of potential biomarkers in serum of four groups1)
3.6.Spearman correlation between gut microbiota and potential biomarkers in serum
As shown in Table 6,a total of 8 correlations were detected at the phylum level between gut microbiota and potential biomarkers in serum,which included 3 phyla and 8 biomarkers in serum. A total of 28 correlations were found between top 10 bacterial genera and potential fecal biomarkers. These correlations included 7 bacterial genera and 19 potential biomarkers. Among these biomarkers,amino acids and their derivatives including three kinds of BCAAs accounted for a large proportion. Correlations between gut microbiota and amino acids biomarkers were more obvious in serum than in feces.
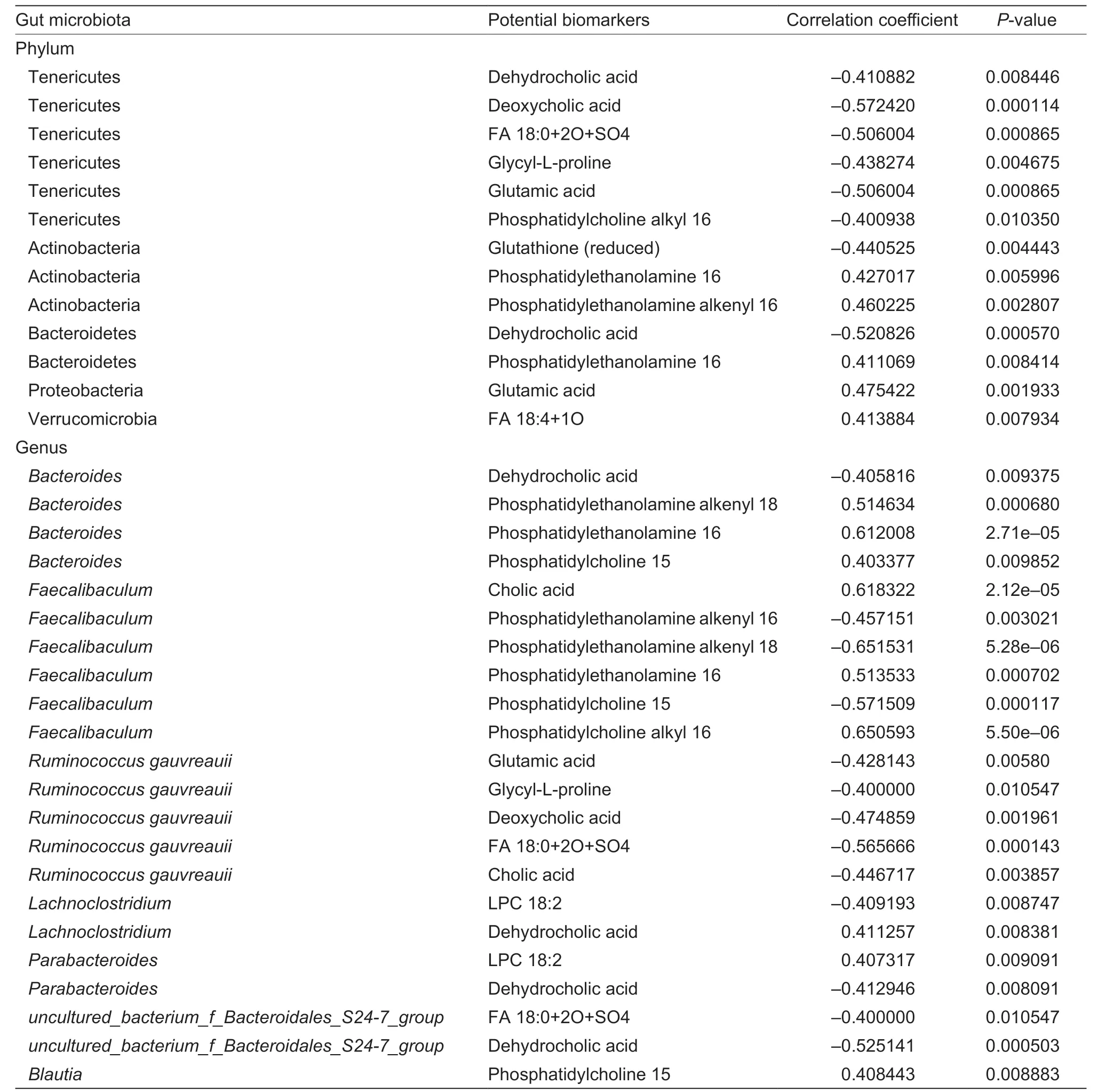
Table 5 The Spearman correlation analysis between relative abundances of gut microbiota at phylum level,top 10 genera and potential biomarkers in feces

Table 6 The Spearman correlation analysis between relative abundances of gut microbiota at phylum level,top 10 genera and potential biomarkers in serum1)
3.7.Pathway analysis of metabolites in serum
All 33 biomarkers were imported to conduct pathway enrichment and analysis of metabolites in the serum.In the present study,0.10 was set as the threshold of pathway impact. Significantly enriched pathways withP-values above this threshold were identified as differential metabolic pathways between the two groups.As shown in Fig.3,three differential pathways (D-glutamine and D-glutamate metabolism pathway,valine,leucine and isoleucine biosynthesis pathway,and alanine,aspartate and glutamate metabolism pathway) were identified during the comparison of M and NC groups.Moreover,five differential pathways (valine,leucine and isoleucine biosynthesis pathway,phenylalanine,tyrosine and tryptophan biosynthesis pathway,phenylalanine metabolism pathway,alanine,aspartate and glutamate metabolism pathway,and arginine and proline metabolism pathway) were identified between HOPO and M groups.Only one differential pathway (Valine,leucine and isoleucine biosynthesis pathway) was identified between EVOO and M groups. Interestingly,Valine,leucine and isoleucine biosynthesis pathway was found in Mvs.NC,HOPOvs.M and EVOOvs.M. Accordingly,these results illustrated that amino acid biosynthesis and metabolismrelated pathways were closely related with MS. The valine,leucine and isoleucine biosynthesis pathway(BCAAs biosynthesis pathway) has huge prospects as a potentially key target pathway.

Fig.3 Pathway impact analysis based on biomarkers in serum. A,M vs. NC. B,HOPO vs.M. C,EVOO vs.M. NC,normal control group;M,model group;HOPO,high-oleic acid peanut oil group;EVOO,extra-virgin olive oil group.
4.Discussion
Metabolic syndrome is one of the most serious global public health challenges of the 21st century,contributing to the occurrence and development of diverse serious metabolic diseases,including CVD and T2DM (Albertiet al.2006;O’Neill and O’Driscoll 2015). According to a study from the Diabetes UK-James Lind Alliance Priority Setting Partnership,the role of dietary fats in the management of metabolic diseases has become one of the top ten research priorities (Fineret al.2017). Unhealthy eating patterns are one of the leading pathogeneses of MS. Both the source and amount of dietary fat intake can affect the phenotype of MS(Clifton 2019). As a main feature of the Mediterranean diet,olive oil is rich in oleic acid and widely regarded as healthy cooking oil. Oleic acid,known as a healthy fatty acid,has basic nutritional functions and health benefits,including anti-inflammation,anti-hypertension,cardiovascular disease and diabetes prevention(Sales-Campos 2013). Previous study has found that HOPO and EVOO could alleviate HFHFD-induced MS and significantly inhibit obesity and insulin resistance,which is involved in regulating gut microbiota (Zhaoet al.2019). However,the metabolic mechanism of HOPO and EVOO supplementation in attenuating diet-induced MS remains unclear.The present study investigated the metabolic effects of HOPO and EVOO supplementation in alleviating MS in rats. It was found that 32 and 33 differential metabolites were involved in many metabolic pathways identified in feces and plasma of rats,respectively. Regulation of the BCAAs metabolic pathway was identified to play a crucial role in alleviating MS after HOPO and EVOO supplementation.
Interestingly,it was found that major metabolic changes associated with insulin sensitivity did not involve lipid metabolism but amino acid metabolism,especially BCAAs,aromatic amino acids (phenylalanine tyrosine),glutamic acid,glutamine,and alanine (Zhaoet al.2016). Several case-control and cross-sectional studies from various geographic and ethnic groups have indicated that BCAAs and their related metabolites are closely related to insulin resistance (Tillinet al.2015;Chenet al.2016). BCAAs,including leucine,isoleucine,and valine,are special amino acids containing branched side chains at a carbon atom. These three essential amino acids act as important nutritional,metabolic signal molecules in the body. Previous studies have shown that BCAAs helped control body mass,promoted muscle protein synthesis and maintained blood glucose homeostasis (Wanget al.2011).However,some studies have found that an increase in BCAA was closely related to increased insulin resistance and T2DM risks in human and rodent animals (Giesbertz and Daniel 2015;Cummingset al.2018). Therefore,the effect of BCAAs on metabolic disorder diseases,such as diabetes,obesity and MS,is still controversial.The present study found that HFHFD induced a decreased abundance of BCAAs,and HOPO and EVOO supplementation reversed this decline in BCAAs. It also found differences in Valine,leucine and isoleucine biosynthesis pathway (BCAAs biosynthesis pathway) in Mvs.NC,HOPOvs.M and EVOOvs.M. This trend was consistent with changes in the relative abundance of BCAAs. The occurrence and development of diet-induced MS and the preventive effect of HOPO and EVOO supplementation were associated with the BCAAs biosynthesis pathways.
BCAAs depend mainly on food intake and the degradation of proteins in tissues. It has been found that the dietary protein intake of insulin-resistant patients was comparable to that of insulin-sensitive people,which indicated that other mechanisms might be responsible for the abnormal increase in BCAAs levels (Bloomgarden 2018). However,the underlying mechanisms are unknown,warranting further investigation. In the present study,the calorie proportions of protein in different diets were the same. An increasing body of evidence suggests that gut microbiota play a fundamental and crucial metabolic function in nutrients processing and maintaining host health (Clementeet al.2012;Henao-Mejiaet al.2012;Marcinkevicius and Shirasu-Hiza 2015;Nehraet al.2016). Accordingly,the present study hypothesized that gut microbiota could regulate BCAAs biosynthesis and metabolism and attenuate HFHFD-induced MS in rats.Spearman correlation analysis showed that at phylum level,Tenericutes was positively correlated with serum Leucine levels,while Bacteroidetes showed a negative correlation with serum Isoleucine levels. Moreover,more correlations between gut microbiota and amino acids levels including three BCAAs in serum were observed at genus level. These correlations provided indirect evidence that BCAAs biosynthesis and metabolism are regulated by gut microbiota in HFHFD-fed rats.
Lipids and their derivatives disturbed greatly among different groups. Lipid metabolism disorder is one of the main symptoms and triggers of MS,and multiple glycerophospholipids are considered as prominent biomarkers of MS (Comteet al.2021).Glycerophospholipid metabolism pathway is observed as the most relevant pathway for lipid metabolisms in T2DM patients (Yanet al.2021). The role of interaction between glycerophospholipids and gut microbiota in metabolic health may be underestimated (Penget al.2020;Gaoet al.2021). Spearman correlation analysis also showed a link between gut microbiota and level of lipids and their derivatives in this study. The different fatty acid and functional ingredient composition of fats restricted the in-depth evaluation of the role of gut microbiota in glycerophospholipid metabolism.
The past decades have seen great achievements in the breeding of high-oleic acid peanuts (Janilaet al.2015).High-oleic acid peanuts have been documented to have stronger oxidation stability,higher nutritional value,better flavor,and greater health benefits than traditional peanuts(Vassiliouet al.2009;Riveroset al.2010;Barbouret al.2017;Limet al.2017;Paulaet al.2018). High-oleic acid peanuts are mountingly popular globally and are expected to replace ordinary peanuts in the future. HOPO has similar or even higher MUFA levels than olive oil and is rich in minor bioactive components,including phytosterols and polyphenol. It is worth mentioning that peanut is one of the most important oil plants globally and is cultivated more widely than olives (Wang 2017). Research on the health efficacy of HOPO is important to provide evidence for consumers to select HOPO as an economically healthy fat source,especially in regions unsuitable for olive planting. In summary,HOPO has huge prospects as a healthy dietary fat source.
5.Conclusion
This study investigated the metabolic effects of HOPO and EVOO supplementation on attenuating MS,which mainly involved the BCAAs biosynthesis pathway. Given the limitations in this study,further research exploring different dietary interventions in humans are essential to substantiate the efficacy of HOPO consumption on MS prevention. Moreover,significant correlations were found between gut microbiota and amino acid metabolism,especially BCAAs and aromatic amino acids. Furtherinvitroandinvivostudies are required to check the robustness of the findings.
Acknowledgements
This research was supported by the Agricultural Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-201XIAPPST),the Top Young Talents of Grain Industry in China (LQ2020202),and the National Natural Science Foundation of China(32172149).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal-related procedures were checked and approved by the Institutional Animal Care and Use Committee of Beijing Vital River Laboratory Animal Technology Co.,Ltd.(approval number:P2018036).
杂志排行
Journal of Integrative Agriculture的其它文章
- First record of the golden potato nematode Globodera rostochiensis in Yunnan and Sichuan provinces of China
- Current state and suggestions for mechanical harvesting of corn in China
- Transcriptome and phytochemical analyses reveal the roles of characteristic metabolites in the taste formation of white tea during the withering process
- The ciliate protozoan Colpoda cucullus can improve maize growth by transporting soil phosphates
- Interactions between phosphorus availability and microbes in a wheat–maize double cropping system:A reduced fertilization scheme
- Dynamics of organic carbon and nitrogen in deep soil profile and crop yields under long-term fertilization in wheat-maize cropping system
