HCl介质中二苯乙酮咪唑啉季铵盐对碳钢的缓蚀行为及机制
2022-02-12李文涛吴浩王璇苑旭雯曹扬杨怀玉
李文涛,吴浩,王璇,苑旭雯,曹扬,杨怀玉
HCl介质中二苯乙酮咪唑啉季铵盐对碳钢的缓蚀行为及机制
李文涛1,2,吴浩1,2,王璇1,苑旭雯1,2,曹扬1,2,杨怀玉1
(1.中国科学院金属研究所,沈阳 110016;2.中国科学技术大学 材料科学与工程学院,合肥 230026)
合成一种二苯乙酮咪唑啉季铵盐(PPLB)化合物,研究其在0.5 mol/L HCl溶液中对Q235钢的缓蚀性能,探讨其吸附动力学过程及可能的缓蚀机制。以有机胺、硬脂酸、苯乙酮和氯化苄等为原料,经脱水环化、曼尼希反应和季铵化制备季铵盐。通过腐蚀失重、动电位极化曲线测试、电化学阻抗和表面形貌分析等手段,研究了二苯乙酮咪唑啉季铵盐在HCl介质中对Q235钢的缓蚀性能。HCl溶液中PPLB对碳钢具有优异的缓蚀性能,缓蚀效率随浓度和介质温度的升高而增加。在353 K下,PPLB添加量为4.8×10–4mol/L时,缓蚀效率可达98.9%,表明缓蚀剂在高温酸性介质中仍可有效抑制碳钢的腐蚀。电化学研究结果证明,PPLB可同时降低阴阳极的腐蚀电流密度,即抑制了腐蚀的阴阳极过程,是一种通过“几何覆盖效应”起作用的混合型缓蚀剂,其在碳钢表面的吸附遵循Langmuir吸附等温规律,为典型的化学吸附。合成的二苯乙酮咪唑啉季铵盐化合物是一种优异的酸性介质缓蚀剂,独特的分子结构使其通过多中心化学吸附在碳钢表面生成保护性吸附膜,从而有效抑制了HCl对碳钢的腐蚀。
缓蚀剂;季铵盐;碳钢;电化学;吸附;酸液腐蚀
近年来,随着生产开发的不断深入,油气井产出液中水含量逐渐增加,油气产量不断下降。酸化压裂已成为增加油气产能、提高采收率、实现油气稳产和增产最普遍的技术手段,尤其是在当前我国油气供应对外依存度不断攀升的形势下,如何保证酸化压裂等增产技术措施地顺利实施,显得愈发重要。除此之外,许多化工过程,如酸洗、除锈、除垢和化学合成等,都需要使用无机或有机酸液,因其具有强烈的腐蚀性,将不可避免地对相关金属设备和装置造成严重腐蚀,不仅大幅缩短了设备的使用寿命,造成重大经济损失,甚至还可能引发严重的安全生产事故[1-2]。有许多手段可用来减缓酸性介质中金属设备的腐蚀,其中添加缓蚀剂因其成本低、可操作性强、保护效果好、适用范围广等特点,在石油、冶金、机械、能源、交通运输和国防工业等领域发挥着重要的防腐作用,已成为应用最广泛的腐蚀防护技术之一[3]。酸性缓蚀剂通常是由含碳氢原子非极性基团和含氮、氧、硫、磷等电负性较大的杂原子极性基团组成,这些有机化合物分子通过与金属表面间的静电相互作用、共享电子或活性位点吸附等方式,在金属表面形成一层保护性吸附膜,有效阻碍侵蚀性物质与金属表面接触,从而达到减缓金属腐蚀的目的。有机化合物在金属表面的吸附不仅取决于化合物的物理和化学性质,还与其分子中官能团、电子云密度、芳香性、空间位阻效应和π电子特性等密切相关[4-5]。
咪唑啉及其季铵盐作为一种优良的缓蚀剂,因其独特的分子结构,以及低成本、高效、易合成和良好的生物降解性等特点,在多种无机、有机酸中,对碳钢、铁基合金、铜和铝及其合金等均具有较好的缓蚀效果[6-8],目前已广泛应用于石油天然气生产、集输和储运中的腐蚀防护过程[9-10]。因此,多年来有关咪唑啉及其衍生物缓蚀效果及缓蚀机理的研究,一直是国内外缓蚀剂领域的一个热点。近期,Kegui Zhang等[11]研究了不同卤代咪唑啉衍生物在0.5 mol/L HCl溶液中对Q235钢的缓蚀性能,发现氯代咪唑啉分子因更倾向于在铁晶面发生平行式吸附,并形成更致密的吸附膜,其缓蚀性能明显优于氟代咪唑啉。Jun Zhang等[12]通过量化计算和分子动力学模拟,系统研究了4种不同亲水基团的十一烷基咪唑啉化合物在3% NaCl饱和CO2溶液中对碳钢的缓蚀效果,结果表明,4种咪唑啉衍生物分子均可在铁表面形成自组装膜,且亲水基团对咪唑啉分子的反应活性、自组装膜与铁表面间结合强度,以及自组装膜的致密性具有明显的影响,其缓蚀性能优劣顺序为—R==CH2COOH > —CH2CH2OH >—CH2CH2NH2> H。此外,曼尼希碱类缓蚀剂始终受到人们的广泛关注。曼尼希碱是一类由醛或酮的α-活泼氢与醛和胺发生缩合反应(曼尼希反应)得到的β-氨基化合物,与其他酸性缓蚀剂相比,它具有结构稳定、缓蚀效果好、耐高温等特点[13-18]。然而,尽管人们对上述两类化合物的缓蚀性能及缓蚀机理进行了大量研究,但目前为止,就其具体分子中吸附活性位点的确定,以及对缓蚀机理的深入理解仍存在很大争议,有待进一步研究。另外,有关曼尼希碱式咪唑啉季铵盐作为缓蚀剂的研究也未见报道。
为此,本文以有机胺、硬脂酸等为原料,经脱水环化与曼尼希反应等过程,制备了一种兼具咪唑啉和曼尼希碱类缓蚀剂特征的二苯乙酮咪唑啉季铵盐,其目的是通过腐蚀失重和电化学等手段,研究该化合物在0.5 mol/L HCl溶液中对碳钢的缓蚀性能,并结合吸附动力学行为和相关动力/热力学参数,分析探讨其可能的缓蚀作用机理,以期为该类缓蚀剂工业应用提供可靠技术支撑。
1 实验
1.1 材料与试剂
实验材料为Q235钢,其化学组成(质量分数)为:C 0.15%,Mn 0.06%,P 0.55%,Si 0.01%,余量Fe。所用试剂为三乙烯四胺、硬脂酸、苯乙酮、甲醛和氯化苄,上述试剂均为分析纯,购自国药集团化学试剂有限公司。腐蚀介质为0.5 mol/L HCl溶液,由蒸馏水和37% HCl配制而成。
1.2 PPLB合成与红外表征
将三乙烯四胺和硬脂酸按物质的量比1∶1混合,放入装有机械搅拌和分水器的三口烧瓶中,通入氮气并加热至433 K,在搅拌条件下反应3 h,而后继续加热至493 K反应4 h,所得产物标记为P1。将P1与苯乙酮和甲醛按物质的量比1∶2∶2混合,加热至358 K并搅拌反应4 h,经曼尼希反应后,所得产物标记为P2。最后将P2与氯化苄混合物加热至353 K回流反应36 h,得到目标产物PPLB,其分子结构如图1所示。
图2为化合物PPLB的红外光谱。由图2可知,1647 cm–1处为C==N伸缩振动峰,为咪唑环的特征吸收峰,3062 cm–1处为咪唑环上C—H伸缩振动,1551 cm–1处为C—H弯曲振动峰,1456 cm–1处为—CH3弯曲振动峰,2854 cm–1和2926 cm–1处为—CH2伸缩振动峰,1743 cm–1处为—C==O伸缩振动峰。上述红外结果表明,所制备产物为目标化合物[19-23]。
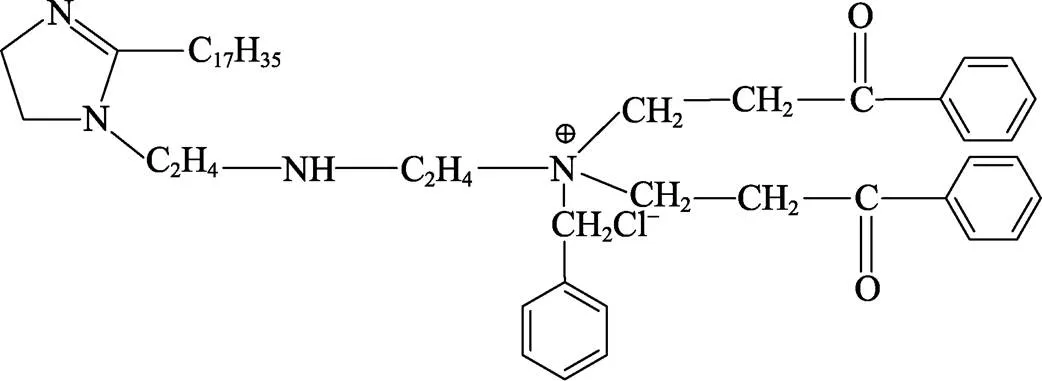
图1 PPLB分子结构
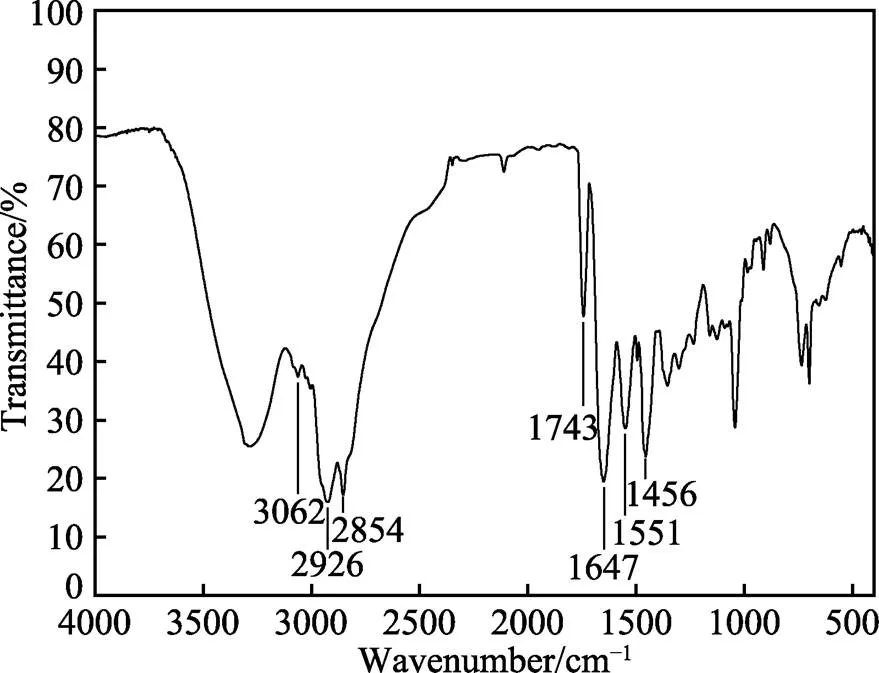
图2 PPLB的红外光谱
1.3 腐蚀失重实验
失重试片由Q235钢板材经线切割后加工而成,尺寸为50 mm×25 mm×2 mm。所有试片表面依次经200、400、800目砂纸逐级打磨,蒸馏水和丙酮清洗,冷风吹干后储存于真空干燥器。将准确称量后的试样放入含有不同浓度缓蚀剂的500 ml 0.5 mol/L HCl溶液中,浸泡4 h后取出,小心去除表面腐蚀产物,并依次用蒸馏水和乙醇清洗,干燥后再次称量。为保证实验数据的重现性,每组实验采用3个平行样同时进行,失重结果取平均值。实验分别在293、313、333、353 K下进行。失重实验的腐蚀速率()由式(1)计算。

式中:1和2分别为失重实验前后试片的质量,为试片面积,为腐蚀时间。缓蚀效率和覆盖度可分别由式(2)和(3)计算[24]。


式中:0和1分别为空白和添加缓蚀剂时试片的腐蚀速率。
1.4 电化学实验
所有电化学实验均在Parstat 2273电化学工作站上进行,采用标准三电极电解池,辅助电极为大面积铂片(Pt),参比电极为饱和甘汞(SCE)电极,工作电极为Q235钢。除0.785 cm2工作面外,电极其余部分用环氧树脂密封在聚四氟乙烯管中。实验前,工作面用SiC砂纸逐级打磨至800目,蒸馏水与丙酮清洗后冷风吹干。为获得稳定的开路电位(OCP),将工作电极放入待测溶液1 h后开始电化学测量。
动电位极化曲线的测量电位范围为相对OCP ‒150 ~ +350 mV,电位扫描速度为0.5 mV/s,实验数据通过Powersuite软件进行分析。电化学交流阻抗测量在OCP下进行,频率范围为100 kHz~10 mHz,激励信号为振幅±10 mV交流信号,实验数据用Zsmipwin软件进行拟合。
1.5 表面形貌观察
将Q235钢制成10 mm×10 mm×2 mm试样并逐级打磨至2000目,用Al2O3研磨膏抛光,蒸馏水和丙酮清洗后,分别浸泡于空白和添加4.8×10–4mol/L PPLB的0.5 mol/L HCl溶液中,4 h后取出,蒸馏水清洗,冷风吹干后,用ESEM XL30 FEG扫描电子显微镜观察试样表面。
2 结果与讨论
2.1 腐蚀失重
不同温度条件下,碳钢在添加不同浓度PPLB的0.5 mol/L HCl溶液中的腐蚀速率及相应缓蚀效率列于表1。从表1可以看出,PPLB在0.5 mol/L HCl溶液中对Q235钢具有优异的缓蚀性能。在293 K,添加浓度仅为1.5×10–6mol/L时,缓蚀效率高达93.3%,且在整个实验浓度范围内,缓蚀效率随缓蚀剂浓度的增加而逐渐升高,当添加浓度为4.8× 10–4mol/L时,缓蚀率达到97.2%。在其他温度条件下,缓蚀效率随缓蚀剂浓度的升高也具有类似的变化规律。这可能是由PPLB分子结构中N和O原子通过其孤对电子与Fe原子空d轨道形成配位键所致。此外,化合物分子结构中的C==O和C==N上的π电子也可与金属空d轨道进行键合,即缓蚀剂分子在金属表面发生化学吸附[25]。另外,随缓蚀剂浓度的增加,缓蚀剂分子在碳钢表面的吸附速率加快,吸附量增大,覆盖度不断增加,表明缓蚀剂分子在碳钢表面形成稳定的吸附保护膜,阻止了酸性介质中的H+与金属表面接触,从而有效抑制了金属腐蚀,缓蚀效率进一步提高。对表1结果需要注意的是,在353 K时,当缓蚀剂浓度为1.5×10–6mol/L时,与低温时相比,缓蚀效率降至82%。这是因为,一方面温度升高加速了缓蚀剂分子的热运动及在溶液中的扩散,弱化了缓蚀剂分子与金属表面间的作用[26];另一方面,介质温度升高还可致使腐蚀加剧,此时无论阳极铁的溶解还是阴极氢的析出,都将不利于缓蚀剂分子的吸附。因此,在高温下,低缓蚀剂浓度(1.5×10–6mol/L)时,缓蚀剂无法在碳钢表面形成致密完整的吸附膜,致使缓蚀效率有所下降。但当缓蚀剂浓度增加至4.8× 10–4mol/L时,缓蚀率增至98.9%,表明缓蚀剂添加量达到一定浓度时,高温下该化合物仍具有优异的缓蚀性能。
此外,对表1结果仔细分析还可以发现,当缓蚀剂浓度低于3.0×10–5mol/L时,缓蚀效率随浓度的增加而明显升高,且随酸液温度的升高,缓蚀效率的提升幅度更大;但当缓蚀剂浓度高于3.0×10–5mol/L时,这种升高的趋势开始减缓,表明PPLB分子在碳钢表面的吸附随其浓度的增加而逐渐趋于饱和。通常情况下,升高温度不仅会加速金属在酸性溶液中的溶解,还会促进缓蚀剂在金属表面脱附,导致缓蚀效率下降。PPLB对碳钢的缓蚀效率随温度的升高而增大,这意味着缓蚀剂分子与碳钢表面间的相互作用并非单一的物理作用,可能存在强烈的化学吸附。这里需要指出的是,虽然缓蚀效率随温度的升高而增大,但与低温时相比,相同缓蚀剂浓度下,碳钢的绝对腐蚀速率有所升高,这主要是由空白溶液中碳钢的绝对腐蚀速率随温度升高而快速增加所致。
表1 不同温度下Q235钢在不同浓度PPLB的0.5 mol/L HCl溶液中的失重结果

Tab.1 Corrosion rate and inhibition efficiency obtained from weight loss measurements for Q235 steel in 0.5 mol/L HCl solution with various concentration of PPLB at different temperatures
2.2 动电位极化
图3为293 K时,碳钢在添加不同浓度缓蚀剂的0.5 mol/L HCl溶液中的动电位极化曲线。通过外推法拟合得到的腐蚀电位(corr)、腐蚀电流密度(corr)等电化学参数列于表2。其中,缓蚀效率由式(4)计算[27]。
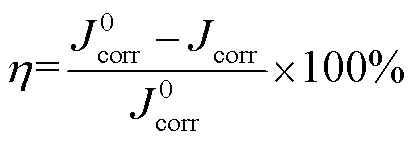
从图3可以看出,随缓蚀剂浓度的增加,碳钢阴阳极极化曲线逐渐向低腐蚀电流密度方向移动,尤其是在低浓度时,这种趋势更明显,表明缓蚀剂的添加对阳极铁的溶解和阴极氢的析出过程均具有很好的抑制作用,且随缓蚀剂浓度的增加,上述抑制作用越来越强。另外,在扫描电位范围内,随缓蚀剂浓度的改变,阴极支极化曲线基本呈平行状态,说明缓蚀剂加入并没有改变电极过程的阴极析氢机理[28]。至于极化的阳极支,当极化电位在自腐蚀电位至–300 mV区间时,随缓蚀剂浓度的增加,阳极corr逐步减小,缓蚀剂表现出优良的阳极抑制特性。但当极化电位高于–300 mV时,随电位的增加,阳极corr出现一个快速增加的“平台”区,尤其在高浓度时,这一过程尤为突出。这是因为随阳极极化电位的升高,铁的阳极溶解速度加快,被缓蚀剂覆盖的电极表面不再稳定,缓蚀剂分子在碳钢表面的吸/脱附平衡被打破,吸附缓蚀剂分子在自身热运动和铁阳极溶解的携带作用下开始离开碳钢表面,导致已吸附在电极表面上的缓蚀剂分子发生阳极脱附,这也是吸附型缓蚀剂在阳极极化电位较高时的突出特征。通常把与该“电流平台”对应的电位称作缓蚀剂的阳极脱附电位,阳极脱附电位越高,表明缓蚀剂吸附越稳定[29-31]。由图3可知,随缓蚀剂浓度的增加,缓蚀剂脱附电位逐渐升高,这表明随PPLB浓度的升高,其在碳钢表面的吸附越稳定[32]。
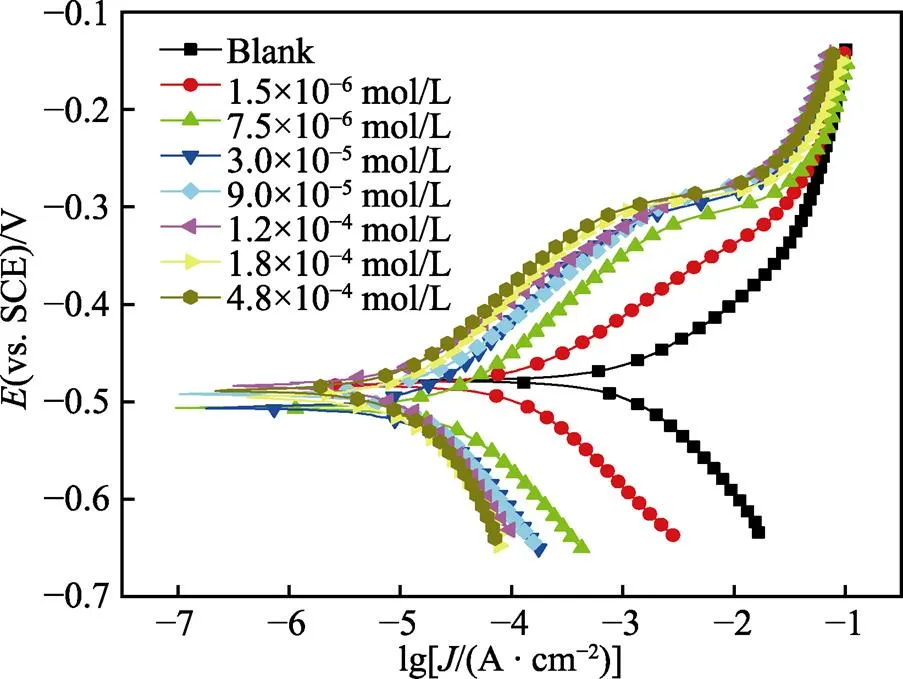
图3 Q235钢在添加不同浓度PPLB的0.5 mol/L HCl溶液中的极化曲线
由表2结果可以看出,添加PPLB后,碳钢的corr显著降低,当添加浓度为4.8×10–4mol/L时,corr值由1518 μA/cm2降至13 μA/cm2,缓蚀率高达99.1%,且随缓蚀剂浓度的增大,碳钢腐蚀电流密度逐渐下降,表明缓蚀剂对HCl溶液中碳钢的腐蚀具有优良的抑制性能。另外,与空白相比,添加缓蚀剂后碳钢的corr没有发生明显变化(Δcorr< 85 mV),且腐蚀反应的阴阳极Tafel斜率变化不大,表明PPLB缓蚀剂为混合型缓蚀剂,其在碳钢表面的吸附为几何覆盖效应,缓蚀剂添加并没有改变碳钢在HCl介质中的腐蚀机理[33-34]。
表2 Q235钢在添加不同浓度PPLB的0.5 mol/L HCl溶液中的动电位极化曲线拟合参数

Tab.2 Electrochemical parameters obtained from potentiodynamic polarization curve for Q235 steel in 0.5 mol/L HCl solutions with different concentrations of PPLB
2.3 电化学阻抗
图4为293 K时,碳钢在添加不同浓度PPLB的0.5 mol/L HCl溶液中的Nyquist和 Bode阻抗图谱。可以看出,随PPLB添加浓度不断增加,无论Nyquist 图中(图4a)的容抗弧半径,还是Bode图中(图4b)的低频阻抗膜值和最大相位角均逐渐增大,尤其在缓蚀剂浓度较低时,这种变化幅度和趋势更为显著,说明缓蚀剂的加入,有效提高了碳钢电极表面的腐蚀反应阻力,且随其浓度的增加,缓蚀剂分子在碳钢电极表面的吸附逐渐增强,吸附量不断增加,所形成的保护性吸附膜越来越完整致密,从而有效地减缓了Q235钢在酸性溶液中的腐蚀,对碳钢起到明显的抑制作用。另外还可发现,Nyquist图由高中频和低频2个容抗弧构成,且在与此相应的Bode图中,随缓蚀剂浓度的增加,相位角也逐渐分化出两个清晰可见的最大相位角峰,表明该体系存在两个时间常数。其中高频端时间常数对应于电极表面法拉第过程,即电荷转移电阻与双电层电容对交流信号的响应,而低频端时间常数则为碳钢表面吸附粒子的弛豫过程[35-36]。此外,在阻抗复平面中的容抗弧呈现为圆心偏下的不完整半圆状,这种现象通常被认为是由电极表面的不均匀性和电场分布不均而产生的弥散效应所致[37]。
基于上述阻抗行为的变化规律及分析,采用图5所示等效电路对阻抗数据进行拟合(图5a、b分别用于拟合空白和添加缓蚀剂后的阻抗数据),其中s为溶液电阻,ct为电荷转移电阻,f为缓蚀剂等物种吸附膜电阻,CPE1和CPE2分别代表双电层电容和缓蚀剂等粒子吸附膜电容,为表征电极表面不均匀程度的参数,拟合所得电化学参数列于表3。在电化学阻抗实验中,缓蚀效率通过公式(5)进行计算[38-39]。
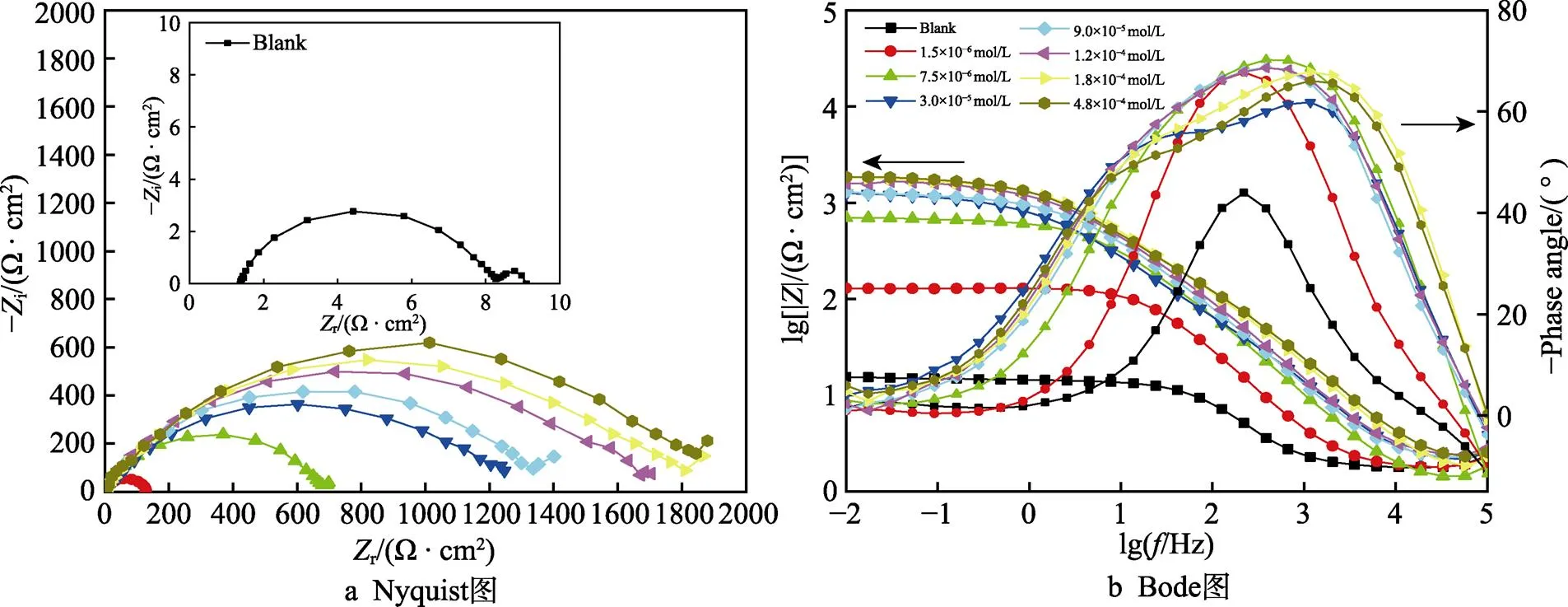
图4 添加不同浓度缓蚀剂时Q235钢电极在0.5 mol/L HCl溶液中的Nyquist图和Bode图
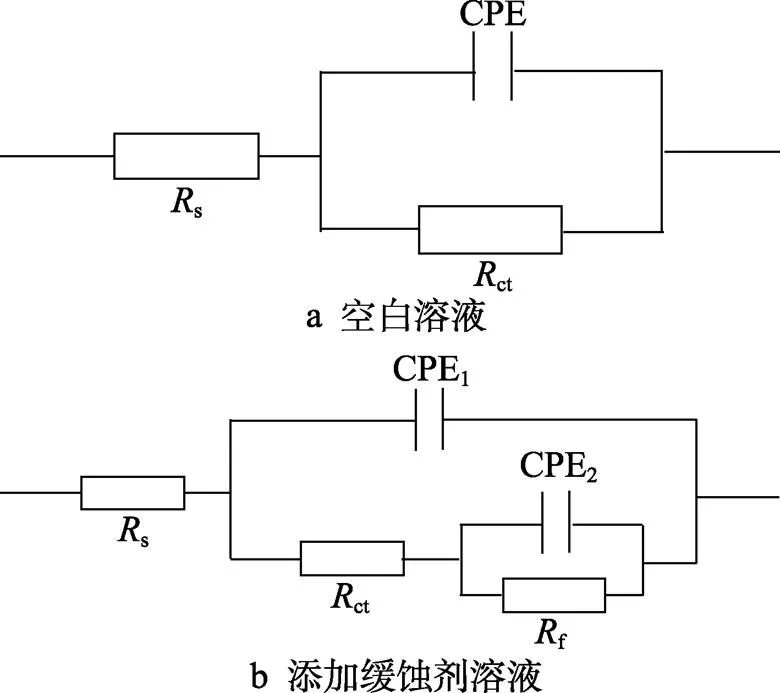
图5 电化学阻抗拟合等效电路
Fig.5 Equivalent circuit model used to fit EIS data: (a) blank; (b) with PPLB

表3 Q235钢在添加不同浓度PPLB的0.5 mol/L HCl溶液中的电化学阻抗拟合参数

Tab.3 Impedance parameters derived from EIS data for Q235 steel in 0.5 mol/L HCl in the absence and the presence of different concentrations of PPLB
2.4 动力学吸附等温式与热力学参数
吸附等温式可反映缓蚀剂分子与碳钢表面间相互作用的本质,通过对不同吸附等温式进行拟合,发现Langmuir吸附等温式拟合结果最佳,其斜率趋近于1。式(6)为Langmuir吸附等温式[42]。

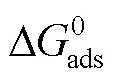
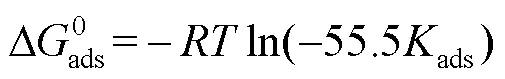
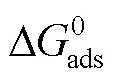
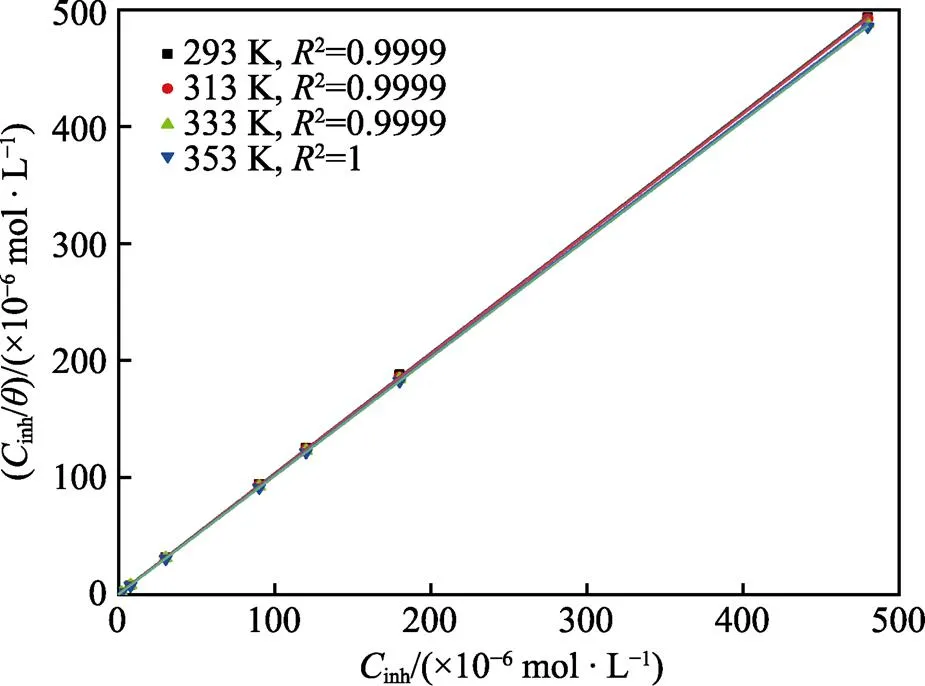
图6 不同温度下PPLB在Q235钢表面的吸附Langmuir拟合结果
表4 不同温度下PPLB在Q235钢表面吸附热力学参数
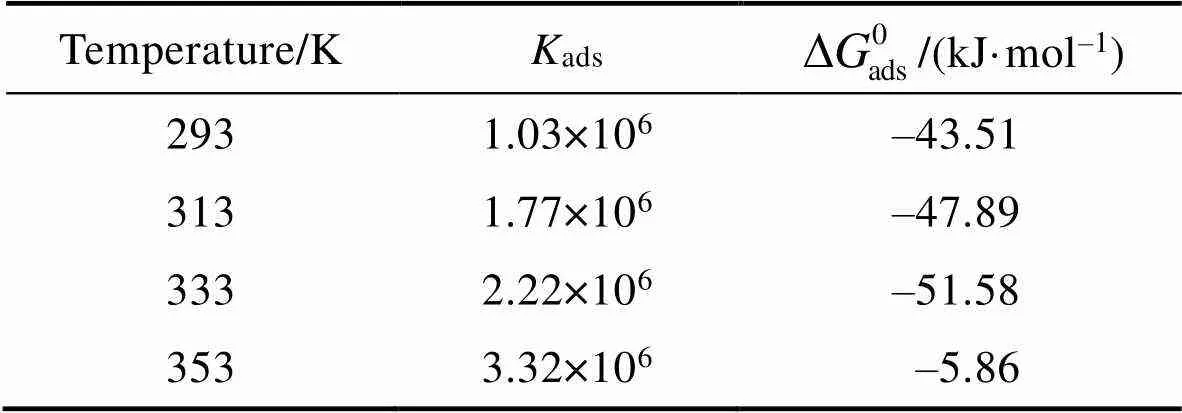
Tab.4 Adsorption parameters obtained from weight loss measurements for PPLB inhibitor at different temperatures
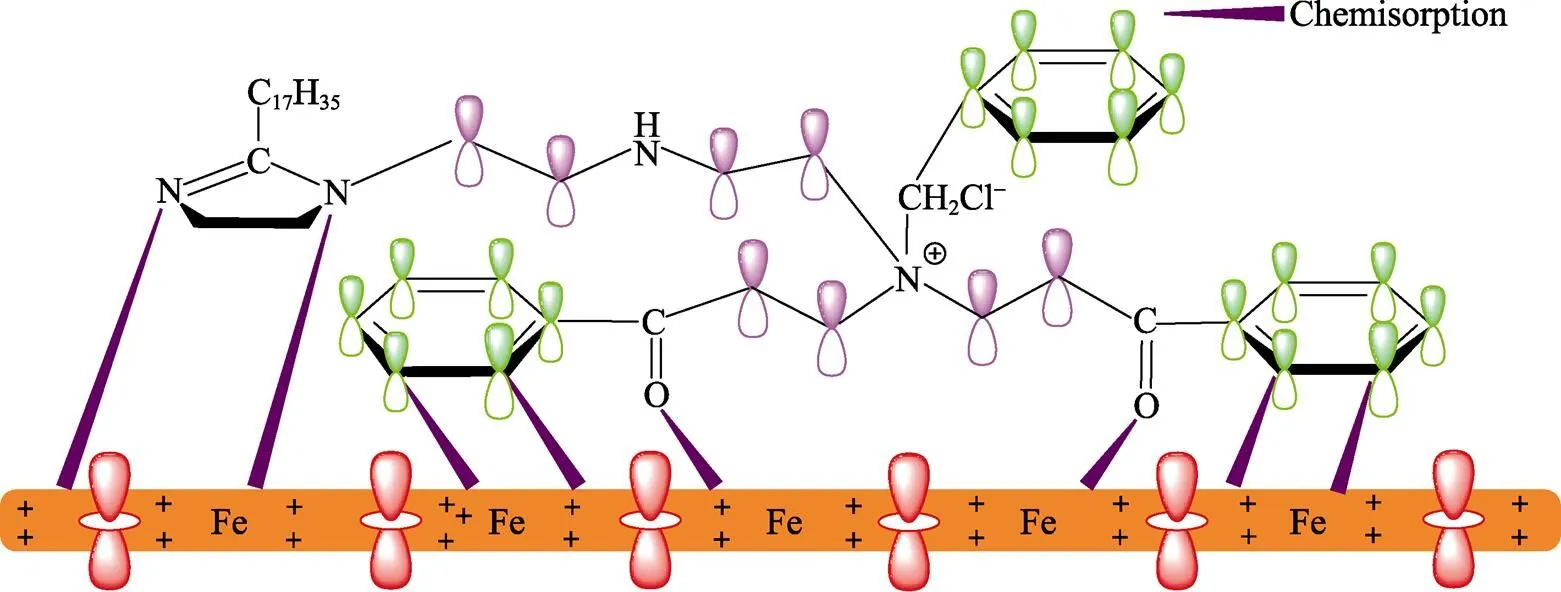
图7 PPLB分子在Q235钢表面吸附示意图
2.5 温度的影响及动力学参数
假定Q235钢在0.5 mol/L HCl溶液中的腐蚀速率和温度之间满足Arrhenius公式(8)及其变换式(9)[47]。
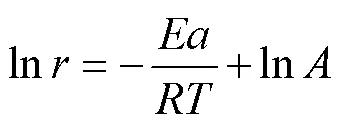
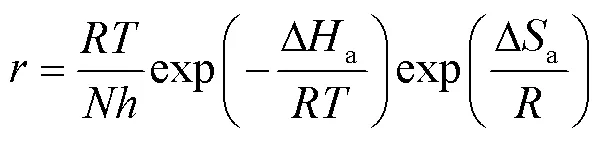
式中:为表观活化能,为指前因子,为摩尔气体常数,为阿伏伽德罗常数,为普朗克常量,∆a和∆a分别为反应的活化焓和活化熵。图8为ln以及ln(/)与1/间的关系图。根据线性拟合结果分别计算得到的、∆a和∆a值列于表5。由表5可知,添加缓蚀剂后,活化能明显下降,说明添加缓蚀剂后,金属表面能垒降低,与缓蚀剂分子间的反应活性随之增大,缓蚀剂更易吸附在金属表面。而空白溶液中,∆a为正值,表明碳钢在酸性溶液中的溶解为吸热过程,温度升高可促进碳钢腐蚀,这与腐蚀失重实验结果相一致。与空白溶液时相比,添加缓蚀剂后,∆a明显降低,表明碳钢表面生成的吸附保护膜可有效抑制金属的阳极溶解。另外,添加缓蚀剂后,与空白相比,∆a值更负,表明缓蚀剂吸附是一个熵减过程,这是因为添加缓蚀剂后,缓蚀剂分子逐渐由溶液中的无序状态转化为在碳钢表面有规则吸附的有序状态,导致体系混乱度减小,活化熵降低[48-49]。
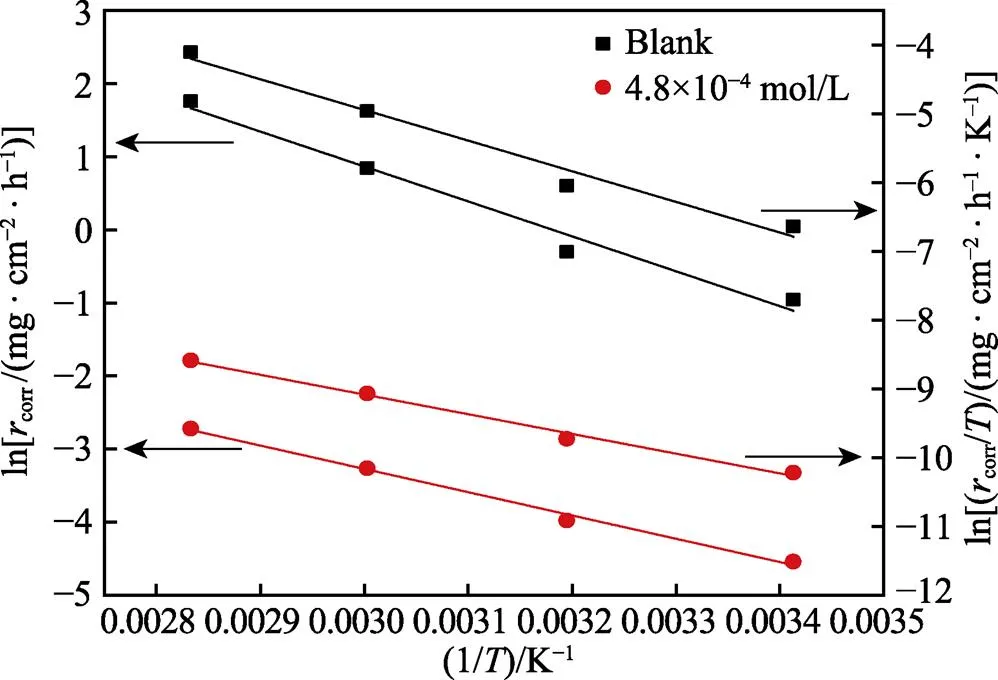
图8 空白和添加PPLB时Q235钢的lnr以及ln(r/T)与1/T间的关系
表5 空白和添加PPLB时Q235钢表面腐蚀过程动力学参数
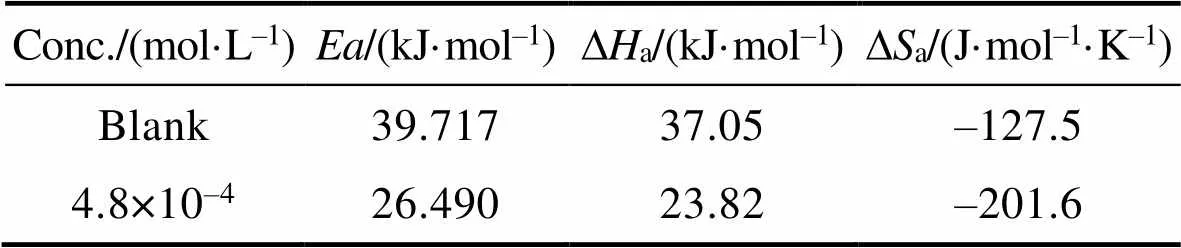
Tab.5 The calculated kinetic parameters from weight loss measurements for Q235 steel in 0.5 mol/L HCl solutions without and with PPLB of 4.8×10–4 mol/L
2.6 腐蚀形貌
图9为293 K时,Q235钢在空白和添加4.8× 10–4mol/L PPLB的0.5 mol/L HCl溶液中浸泡4 h后的表面形貌。由图9可以看出,在空白HCl溶液中(图9b),试片表面遭受严重腐蚀,表面粗糙度明显增加。而在添加缓蚀剂的酸液中(图9c),试样经4 h腐蚀后,表面依旧光滑均匀,腐蚀程度显著下降,这表明PPLB对HCl溶液中Q235钢的腐蚀具有优异的抑制效果。

图9 Q235钢表面的SEM形貌
3 结论
1)合成的二苯乙酮咪唑啉季铵盐对Q235钢在0.5 mol/L HCl溶液中的腐蚀具有优异的缓蚀性能,缓蚀效率随其浓度和介质温度的升高而增加。
2)电化学研究结果表明,随缓蚀剂浓度的增加,Q235钢的腐蚀电流密度与双电层电容逐渐下降,但其电荷转移电阻和吸附膜电阻随浓度的升高而逐渐增大。PPLB可同时抑制腐蚀的阴、阳极过程,为具有“几何覆盖效应”的混合型缓蚀剂。失重与电化学研究结果具有很好的一致性。
3)PPLB化合物在碳钢表面的吸附符合Langmuir吸附等温规律,吸附为自发的化学过程,添加缓蚀剂后,吸附反应的活化能、活化焓和活化熵降低,表明温度升高有利于化合物在碳钢表面形成致密有序的吸附保护膜。
[1] FINŠGAR M, JACKSON J. Application of Corrosion Inhibitors for Steels in Acidic Media for the Oil and Gas Industry: A Review[J]. Corrosion Science, 2014, 86: 17- 41.
[2] HOOSHMAND ZAFERANI S, SHARIFI M, ZAAREI D, et al. Application of Eco-Friendly Products as Corrosion Inhibitors for Metals in Acid Pickling Processes: a Review[J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 652-657.
[3] UMOREN S A, SOLOMON M M. Effect of Halide Ions on the Corrosion Inhibition Efficiency of Different Organic Species: a Review[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 81-100.
[4] GOYAL M, KUMAR S, BAHADUR I, et al. Organic Corrosion Inhibitors for Industrial Cleaning of Ferrous and Non-Ferrous Metals in Acidic Solutions: A Review[J]. Journal of Molecular Liquids, 2018, 256: 565- 573.
[5] ITUEN E, AKARANTA O, JAMES A. Evaluation of Performance of Corrosion Inhibitors Using Adsorption Isotherm Models: An Overview[J]. Chemical Science International Journal, 2017, 18(1): 1-34.
[6] GONZÁLEZ-RODRÍGUEZ C A, RODRÍGUEZ-GÓMEZ F J, GENESCÁ-LLONGUERAS J. The Influence of Desulfovibrio Vulgaris on the Efficiency of Imidazoline as a Corrosion Inhibitor on Low-Carbon Steel in Seawater[J]. Electrochimica Acta, 2008, 54(1): 86-90.
[7] OKAFOR P C, ZHENG Yu-gui. Synergistic Inhibition Behaviour of Methylbenzyl Quaternary Imidazoline Derivative and Iodide Ions on Mild Steel in H2SO4Solutions[J]. Corrosion Science, 2009, 51(4): 850-859.
[8] FARELAS F, RAMIREZ A. Carbon Dioxide Corrosion Inhibition of Carbon Steels through Bis-Imidazoline and Imidazoline Compounds Studied by EIS[J]. International Journal of Electrochemical Science, 2010, 5(6): 797-814.
[9] JEVREMOVIĆ I, SINGER M, NEŠIĆ S, et al. Inhibition Properties of Self-Assembled Corrosion Inhibitor Talloil Diethylenetriamine Imidazoline for Mild Steel Corrosion in Chloride Solution Saturated with Carbon Dioxide[J]. Corrosion Science, 2013, 77: 265-272.
[10] SHAMSA A, BARKER R, HUA Yong, et al. Performance Evaluation of an Imidazoline Corrosion Inhibitor in a CO2-Saturated Environment with Emphasis on Localised Corrosion[J]. Corrosion Science, 2020, 176: 108916.
[11] ZHANG Ke-gui, XU Bin, YANG Wen-zhong, et al. Halogen-Substituted Imidazoline Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution[J]. Corrosion Science, 2015, 90: 284-295.
[12] ZHANG Jun, QIAO Gui-min, HU Song-qing, et al. Theoretical Evaluation of Corrosion Inhibition Performance of Imidazoline Compounds with Different Hydrophilic Groups[J]. Corrosion Science, 2011, 53(1): 147- 152.
[13] AHAMAD I, PRASAD R, QURAISHI M A. Adsorption and Inhibitive Properties of some New Mannich Bases of Isatin Derivatives on Corrosion of Mild Steel in Acidic Media[J]. Corrosion Science, 2010, 52(4): 1472-1481.
[14] TANG Ming-jin, LI Jian-bo, LI Zhi-da, et al. Mannich Base as Corrosion Inhibitors for N80 Steel in a CO2Saturated Solution Containing 3wt% NaCl[J]. Materials, 2019, 12(3): 449.
[15] QURAISHI M A, AHAMAD I, SINGH A K, et al. N-(Piperidinomethyl)-3-[(Pyridylidene)Amino] Isatin: A New and Effective Acid Corrosion Inhibitor for Mild Steel[J]. Materials Chemistry and Physics, 2008, 112(3): 1035-1039.
[16] VERMA C, QURAISHI M, EBENSO E. Mannich Bases Derived from Melamine, Formaldehyde Alkanoleamines as Novel Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Medium[J]. International Journal of Electrochemical Science, 2013, 8(8): 10851-10863.
[17] YADAV M, SHARMA U, YADAV P. Corrosion Inhibitive Properties of some New Isatin Derivatives on Corrosion of N80 Steel in 15% HCl[J]. International Journal of Industrial Chemistry, 2020, 11(3): 203.
[18] JEEVA M, VENKATESA PRABHU G, RAJESH C M. Inhibition Effect of Nicotinamide and Its Mannich Base Derivatives on Mild Steel Corrosion in HCl[J]. Journal of Materials Science, 2017, 52(21): 12861-12888.
[19] FARAHATI R, GHAFFARINEJAD A, MOUSAVI- KHOSHDEL S M, et al. Synthesis and Potential Applications of some Thiazoles as Corrosion Inhibitor of Copper in 1 M HCl: Experimental and Theoretical Studies[J]. Progress in Organic Coatings, 2019, 132: 417- 428.
[20] MIGAHED M A, RASHWAN S M, KAMEL M M, et al. Synthesis, Characterization of Polyaspartic Acid-Glycine Adduct and Evaluation of Their Performance as Scale and Corrosion Inhibitor in Desalination Water Plants[J]. Journal of Molecular Liquids, 2016, 224: 849-858.
[21] FENG Li-juan, YANG Huai-yu, WANG Fu-hui. Experimental and Theoretical Studies for Corrosion Inhibition of Carbon Steel by Imidazoline Derivative in 5% NaCl Saturated Ca(OH)2Solution[J]. Electrochimica Acta, 2011, 58: 427-436.
[22] 张晨峰, 扈俊颖, 钟显康, 等. 双咪唑啉在CO2/O2环境中的缓蚀行为及其与巯基乙醇的复配性能[J]. 表面技术, 2020, 49(11): 66-74.
ZHANG Chen-feng, HU Jun-ying, ZHONG Xian-kang, et al. Bis-Imidazoline Compound as a Corrosion Inhibitor in CO2/O2Environment and Its Synergistic Effect with 2-Mercaptoethanol[J]. Surface Technology, 2020, 49(11): 66-74.
[23] 饶兴兴, 王璇, 王言建, 等. 环境友好型含辣椒素缔合物的缓蚀性能评价[J]. 表面技术, 2020, 49(11): 252-261.
RAO Xing-xing, WANG Xuan, WANG Yan-jian, et al. Corrosion Inhibition Evaluation of Environmentally Friendly Capsaicin-Containing Associations[J]. Surface Technology, 2020, 49(11): 252-261.
[24] ELAYYACHY M, IDRISSI A E, HAMMOUTI B. New Thio-Compounds as Corrosion Inhibitor for Steel in 1 M HCl[J]. Corrosion Science, 2006, 48(9): 2470-2479.
[25] 邓书端, 李向红, 杜官本. 2-巯基嘧啶对冷轧钢在柠檬酸中的缓蚀性能[J]. 腐蚀科学与防护技术, 2017, 29(6): 597-602.
DENG Shu-duan, LI Xiang-hong, DU Guan-ben. Corrosion Inhibition of 2-Mercaptopyrimidine for Cold Rolled Steel in Citric Acid Solution[J]. Corrosion Science and Protection Technology, 2017, 29(6): 597-602.
[26] EL H F, BELKHMIMA R A, ZERGA B, et al. Temperature Performance of a Thione Quinoxaline Compound as Mild Steel Corrosion Inhibitor in Hydrochloric Acid Medium[J]. International Journal of Electrochemical Science, 2014, 9(9): 4721-4731.
[27] 温福山, 杜永霞, 张涵, 等. 双咪唑啉缓蚀剂的缓蚀性能评价[J]. 腐蚀与防护, 2019, 40(2): 92-100, 109. WEN Fu-shan, DU Yong-xia, ZHANG Han, et al. Evaluation of Corrosion Inhibition of a Bis-Imidazoline Corrosion Inhibitor[J]. Corrosion & Protection, 2019, 40(2): 92-100, 109.
[28] DAHIYA S, LATA Su-man, KUMAR R, et al. Comparative Performance of Uroniums for Controlling Corrosion of Steel with Methodical Mechanism of Inhibition in Acidic Medium: Part 1[J]. Journal of Molecular Liquids, 2016, 221: 124-132.
[29] AMIN M A, KHALED K F, FADL-ALLAH S A. Testing Validity of the Tafel Extrapolation Method for Monitoring Corrosion of Cold Rolled Steel in HCl Solutions: Experimental and Theoretical Studies[J]. Corrosion Science, 2010, 52(1): 140-151.
[30] LI Xiang-hong, DENG Shu-duan, FU Hui, et al. Adsorption and Inhibition Effect of 6-Benzylaminopurine on Cold Rolled Steel in 1.0 M HCl[J]. Electrochimica Acta, 2009, 54(16): 4089-4098.
[31] CHETOUANI A, AOUNITI A, HAMMOUTI B, et al. Corrosion Inhibitors for Iron in Hydrochloride Acid Solution by Newly Synthesised Pyridazine Derivatives[J]. Corrosion Science, 2003, 45(8): 1675-1684.
[32] DÖNER A, KARDAŞ G. N-Aminorhodanine as an Effective Corrosion Inhibitor for Mild Steel in 0.5 M H2SO4[J]. Corrosion Science, 2011, 53(12): 4223-4232.
[33] BOBINA M, KELLENBERGER A, MILLET J P, et al. Corrosion Resistance of Carbon Steel in Weak Acid Solutions in the Presence of L-Histidine as Corrosion Inhibitor[J]. Corrosion Science, 2013, 69: 389-395.
[34] 王秀梅, 杨怀玉, 王福会. 两种季铵盐化合物在0.5 mol/L HCl溶液中对碳钢缓蚀效果的对比研究[J]. 腐蚀科学与防护技术, 2010, 22(4): 338-342.
WANG Xiu-mei, YANG Huai-yu, WANG Fu-hui. A Comparative Investigation on Inhibition Performance of Two Quaternary Ammonium Salts for Mild Steel in 0.5 mol/L HCl Solution[J]. Corrosion Science and Protection Technology, 2010, 22(4): 338-342.
[35] SINGH A, LIN Yuan-hua, ANSARI K R, et al. Electrochemical and Surface Studies of some Porphines as Corrosion Inhibitor for J55 Steel in Sweet Corrosion Environment[J]. Applied Surface Science, 2015, 359: 331-339.
[36] LI Xiang-hong, DENG Shu-duan, FU Hui. Sodium Molybdate as a Corrosion Inhibitor for Aluminium in H3PO4Solution[J]. Corrosion Science, 2011, 53(9): 2748- 2753.
[37] AHAMAD I, QURAISHI M A. Mebendazole: New and Efficient Corrosion Inhibitor for Mild Steel in Acid Medium[J]. Corrosion Science, 2010, 52(2): 651-656.
[38] YLDZ R. An Electrochemical and Theoretical Evaluation of 4,6-Diamino-2-Pyrimidinethiol as a Corrosion Inhibitor for Mild Steel in HCl Solutions[J]. Corrosion Science, 2015, 90: 544-553.
[39] TEZCAN F, YERLIKAYA G, MAHMOOD A, et al. A Novel Thiophene Schiff Base as an Efficient Corrosion Inhibitor for Mild Steel in 1.0 M HCl: Electrochemical and Quantum Chemical Studies[J]. Journal of Molecular Liquids, 2018, 269: 398-406.
[40] WANG B, DU M, ZHANG J, et al. Electrochemical and Surface Analysis Studies on Corrosion Inhibition of Q235 Steel by Imidazoline Derivative Against CO2Corrosion[J]. Corrosion Science, 2011, 53(1): 353-361.
[41] 张银辉, 王玮, 常洪莉, 等. 盐酸溶液中Tween-80和CTAB对Q235钢的缓蚀协同作用[J]. 表面技术, 2018, 47(10): 11-21.
ZHANG Yin-hui, WANG Wei, CHANG Hong-li, et al. Synergistic Effect of Tween-80 and CTAB on Corrosion Inhibition of Q235 Steel in HCl Solution[J]. Surface Technology, 2018, 47(10): 11-21.
[42] WANG Xiu-mei, YANG Huai-yu, WANG Fu-hui. An Investigation of Benzimidazole Derivative as Corrosion Inhibitor for Mild Steel in Different Concentration HCl Solutions[J]. Corrosion Science, 2011, 53(1): 113-121.
[43] YU Zong-xue, LIU Yu-chuan, LIANG Ling, et al. Inhibition Performance of a Multi-Sites Adsorption Type Corrosion Inhibitor on P110 Steel in Acidic Medium[J]. Chemical Physics Letters, 2019, 735: 136773.
[44] 吕艳丽, 王艳秋, 周丽, 等. 邻菲罗啉及其衍生物在1 mol/L盐酸中的缓蚀性能研究[J]. 表面技术, 2018, 47(10): 51-58.
LYU Yan-li, WANG Yan-qiu, ZHOU Li, et al. Corrosion Inhibition of 1,10-Phenanthroline and Its Derivative on Mild Steel in 1 mol/L HCl[J]. Surface Technology, 2018, 47(10): 51-58.
[45] ZARROUK A, HAMMOUTI B, DAFALI A, et al. Inhibitive Properties and Adsorption of Purpald as a Corrosion Inhibitor for Copper in Nitric Acid Medium[J]. Industrial & Engineering Chemistry Research, 2013, 52(7): 2560-2568.
[46] 王秀梅, 万晔, 杨怀玉. 双苯并咪唑化合物对碳钢在H2SO4溶液中的缓蚀作用[J]. 腐蚀科学与防护技术, 2012, 24(4): 296-300.
WANG Xiu-mei, WAN Ye, YANG Huai-yu. Corrosion Inhibition of Mild Steel in H2SO4Solution by Bis- Benzimidazole Compound[J]. Corrosion Science and Protection Technology, 2012, 24(4): 296-300.
[47] ZARROK H, AL D S S, ZARROUK A, et al. Thermodynamic Characterisation and Density Functional Theory Investigation of 1,1′,5,5′-Tetramethyl-1H,1′H-3,3′-Bipyrazole as Corrosion Inhibitor of C38 Steel Corrosion in HCl[J]. International Journal of Electrochemical Science, 2012, 7(5): 4047-4063.
[48] OBOT I B, EBENSO E E, AKPAN I A, et al. Thermodynamic and Density Functional Theory Investigation of Sulphathiazole as Green Corrosion Inhibitor at Mild Steel/Hydrochloric Acid Interface[J]. International Journal of Electrochemical Science, 2012, 7(3): 1978-1996.
[49] HOSEIN ZADEH A R, DANAEE I, MADDAHY M H. Thermodynamic and Adsorption Behaviour of Medicinal Nitramine as a Corrosion Inhibitor for AISI Steel Alloy in HCl Solution[J]. Journal of Materials Science & Technology, 2013, 29(9): 884-892.
Corrosion Inhibition Property and Mechanism of Diacetophenone Imidazoline Quaternary Salt for Carbon Steel in HCl Solution
1,2,1,2,1,1,2,1,2,1
(1. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China; 2. School of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, China)
The work aimed to synthesize a new diacetophenone imidazoline quaternary ammonium salt compound (labeled PPLB) and evaluate its corrosion inhibition performance for Q235 steel in 0.5 mol/L HCl solution by weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and scanning electron microscope (SEM). The inhibition mechanism of PPLB was also discussed. The diacetophenone imidazoline quaternary ammonium salt compound was prepared from organic amine, stearic acid, acetophenone and benzyl chloride by dehydration, cyclization, Mannich reaction and quaternization. The weight loss measurement results showed that with the increasing of PPLB concentration and medium temperature, the inhibition efficiency increased gradually, and when the inhibitor dosage was 4.8×10‒4mol/L, the inhibition efficiency reached to 98.9% at 353 K, proving that the PPLB was an excellent corrosion inhibitor for Q235 steel in HCl solution. Electrochemical studies indicated that the inhibitor simultaneously retarded both cathodic and anodic processes and acted as a mixed type inhibitor. The PPLB molecules absorbed on metal surface merely by geometric blocking mechanism and the corrosion reaction mechanism was not changed. Besides, the adsorption behavior of PPLB was in accordance with the Langmuir adsorption isotherm. After the addition of PPLB, the charge transfer resistance and film resistance values obviously enhanced, while the double layer capacitance and film capacitance significantly reduced, suggesting that the inhibitor molecules took effects by adsorbing at the metal/solution interface. The thermodynamic parameters deduced from experimental data revealed that the adsorption of inhibitor PPLB was a spontaneously chemical process. The inhibitor molecules may take effects by adsorbing on mild surface via lone pair electrons of nitrogen and oxygen atoms, which could lead to the increase of surface coverage of PPLB molecules on the Q235 steel surface. The inhibition performance was also evidenced by scanning electron microscope. Compared with the sample in 0.5 mol/L HCl solution, the surface of specimen in the presence of PPLB was much smoother. These results confirmed that the PPLB can effectively protect mild steels from corrosion in 0.5 mol/L HCl solution.
inhibitor; quaternary ammonium salt; carbon steel; electrochemistry; adsorption; acidic corrosion
2021-03-20;
2021-05-26
LI Wen-tao (1996—), Female, Postgraduate, Research focus: corrosion inhibitor.
杨怀玉(1963—),男,博士,研究员,主要研究方向为缓蚀剂。
Corresponding author:YANG Huai-yu (1963—), Male, Doctor, Researcher, Research focus: corrosion inhibitor.
李文涛, 吴浩, 王璇, 等. HCl介质中二苯乙酮咪唑啉季铵盐对碳钢的缓蚀行为及机制[J]. 表面技术, 2022, 51(1): 121-130.
TG172
A
1001-3660(2022)01-0121-10
10.16490/j.cnki.issn.1001-3660.2022.01.013
2021-03-20;
2021-05-26
李文涛(1996—),女,硕士研究生,主要研究方向为缓蚀剂。
LI Wen-tao, WU Hao, WANG Xuan, et al. Corrosion Inhibition Property and Mechanism of Diacetophenone Imidazoline Quaternary Salt for Carbon Steel in HCl Solution[J]. Surface Technology, 2022, 51(1): 121-130.
