The ciliate protozoan Colpoda cucullus can improve maize growth by transporting soil phosphates
2022-02-15ZHANGWenliLINQimeiLIGuitongZHAOXiaorong
ZHANG Wen-li,LIN Qi-mei,LI Gui-tong,ZHAO Xiao-rong
College of Land Science and Technology,China Agricultural University,Beijing 100193,P.R.China
Abstract Little is known regarding the ability of protozoans to transfer phosphates and improve maize growth. The objective of this study was to determine whether Colpoda cucullus could improve the maize phosphorus (P) level by transferring phosphate. In this three-compartment root box study,the soil in the outer compartment was inoculated with the common ciliate,C.cucullus,together with the addition of either KH232PO4,rock phosphate (RP),super phosphate (SP)or ammonium phosphate (AP),and then maize was grown in the inner compartment. The results showed that the maize plants grown in the soil inoculated with C.cucullus had much higher32P radioactivity than the control. Colpoda cucullus inoculation resulted in significant increases in dry matter by up to 25.07%,and nitrogen (N),P and potassium (K)absorption by 1–36% (P<0.05). Soil available P in the inner compartment of the root box was also enhanced by at least 30% due to the ciliate inoculation (P<0.05). It was therefore suggested that phosphates might be transported from the outer to inner compartments by the inoculated C.cucullus and then absorbed by the maize plant.
Keywords:Colpoda cucullus,root box,32P,phosphate fertilizer,maize
1.Introduction
Protozoa,as the keystone organisms in soil ecosystems,are at a pivotal position within the soil food web (Geisenet al.2017). They can regulate essential processes of soil fertility,such as nutrient cycling and plant growth (Abrahamet al.2019). Inoculation with protozoa often induces rapid mineralization of organic nitrogen (N) and phosphorus(P) nutrients and enhances soil nutrition bioavailability(Freyet al.2001;Sunet al.2003a). Their predation can alter the structure and activity of microbial communities,and thus enhance the survival of beneficial microbes that suppress pathogens (Bonkowski 2004;Weidneret al.2017). Some protozoa may stimulate the production of plant growth hormones (Kromeet al.2010) and the compounds related to disease suppression,such as antibiotics (Jousset and Bonkowski 2010) or siderophores(Levratet al.1989).
It is believed that most of these functions are related to the mobility of protozoa in soil. Protozoa can move along pore water membranes by even several centimeters per day (Adl 2007). The movement of protozoa may be conducive to microbial dissemination and by increasing contact with organic matter they may facilitate its decomposition (Crottyet al.2012).Ammonium added into surface soil can be translocated to the subsoil by inoculated flagellates (Verhagenet al.1993). Three mechanisms might be involved in this ammonium redistribution within the soil pedon. Firstly,flagellate motion improves soil pore conditions and thereby facilitates diffusion of ammonium ions. Secondly,flagellates can absorb ammonium and then move down into the deep soil. Thirdly,flagellates lead to downward movement of microbial biomass N.
It is widely known that soils often have a very low concentration of phosphate,usually less than 1 mg L–1,because of their strong fixation for phosphates (Gérard 2016). Most plants largely rely on root interception to obtain phosphates,while mass flow and ion diffusion contribute little to P uptake (Ravenet al.2018). As a result,P usually becomes a limiting factor for most plant production (Kochian 2012). It is therefore a great challenge to develop novel measures for improving plant P levels.
A variety of measures,such as soil improvement(Liet al.2016),breeding (van de Wielet al.2016),phosphate-dissolving microbes (Bargazet al.2018) and others,have been used for enhancing soil P availability and improving plant P levels. Of these measures,both ecto-and endo-mycorrhizal hyphae can not only enhance plant phosphate uptake,but also transfer polyphosphate from bulk soil to the roots (Ergin and Gulser 2016).Protozoa can move toward the rhizosphere (Sunet al.2003b),but it is not known whether the motion of protozoa can bring phosphate from bulk soil to the rhizosphere and then improve the plant P level.
Ciliates are considered to be the most complex and highly differentiated unicellular eukaryotes,with a short generation time,rapid reproduction and high respiration (Liet al.2010;Ninget al.2016). They are widely distributed in soils (Ninget al.2016). Around 39% of ciliate species feed on bacteria and 34% feed on other microbes,while 20% are omnivorous (Slavaet al.1992;Foissner 1999).As an important group in the soil food web,ciliates play multiple important roles in energy flow and nutrient circulation of the soil ecosystem (Janssen and Heijmans 1998;Christoffersen and Gonzalez 2003;Liet al.2010).
Colpodacucullusis a typical ciliate which is widely found in soils (Akematsu and Matsuoka 2008) and it tends to be enriched in the rhizosphere. However,so far it is not known whetherC.cuculluscan transport phosphate from bulk soil to the rhizosphere. Herein we tested the following hypotheses thatC.cuculluscould transfer nutrients (like P) from bulk soil in the outer compartment of a root box to the root zone in the inner compartment of the root box and thereby improve the maize P level and enhance maize dry matter. The capacity of P transport may depend on the added phosphates. This is the first study to explore the association of ciliate movement with phosphate translocation and the improvement of the plant P level. The aim was to find direct evidence showing the32P-labelled phosphate movement and subsequent absorption by the maize plant.
2.Materials and methods
2.1.Soil
A silt loam soil (Alfisol) was collected from the A horizon(about 0–20 cm) of forest land in Beijing Baiwangshan Mountain. The soil pH was 7.2,organic matter was 14.40 g kg–1,total N was 0.78 g kg–1,available P was 0.34 mg kg–1,microbial biomass carbon (MBC) was 71.41 mg kg–1,flagellates was 1 140 g–1,ciliates was 1 400 g–1and amoebas was 14 500 g–1. Plant residues and gravel were removed from the soil which was then sifted through a 2-mm screen. The fresh soil was adjusted to about 60%of water-holding capacity and then stored at 4°C prior to use.
2.2.Root box
The root box was made with a grey plexiglass sheet that was 15 cm high,21 cm long and 10 cm wide (Fig.1). It consisted of three compartments which were separated by 50 μm nylon mesh in order to block maize root penetration(usually >100 μm in diameter) while allowingC.cucullusto move through the net. The inner compartment was 5 cm wide,while both outer compartments were 8 cm wide. The32P-labelled phosphate was amended at 5 cm from the end of the outer compartment.

Fig.1 The three-compartment root box of 15 cm high,21 cm long and 10 cm wide,was prepared from a grey plexiglass sheet. The three compartments were separated by 50-μm nylon net.
2.3.Protozoans
Colpoda cuculluswas isolated from the cropland soil at Science and Technology Park of China Agricultural University. A total of 10 mL of the soil suspension (1:10 soil to sterilized water,w/v) was added into 100 mL dry grass extract and then incubated at 25°C for 4 days.The cells ofC.cuculluswere collected by a capillary pipette (about 20 μL) under an inverted microscope and incubated again as above for 2 days. This procedure was repeated until onlyC.cuculluscells were obtained (Yin 1998).
2.4.Maize seed
Maize seed (ZeamaysL.) was firstly sterilized with 10%H2O2for 10 min and then rinsed five times with sterilized water. The seeds were germinated in sterile Petri dishes at 25°C for 3 days.
2.5.Root box incubation test with KH232PO4
Two treatments ofC.cucullusinoculation and a control with no inoculation (with the same volume of filtered suspension),each treatment with three replicates,were involved in this test. The bulk of the fresh soil was thoroughly mixed with corn straw powder (8 g kg–1soil),urea (0.2 g N kg–1soil) and rock phosphate (RP)(50 g kg–1soil),and then inoculated withC.cucullus(2.8×103cells g–1soil). The soil was carefully used to fill the outer compartments of the root box layer by layer(each time about 1 cm). The KH232PO4-labelled soil(7 400 kBq kg–1soil) was filled into a 2 mm thick layer at 5 cm away from the end of both outer compartments(Fig.1). Only urea was added to the inner compartment soil. One well-germinated maize seed was planted in the inner compartment and then incubated in a glasshouse under natural autumn conditions for 2 months. The aboveground plant was tightly attached to a Kodak film and kept in a dark chamber for 10 days. The32P radiation intensity in the inner compartment soil of the root box was also measured by a double channel liquid scintillation counter (FJ-2101,Xi’an 262 Factory,China).
2.6.Root box incubation test with different phosphate fertilizers
Two sets ofC.cucullusinoculations and a control noninoculation (with the same volume of filtered suspension)were implemented in this test. Each set had four treatments of the addition of either RP (50 g kg–1soil),super phosphate (SP) (10 g kg–1soil),ammonium phosphate (AP) (5 g kg–1soil) or no phosphate (CK). All treatments were replicated three times,totally 24 root boxes. As described above,bulk fresh soil was thoroughly mixed with corn straw powder (8 g kg–1soil),urea (0.2 g N kg–1soil) and one of the three phosphates. Following inoculation withC.cucullussuspension (2.8×103g–1soil),the soils were used to fill the two outer compartments of the root box layer by layer. Only urea was added to the inner compartment soil. One well-germinated maize seed was planted in the inner compartment and then incubated in a glasshouse under natural autumn conditions for 2 months. The aboveground plant was measured for dry matter,N,P and potassium (K). The levels of soil available phosphorus in both outer and inner compartments were also determined.
2.7.Assays
Soil pH were determined in a suspension of 1:2.5 soil to water (w/v) by a glass electrode. Organic matter and total N were assayed by the potassium dichromate oxidation method and Kjeldahl method,respectively. Soil available P was quantified by the Olsen method. MBC was estimated by a fumigation-extraction method (Lu 1999).The contents of N,P and K in the plants were determined by the H2SO4-H2O2digestion method. The total numbers of flagellates,ciliates and amoebas in the original soil were measured by a glass ring culture counting method(Ning and Shen 1996). The number ofC.cucullusin the culture suspension was directly counted under an inverted microscope (Guoet al.2001).
2.8.Data processing and statistical analysis
The differences amongC.cucullusinoculation and phosphate treatments were tested by a two-way analysis of variance (ANOVA) with the software of SPSS 22. A significant difference was determined by Duncan multiple comparisons atP<0.05.
3.Results
3.1.32P autoradiographs
The autoradiographs showed that a large amount of32P-labelled phosphate was absorbed by the maize plants grown in the soil inoculated withC.cucullus(Fig.2). WhenC.cuculluswas inoculated in the outer compartments,the soil in the inner compartment had a32P radioactivity of 10.69 kBq kg–1,10 times higher than the control without inoculation (1.02 kBq kg–1),which indicated that much of the32P-labelled phosphate was transferred from the outer compartment to the inner compartment.
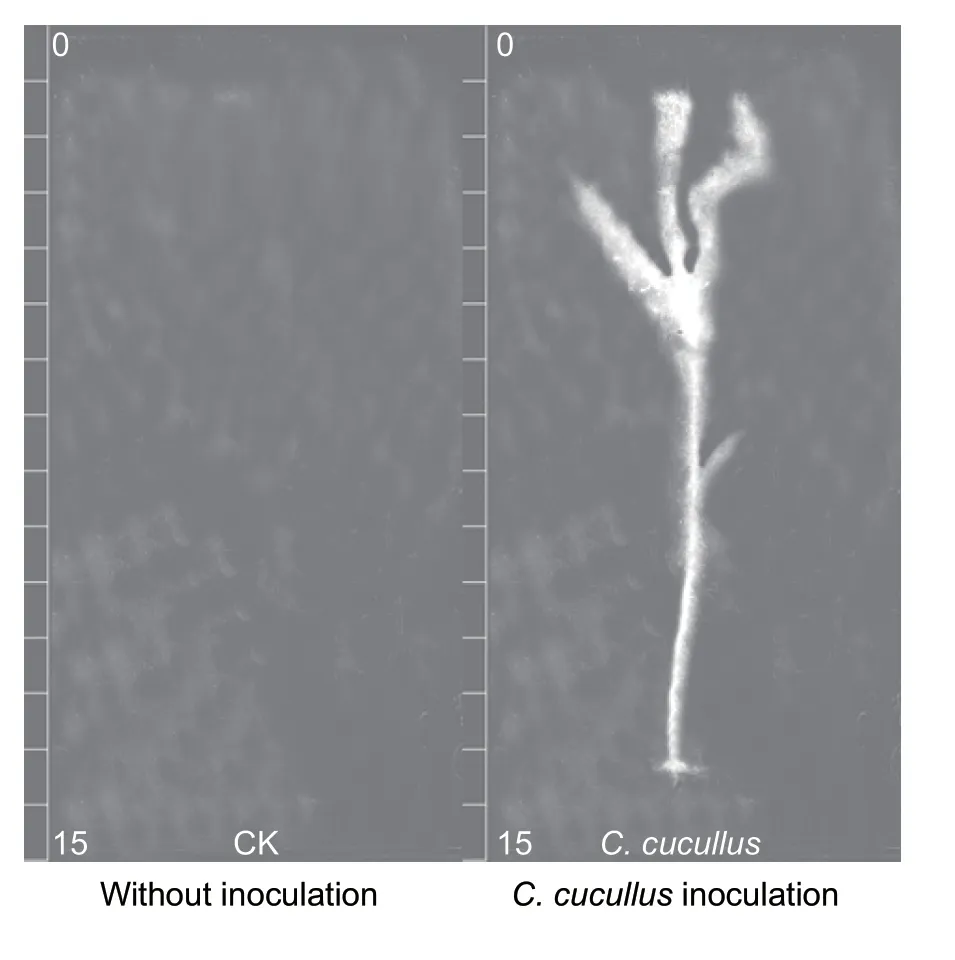
Fig.2 32P autoradiographs of the maize plants which were grown in the soils without (left) or with (right) Colpoda cucullus inoculation in the outer compartment of the root box,and incubated in a glasshouse under natural conditions for 2 months.
3.2.Plant dry matter and NPK level
The dry matter of maize plants grown in the soils with either SP or AP added was 3–5 times greater than that of control and soil with RP added (Fig.3). Similarly,the contents and uptakes of N,P and K in maize plants grown in the SP-soils and the AP-soils were enhanced by 186–297,469–513 and 75–92%,respectively,compared with the control (Fig.4-A–C).
Inoculation withC.cucullusin the soil,either with or without adding RP,did not show any significant effect on the dry matter of maize plants (Fig.3). However,inoculating this ciliate in the soils together with either SP or AP addition significantly increased maize dry matter by 17.27 and 25.07%,respectively (P<0.05),compared with the control treatment. Similarly,C.cucullusinoculation led to significant increases (P<0.05) in N,P and K uptake of maize plants by 22–34,23–56 and 26–43%,respectively(Fig.4-A–C).
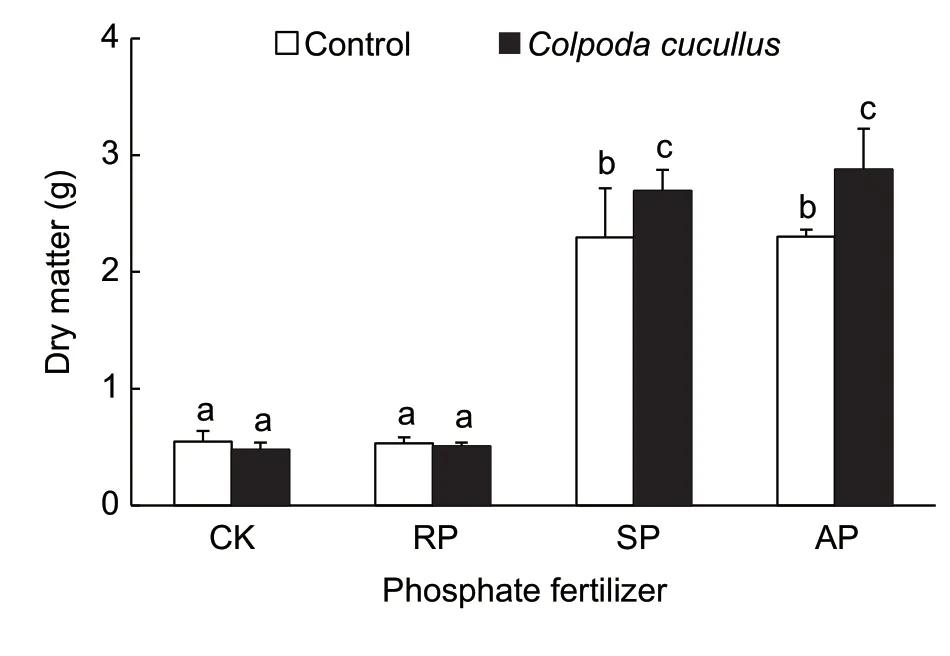
Fig.3 The aboveground dry matter of the maize plants.The plants were grown in the soils amended with either no phosphate (CK),rock phosphate (RP),super phosphate (SP)or ammonium phosphate (AP),and inoculated with Colpoda cucullus,and then incubated in a glasshouse under natural conditions for 2 months. The bars above the columns are the standard deviations of the means (n=3). Different lowercase letters above the columns represent the significant differences at P<0.05.

Fig.4 The absorbed quantities of N (A),P (B) and K (C) by maize aboveground biomass. The plants were grown in the soils amended with no phosphate (CK),rock phosphate (RP),super phosphate (SP) or ammonium phosphate (AP),inoculated with Colpoda cucullus and then incubated in a glasshouse under natural conditions for 2 months. The bars above the columns are the standard deviations of the means (n=3). Different lowercase letters above the columns represent the significant differences at P<0.05.
3.3.Soil available P
Adding phosphate fertilizer as either RP,SP or AP resulted a large increase of soil available P in the outer compartment of the root box,which was obviously from the transformation of both added phosphates and the maize straw (Fig.5-A). Inoculation ofC.cucullusalso caused an enhancement of soil available P content by 0.12–3.0 mg kg–1in the outer compartments.

Fig.5 Soil available P content in the outer (A) and inner (B) compartments of the root box. The soil in the outer compartment was amended with no phosphate (CK),rock phosphate (RP),super phosphate (SP) or ammonium phosphate (AP) together with Colpoda cucullus inoculation. Maize plants were grown in the inner compartment for 2 months in a glasshouse under natural conditions. The bars above the columns are the standard deviations of the means (n=3). Different lowercase letters above the columns represent the significant differences at P<0.05.
For all the treatments,the soils in the inner compartment had much lower available P than those in the outer compartments. Adding phosphate fertilizer as RP,SP or AP significantly increased soil available P (P<0.05),indicating successful soil phosphate transformation during maize growth. Inoculation ofC.cucullusalso resulted in a significant increase in soil available P by 0.14–0.49 mg kg–1(P<0.05).
4.Discussion
Numerous studies have shown that protozoans,including flagellates,ciliates and amoebae,play indispensable roles in improving soil fertility and plant growth (Bamforth 1980;Foissner 1999;Freyet al.2001;Abrahamet al.2019).Colpoda cucullusis a typical ciliate which is widely distributed in soils (Akematsu and Matsuoka 2008). The root box tests conducted here showed that inoculation withC.cuculluscaused a much stronger intensity of32P radioactivity and higher P content in maize plants than in non-inoculation controls. InoculatingC.cucullusin the outer compartment resulted an increase of soil P content in the inner compartment. These data suggested thatC.cucullustransferred P from the bulk soil to the root zone,and then enhanced plant uptakes of P and dry matter of the maize plants.
Four potential explanations may be involved in the association of the ciliate motion with phosphate redistribution in the root box. In the first mechanism,the inoculatedC.cucullusmight devour the P-containing particles (such as the added RP,SP and AP,minerals and microbial tissues),and then simply carry the P from the outer to inner compartment through the nylon net.It is widely known that most protozoans have mouthlike organs and can swallow granular substances in a size similar to bacteria (0.5–5 μm) (Bonkowski 2004;Weidneret al.2017). Furthermore,they can move by up to several centimeters per day along the waterfilm inside soil pores using cilia,flagella or pseudopods (Darbyshire 2005;Adl 2007). It was undoubtable that the inoculated ciliate ofC.cucullusin outer compartment could move through the net and reach the inner root zone through its chemotactic movement (Sunet al.2003b). The devoured phosphates would thereby be transported from the outer to inner compartment of the root box and then absorbed by the maize plant. Future study on quantify how manyC.cululusare involved in phosphate transfer and differentiate the transported phosphate forms would help to explain details of the mechanism.
In addition,one of the possible explanations for this result is that inoculatedC.cuculluscould promote the decomposition of soil organic matter,and then release more nutrients for the maize plants. A few studies have shown that protozoa inoculation often induces rapid mineralization of organic matter,resulting in the release of more N and P nutrients for plant growth (Freyet al.2001). Researchers suggested that this may be caused by altering the structure and activity of soil microbial communities (Mülleret al.2013;Weidneret al.2017).According to previous studies,one possible explanation for the improvement of the soil available P and maize P level could be the contribution of increased organic P decomposition byC.cucullus.
What’s more,existing studies supported our findings thatC.cuculluscould increase phosphate bioavailability.Sunet al.(2003a) found that some protozoans,such as earthworms,devoured and digested RP particles and thereby enhanced phosphate bioavailability. However,direct evidence is required for understanding the phosphate dissolution by protozoans.
Another potential explanation is thatC.cucullusmight secrete plant growth regulators,such as indoleacetic acid,which promote maize plant growth. Kromeet al.(2010) reported that some protozoans could stimulate the production of plant growth hormones and the compounds linked to disease suppression,such as antibiotics(Joussetet al.2010) or siderophores (Levratet al.1989).However,it is not known ifC.cucullusproduces these sorts of regulatory substances. More work is required to discover the production of regulators byC.cucullus.
5.Conclusion
InoculatingC.cucullussignificantly enhanced the dry matter of maize plants,by improving the P level of the plant. The inoculated ciliate,during its chemotactic movement to the roots,could carry phosphates from the phosphate-enhanced soil in the outer compartment of the root box to the inner compartment where the maize plants were growing. However,further study is needed to understand the details of the mechanism.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (41877059). We thank the College of Land Science and Technology,China Agricultural University for providing the necessary facilities and infrastructure for the research.
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- First record of the golden potato nematode Globodera rostochiensis in Yunnan and Sichuan provinces of China
- Current state and suggestions for mechanical harvesting of corn in China
- Protective effect of high-oleic acid peanut oil and extra-virgin olive oil in rats with diet-induced metabolic syndrome by regulatingbranched-chain amino acids metabolism
- Transcriptome and phytochemical analyses reveal the roles of characteristic metabolites in the taste formation of white tea during the withering process
- Interactions between phosphorus availability and microbes in a wheat–maize double cropping system:A reduced fertilization scheme
- Dynamics of organic carbon and nitrogen in deep soil profile and crop yields under long-term fertilization in wheat-maize cropping system
