暹罗炭疽菌转录因子CsAtf1互作蛋白的筛选和鉴定
2022-02-10宋苗方思齐李潇刘文波林春花缪卫国
宋苗 方思齐 李潇 刘文波 林春花 缪卫国

摘 要:堿性亮氨酸拉链bZIP是真核生物转录因子家族之一,通过调控基因的表达来参与调控生长发育及生物、非生物胁迫应答等生理过程。已有报道表明bZIP转录因子Atf1与多种病原真菌的生长发育及致病力相关。由于转录因子通常能够在其他互作蛋白的参与下与顺式作用元件特异性结合,从而调节靶基因表达,因此筛选其互作蛋白对深入了解转录因子的调控机制有重要意义。本研究克隆获得来自橡胶树炭疽病的暹罗炭疽菌(Colletotrichum siamense)的一个bZIP转录因子CsAtf1,并对CsAtf1互作蛋白进行了筛选和鉴定。研究结果显示,暹罗炭疽菌CsAtf1的DNA大小为1758 bp,cDNA为1611 bp,编码536个氨基酸,包含2个内含子,具有3个Aft1结构域和1个BRLZ结构域;利用酵母双杂技术,以pGBKT7-CsAtf1为诱饵,从暹罗炭疽菌cDNA酵母文库中筛选获得12个候选互作蛋白,包括细胞壁蛋白PhiA、Rodlet蛋白、乙醇脱氢酶、线粒体缺氧反应区蛋白、发育调控的MAPK相互作用蛋白、自噬相关蛋白、葡萄糖-甲醇-胆碱氧化还原酶、β-葡萄糖苷酶、c-4甲基固醇氧化酶、甲基转移酶和2个假定蛋白;研究还进一步利用免疫共沉淀技术证实了转录因子CsAtf1能够与线粒体缺氧反应区蛋白CsHIG发生体内互作。
关键词:橡胶树;暹罗炭疽菌;转录因子;互作蛋白
中图分类号:S763.7 文献标识码:A
Identification and Screening of Proteins Interacting with CsAtf1 Transcription Factor in Colletotrichum siamense
SONG Miao, FANG Siqi, LI Xiao, LIU Wenbo, LIN Chunhua*, MIAO Weiguo*
College of Plant Protection, Hainan University / Key Laboratory of Green Forest Control and Prevention, Ministry of Education Haikou, Hainan 570228, China
Abstract: The basic leucine zipper (bZIP) is one of the eukaryotic transcription factor families. It participates in the regulation of biological, abiotic stress responses and developmental physiological processes by regulating the expression of genes. It has been reported that bZIP transcription factor Atf1 is related to the growth, development and pathogenicity of many pathogenic fungi. Transcription factors can specifically bind to cis-acting elements with the participation of other interacting proteins, thereby regulating the expression of target genes. Thus, screening the interacting proteins is of great significance to further understand the regulatory mechanism of bZIP transcription factor. In this study, a bZIP transcription factor CsAtf1 from Colletotrichum siamense (the causative species of rubber tree anthracnose in China) was cloned and the proteins interacting with CsAtf1 were screened and identified. The homologous sequence of Atf1 was searched from the transcriptome database of C. siamense HN08 by local BLAST, using genomic DNA and cDNA as the template for PCR amplification respectively. Sequence analysis revealed that CsAtf1 was 1758 bp, a cDNA of 1611 bp, encoding 535 amino acids, including two introns, three Aft1 domains and one BRLZ domain. Then, the CsAtf1 gene was cloned into the yeast expression vector pGBKT7, and identified by PCR and sequencing. The recombinant bait plasmid pGBKT7-CsAtf1 was transformed into yeast strain Y2H Gold competent cells. Besides, self-activation and toxicity detection of bait plasmid yeast showed that pGBKT7-CsAtf1 didn’t have self-activation and toxicity in yeast strains. These results demonstrated that the yeast bait protein expression vector was successfully constructed, then yeast two-hybrid (Y2H) system was adopted to screen interacting proteins of baiting vector pGBKT7-CsAtf1 in cDNA library of C. siamense. The results confirmed that 12 proteins may interact with CsAtf1, including cell wall protein PhiA, rodlet protein, alcohol dehydrogenase, mitochondrial hypoxia responsive domain containing protein, developmentally regulated MAPK interacting protein, autophagy-related protein, glucose-methanol-choline oxidoreductase, c-4 methylsterol oxidase, methyltransferase, secreted beta-glucosidase sun1 and two hypothetical proteins. Furthermore, in order to verify that CsAtf1 can interact with CsHIS in vivo, the expression vector pCB1532-CsAtf1-G containing the sulfonylurea resistant gene ILV1 and CsAtf1-GFP fusion protein was constructed by double restriction-enzyme digestion, meanwhile, the expression vector pXY203-CsHIG-S containing the hygromycin transferase gene HPH and CsHIG-S fusion protein was constructed using homologous recombination in yeast. Plasmids pCB1532-CsAtf1-G and pXY203-CsHIG-S were co-transformed into wild-type strain to obtain transformants. The results of co-IP indicated that the mitochondrial hypoxia response region protein CsHIG could interact with CsAtf1 in C. siamense. In summary, this research screened candidate proteins by the yeast two-hybrid system and confirmed an interaction between the transcription factor CsAtf1 and CsHIG, which would lay a foundation for further study of the function and regulation mechanism of CsAtf1.
Keywords: rubber tree; Colletotrichum siamense; transcription factor; interaction protein
DOI: 10.3969/j.issn.1000-2561.2022.01.008
转录因子(transcription factor, TF)通过与特定位点结合在基因的表达和调控中发挥重要作用。ATF类转录因子是ATF/CREB蛋白家族中的一员,在真菌中,该类转录因子能够在多种应激胁迫中发挥作用,还参与真菌毒素的产生以及菌体生长发育、致病等过程。已经证实VdAtf1是通过介导菌体对氮元素的代谢调节大丽轮枝菌的致病力[1]。稻瘟菌中的MoAtf1及镰刀菌中的Foatf1均通过调节过氧化物酶的转录来抑制活性氧介导的植物防御,进而影响致病性[2-3]。寄生曲霉中的AtfB基因不仅参与氧化应激反应,还影响黄曲霉毒素的合成[4]。在裂殖酵母中已证实ATF家族蛋白为丝裂原活化蛋白激酶(mitogen-activated protein kinases, MAPK)信号途径的下游调控因子[5]。曲霉中的AtfA为HOG MAPK途径的下游转录因子[6-7],该通路参与调节分生孢子及菌丝的胁迫耐受,影响分生孢子的休眠和萌发[8-10]。
橡胶树炭疽病是橡胶树的重要叶部病害[11]。在开春时节,该病为害严重会造成橡胶树新抽嫩叶的反复落叶,导致割胶时间推迟,造成经济损失[12]。橡胶树炭疽病的病原菌有多种,其中胶孢炭疽菌复合群中的暹罗炭疽菌(Colletotrichum siamense)为我国橡胶树田间主要病原菌[13]。课题组前期克隆了胶孢炭疽菌HOG MAPK途径的CgPbs2基因,证实了该途径参与调节胶孢炭疽菌抵御渗透胁迫,影响菌株对吡咯类杀菌剂咯菌腈(fludioxioil)的抗性[14],但该信号途径参与调控应答的机制尚不清楚。本研究克隆该信号途径下游一个bZIP转录因子,即AtfA同源基因CsAtf1,并利用酵母双杂交的方法在暹罗炭疽菌cDNA文库中对暹罗炭疽菌转录因子CsAtf1互作蛋白进行筛选,并对其中一个互作蛋白进行免疫共沉淀验证体内互作。该研究结果为进一步了解HOG MAPK下游转录因子的互作蛋白及功能奠定基础,有助于深入了解炭疽菌对各种胁迫应答的调控机制,为进一步挖掘病原菌防治新靶标提供依据。
1 材料与方法
1.1 供试菌株与质粒
暹罗炭疽菌(C. siamense)菌株HN08由本实验室前期分离、鉴定和保存[14];酵母感受态细胞Y2H Gold和大肠杆菌DH5α感受态细胞购自上海唯地生物技术有限公司;载体PMD-18T购自大连TaKaRa公司;质粒pGBKT7、pGADT7、pXY203和pBC1532-G以及酵母菌株XK1-25,由本实验室保存。暹罗炭疽菌酵母文库,由本实验室前期构建保存[15]。
1.2 暹罗炭疽菌CsAtf1基因的克隆和分析
根據禾谷炭疽(C. graminicola)转录因子bZIP基因(No. XM008094996.1),在暹罗炭疽菌HN08转录组数据库(由本实验室测序保存)中搜索获得同源序列,设计引物pGBKT7-CsAtf1-F/pGBKT7- CsAtf1-R(引物序列见表1),从菌株HN08基因组DNA和cDNA中扩增获得目的条带,参照方思齐等[16]的方法对基因序列进行比对分析。
1.3 pGBKT7-CsAtf1诱饵载体的构建及重组质粒毒性、自激活检测
用限制性内切酶BamHⅠ和PstⅠ将扩增获得的CsAtf1基因的cDNA序列与质粒pGBKT7进行双酶切,参照方思齐等[16]的方法将质粒pGBKT7与CsAtf1基因连接构建质粒pGBKT7-CsAtf1。
参照方思齐等[16]方法验证诱饵载体pGBKT7- CsAtf1对酵母菌的毒性及在SD/-Trp-Leu-His-Ade缺陷培养基上是否有自激活情况。
1.4 酵母双杂交文库筛选与回交试验验证
参照方思齐等[16]方法,用含诱饵载体pGBKT7- CsAtf1的酵母菌液与菌株HN08 cDNA文库(Y187)杂交共培养,筛选和验证阳性克隆。
1.5 CsAtf1与线粒体缺氧反应区蛋白CsHIG免疫共沉淀实验
为构建含有氯嘧磺隆抗性基因ILV1和CsAtf1-GFP融合蛋白表达载体pCB1532-CsAtf1- G,设计引物对pCB1532-G-CsAtf1-F/pCB1532-G- CsAtf1-R,从诱饵载体pGBKT7-CsAtf1中扩增含有PstⅠ和HindⅢ酶切位点的CsAtf1序列,用PstⅠ和HindⅢ对目的片段和载体pCB1532-G进行双酶切。酶切后连接、转化步骤同1.3。
使用酵母体内同源重组的方式构建含有潮霉素转移酶基因HPH和CsHIG-S融合蛋白表达载体pXY203-CsHIG-S,设计引物PXY203-CsHIG-F/ PXY203-CsHIG-R,以暹罗炭疽菌cDNA为模板进行PCR。对pXY203质粒(该载体含有潮霉素转移酶基因HPH和S tag标签)进行酶切后回收。将线性pXY203质粒和PCR扩增得到的CsHIG基因共同转化至酵母XK1-25感受态中,涂布于SD/-Trp营养缺失培养基平板,30℃静置培养3 d,选取正常生长的酵母单克隆进行PCR验证,检测正确的酵母单克隆质粒转化大肠杆菌DH5α,待用。
参照LIN等[14]的方法制备暹罗炭疽菌原生质体。使用PEG介导原生质体转化的方法进行原生质体转化,利用氯嘧磺隆(100 μg/mL)抗性筛选含有pCB1532-CsAtf1-G的转化子,将筛选到的转化子接种于含有潮霉素(600 μg/mL)的PDS平板中筛选含有pXY203-CsHIG-S质粒的转化子,3~5 d后正常生长的为含有2种抗性的转化子。
将含有2种表达载体的转化子菌株接种于CM液体培养基中28℃ 180 r/min培养2~3 d后收集菌体,用丝状真菌蛋白提取试剂盒(BestBio)进行总蛋白提取。将提取的真菌总蛋白与15 μL anti-GFP琼脂糖珠(ChromoTek)在4℃ 50 r/min条件下孵育4 h后,4℃ 1000 r/min离心1 min弃上清收集珠子,用1×PBS溶液洗涤3~4次,加入15 μL 1×SDS Loading Buffer重悬介质,煮沸,进行western blot验证。
2 结果与分析
2.1 暹罗炭疽菌CsAtf1基因的克隆及序列分析
扩增获得的含完整开放阅读框的CsAtf1基因DNA序列大小为1758 bp,cDNA序列大小为1611 bp(图1),该基因编码536个氨基酸,含有2个内含子,包含3个Aft1结构域和1个BRLZ(碱性亮氨酸拉链)结构域(图2),说明CsAtf1为bZIP转录因子家族中的ATF类转录因子。将序列上传至GenBank数据库,登陆号为:MT084770。
2.2 诱饵载体pGBKT7-CsAtf1构建及重组质粒毒性、自激活检测
将上述扩增获得的CsATF1 cDNA序列和载体pGBKT7连接,构建诱饵载体pGBKT7-CsAtf1。经引物对pGBKT7-F/pGBKT7-R进行菌落PCR和测序验证获得重组诱饵载体pGBKT7-CsAtf1。载体pGBKT7-CsAtf1与pGADT7共转化酵母Y2HGlod感受态,获得的转化子扩增培养后于SD/-Trp-Leu平板上可正常生长,说明pGBKT7-CsAtf1能够在酵母中成功表达且对宿主不产生毒性。将菌液涂布于SD/-Trp-Leu-His-Ade缺陷培养基平板上显示没有自激活现象。
2.3 酵母文库的筛选和阳性克隆测序分析
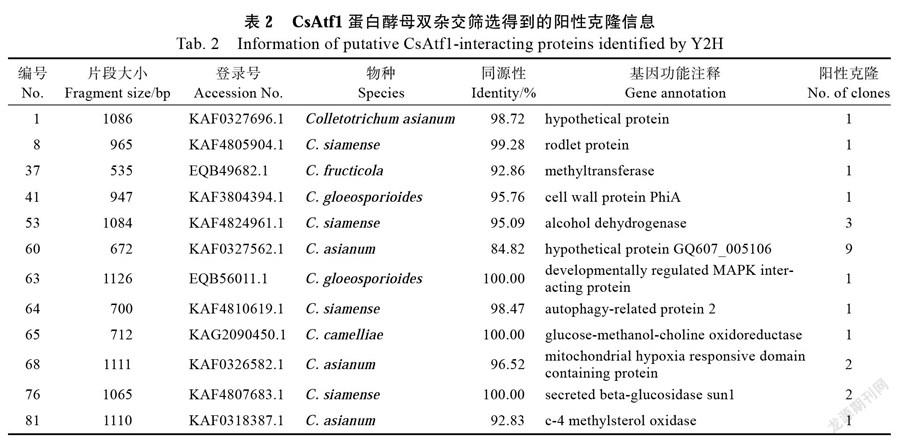
用诱饵载体pGBKT7-CsAtf1和炭疽菌HN08 cDNA酵母文库杂交筛选,获得阳性克隆。提取阳性克隆的质粒,转入大肠杆菌DH5α中进行扩增后测序。结果显示,从文库中共筛选出49个阳性克隆,排除重复和测序失败的克隆子,实际得到的阳性克隆有12个(表2)。它们分别是细胞壁蛋白PhiA、Rodlet蛋白、乙醇脱氢酶、线粒体缺氧反应区蛋白、发育调控的MAPK相互作用蛋白、自噬相关蛋白、β-葡萄糖苷酶、c-4甲基固醇氧化酶、甲基转移酶和假定蛋白等。
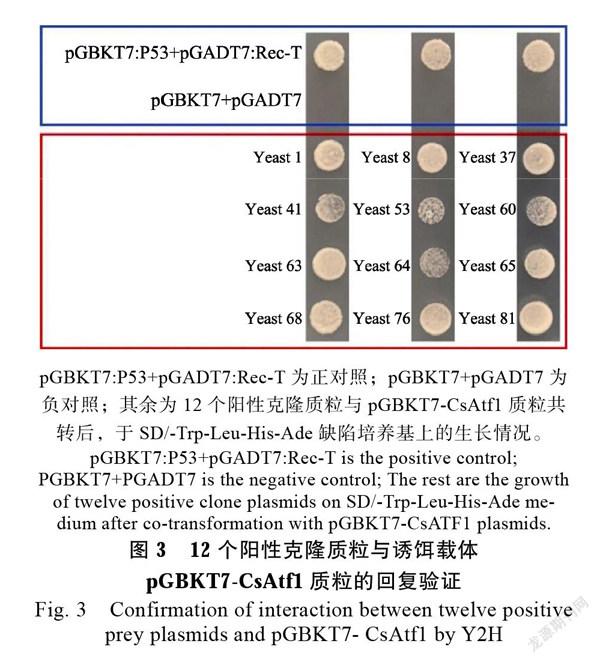
筛选出的12个阳性克隆质粒与诱饵载体pGBKT7-CsAtf1共转入处于同一酵母菌Y2HGlod时均能正常生长(图3),该结果进一步验证了阳性克隆的可靠性。
2.4 CsATF1与线粒体缺氧反应区蛋白CsHIG体内互作鉴定
为了鉴定线粒体缺氧反应区蛋白CsHIG与CsAtf1是否互作,分别构建了pCB1532-CsAtf1-G和pXY203-CsHIG-S融合蛋白表达载体,重组质粒经PCR验证、酶切鉴定和测序鉴定。将质粒pCB1532-CsAtf1-G和pXY203-CsHIG-S共同转化于野生型菌株HN08中,经过含氯嘧磺隆和潮霉素的培养基筛选,获得转化子5个,进行验证后挑取其中1个进行后续实验。
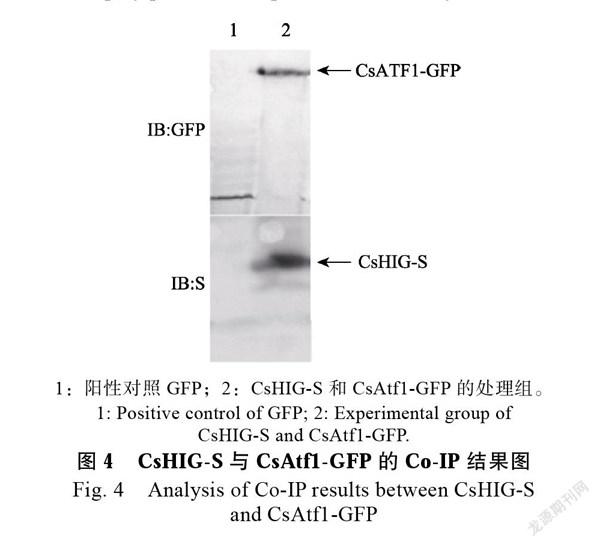
提取转化子总蛋白,用anti-GFP亲和凝胶珠免疫沉淀GFP-CsAtf1蛋白复合体,以GFP- pCB1532空载体蛋白作为阳性对照。利用anti- GFP及anti-Stag抗体检测免疫沉淀复合物中的蛋白(图4),从图中可以看出,用anti-GFP珠子抓取的蛋白中,用GFP抗体孵育可显示出含有一条大小约为90 kDa的目的条带CsAtf1-GFP,用Stag抗体孵育可显示出一条大小约为30 kDa的条带CsHIG-S,而在对照GFP中用Stag抗体孵育不出条带。结果说明线粒体缺氧反应区蛋白CsHIG可以与CsAtf1在炭疽菌菌体内发生互作。
3 讨论
转录因子通常能够在其他互作蛋白的参与下与靶基因的顺式作用原件特异性结合,从而调节靶基因在特定的时间和空间表达,调控生物体的生理代谢和生长发育[17]。因此,分析转录因子的互作蛋白,对深入研究转录因子的调控机制具有重要意义。已知ATF/CREB家族蛋白是一类bZIP转录因子,参与调控真菌胁迫应答、毒素的产生以及致病性等[2-3, 9]。但除了已经证实曲霉A. nidulans中的SakA蛋白能与转录因子AftA互作外[9],对转录因子ATF的互作蛋白还知之甚少。实验室前期已对转录因子CsAtf1的功能进行研究,结果显示CsAtf1基因影响菌体致病力,并能对吡咯类药剂的敏感性进行调控[18]。本研究克隆了炭疽菌C. siemense中的Atf1同源基因CsAtf1,通过生物信息学分析显示,CsAtf1基因含有3个Aft1结构域和1个碱性亮氨酸拉链结构域,说明该基因是bZIP转录因子家族中的Atf1基因。进一步通过酵母双杂法从炭疽菌cDNA酵母文库中筛选获得CsAtf1的12个候选互作蛋白,并使用Co-IP技术证实了其中一个线粒体缺氧反应区蛋白CsHIG1和转录因子CsAtf1的体内互作,研究结果将为深入研究炭疽菌转录因子CsAtf1的调控机制奠定基础。
已知ATF类转录因子与MAPK信号途径相关,为多个MAPK信号途径下游转录因子,比如有Sty1 MAPK、Pmk1 MAPK等[19]。在本研究筛选获得的候选互作蛋白中,Yeast-63为发育调控的MAPK相互作用蛋白。该结果进一步验证了转录因子CsAtf1与MAPK途徑存在联系,Yeast-63与转录因子CsAtf1的互作生物功能值得进一步深入研究。
此外,筛选获得的Yeast-68为线粒体缺氧反应区蛋白(CsHIG1),该蛋白含有一个HIG_1_N蛋白结构域,而HIG1蛋白家族成员的特征就在于N端的HIG1结构域,它是由2个疏水螺旋组成的完整膜蛋白,可能会在脂质双分子层形成发夹状环[20-21]。HIG1家族在整个进化过程中都是保守的,成员包括真菌、线虫和哺乳动物等多种真核生物[21-23]。酿酒酵母中的HIG1家族蛋白Rcf1被发现在线粒体氧化磷酸化过程中与细胞色素c氧化酶互作[24],其后的研究表明Rcf1的功能是通过与线粒体呼吸链复合体IV的动态瞬时关联来改变其稳定性和催化特性[25]。本研究通过酵母双杂和Co-IP证实了CsAtf1和CsHIG1间存在互作关系,这为进一步分析它们互作的生物学功能奠定基础。
候選互作蛋白Yeast-41为细胞壁蛋白PhiA,研究显示构巢曲霉中的PhiA主要与菌丝及分生孢子的生长有关,该基因的缺失突变体表现为菌丝和分生孢子生长下降[26]。而烟曲霉中的PhiA同源蛋白是细胞壁上的主要抗原,推测其可能作为激发子,引起植物对病原物的抗性反应[27]。对大丽轮枝菌Vd991的毒素进行分离纯化后发现细胞壁蛋白PhiA可能与致病相关[28],而ATF类转录因子也通常与病原菌的致病性相关[2-3]。在暹罗炭疽菌中细胞壁蛋白phiA和CsAtf1产生互作,它们是否共同参与调节病原菌致病性值得深入研究。
除此之外,本研究还筛选到Rodlet蛋白、乙醇脱氢酶、甲基转移酶、β-葡萄糖苷酶、c-4甲基固醇氧化酶、自噬相关蛋白等,今后进一步深入研究它们互作的生物学功能,为深入了解炭疽菌应答胁迫反应以及致病机制具有重要意义。
参考文献
[1] TANG C, LI T, KLOSTERMAN S J, TIAN C, WANG Y. The bZIP transcription factor VdAtf1 regulates virulence by mediating nitrogen metabolism in Verticillium dahlia[J]. New Phytologist, 2020, 226(5):1461-1479.
[2] GUO M, GUO W, CHEN Y, DONG S M, ZHANG X, ZHANG H F, SONG W W, WANG W, WANG Q, LV R., ZHANG Z G, WANG Y C, ZHENG X B. The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae[J]. Molecular Plant-Microbe Interactions, 2010, 23(8): 1053-1068.
[3] QI X Z, GUO L J, YANG L Y, HUANG J S. Foatf1, a bZIP transcription factor of Fusarium oxysporum f. sp. cubense, is involved in pathogenesis by regulating the oxidative stress responses of Cavendish banana (Musa spp.)[J]. Physiological and Molecular Plant Pathology, 2013, 84: 76-85.
[4] WEE J, HONG S Y, ROZE L V, DAY D M, CHANDA A, LINZ J E. The fungal bZIP transcription factor AtfB controls virulence-associated processes in Aspergillus parasiticus[J]. Toxins (Basel), 2017, 9(9): 287.
[5] SHIOZAKI K, RUSSELL P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast[J]. Genes & Development, 1996, 10(18): 2276-2288.
[6] HAGIWALA D, MIZUNO T, ABE K. Characterization of NikA histidine kinase and two response regulators with special reference to osmotic adaptation and asexual development in Aspergillus nidulans[J]. Bioscience, Biotechnology, and Biochemistry, 2009, 73(7): 1566-1571.
[7] WONG SAK HOI J, LAMARRE C, BEAU R, MENEAU I, BEREPIKI A, BARRE A, MELLADO E, READ N D, LATGÉ J P. A novel family of dehydrin-like proteins is involved in stress response in the human fungal pathogen Aspergillus fumigatus[J]. Molecular Biology of the Cell, 2011, 22(11): 1896-1906.
[8] HAGIWARA D, SUZUKI S, KAMEI K, GONOI T, KAWAMOTO S. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus[J]. Fungal Genetics and Biology, 2014, 73: 138-149.
[9] LARA-ROJAS F, SÁNCHEZ O, KAWASAKI L, AGUIRRE J. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions[J]. Molecular Microbiology, 2011, 80(2): 436-54.
[10] SAKAMOTO K, IWASHITA K, YAMADA O, KOBAYASHI K, MIZUNO A, AKITA O, MIKAMI S, SHIMOI H, GOMI K. Aspergillus oryzae AtfA controls conidial germination and stress tolerance[J]. Fungal Genetics and Biology, 2009, 46: 887-897.
[11] 蔡志英, 黃贵修. 巴西橡胶树炭疽病研究进展[J]. 西南林业大学学报, 2011, 31(1): 89-93.
CAI Z Y, HUANG G X. Research advances in anthracnose of Hevea brasiliensis[J]. Journal of Southwest Forestry University, 2011, 31(1): 89-93. (in Chinese)
[12] 李加智, 张春霞, 何明霞. 云南橡胶树叶炭疽病病状及发生近况[J]. 热带农业科技, 2008, 31(3): 13-16.
LI J Z, ZHANG C X, HE M X. Symptoms of anthracnose on rubber leave and its incidence in Yunnan[J]. Tropical Agricultural Science & Technology, 2008, 31(3): 13-16. (in Chinese)
[13] LIU X B, LI B X, CAI J M, ZHENG X L, FENG Y L, HUANG G X. Colletotrichum species causing anthracnose of rubber trees in China[J]. Scientific Reports, 2018, 8(1): 10435.
[14] LIN C H, HUANG G X, ZHENG F C, MIAO W G. Functional characterization of CgPBS2, a MAP kinase in Colletotrichum gloeosporioides, using osmotic stress sensitivity as a selection marker[J]. European Journal of Plant Pathology, 2018, 152(3): 801-813.
[15] WANG J Y, ZHAO X Y, LIAO X M, HE Q G, LI X, LIU W B, YANG Z P, ZHANG Y, LIN C H, MIAO W G. Screening for proteins interacting with the perilipin-like protein CAP20 by a yeast two-hybrid system and identification of a protein kinase a catalytic subunit as an interacting protein in Colletotrichum siamense[J]. European Journal of Plant Pathology, 2020, 156: 971-977.
[16] 方思齐, 李泽栋, 王记圆, 何其光, 李 潇, 刘文波, 张 宇, 林春花, 缪卫国. 利用酵母双杂交技术筛选炭疽菌中与CsSSK1相互作用的蛋白质[J]. 热带作物学报, 2021, 42(1): 198-204.
FANG S Q, LI Z D, WANG J Y, HE Q G, LI X, LIU W B, ZHANG Y, LIN C H, MIAO W G. Screening of proteins interacting with CsSSK1 in Colletotrichum siamense by yeast two-hybrid technique[J]. Chinese Journal of Tropical Crops, 2021, 42(1): 198-204. (in Chinese)
[17] LATCHMAN D S. Transcription factors: an overview[J]. International Journal of Biochemistry & Cell Biology, 1997, 29(12): 1305-1312.
[18] SONG M, FANG S Q, LI Z G, WANG N, LI X, LIU W B, ZHANG Y, LIN C H, MIAO W G. CsAtf1, a bZIP transcription factor, is involved in fludioxonil sensitivity and virulence in the rubber tree anthracnose fungus Colletotrichum siamense[J]. Fungal Genetics and Biology, 2022, 158, 103649.
[19] ZHOU X, MA Y, KATO T, KUNO T. A measurable activation of the bZIP transcription factor Atf1 in a fission yeast strain devoid of stress-activated and cell integrity mitogen-activated protein kinase (MAPK) activities[J]. Journal of Biological Chemistry, 2012, 287(28): 23434-23439.
[20] WANG J, CAO Y, CHEN Y, CHEN Y M, GARDNER P, STEINER D F. Pancreatic beta cells lack a low glucose and O2-inducible mitochondrial protein that augments cell survival[J]. Proceedings of the National Academy of Sciences, 2006, 103: 10636-10641.
[21] BEDO G, VARGAS M, FERREIRO M J, CHALAR C, AGRATI D. Characterization of hypoxia induced gene 1: expression during rat central nervous system maturation and evidence of antisense RNA expression[J]. International Journal of Developmental Biology, 2005, 49(4): 431-436.
[22] STROGOLOVA V, FURNESS A, ROBB-MCGRATH M, GARLICH J, STUART R A. Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex[J]. Molecular and Cellular Biology, 2012, 32(8): 1363-1373.
[23] SHEN C, NETTLETON D, JIANG M, KIM S T, POWELL- COFFMAN J A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans[J]. Journal of Biological Chemistry, 2005, 280(21): 20580-20588.
[24] GARLICH J, STRECKER V, WITTIG I, STUART R A. Analysis of the QRRQ Motif in the yeast Hig1 type 2 protein Rcf1 reveals a regulatory role for the cytochrome c oxidase complex[J]. Journal of Biological Chemistry, 2017, 292(13): 5216-5226.
[25] DAWITZ H, SCHÄFER J, SCHAART J M, MAGITS W, BRZEZINSKI P, OTT M. Rcf1 modulates cytochrome c oxidase activity especially under energy-demanding conditions[J]. Frontiers in Physiology, 2020, 10: 1555.
[26] MELIN P, SCHNÜRER J, WAGNER E G. Characterization of phiA, a gene essential for phialide development in Aspergillus nidulans[J]. Fungal Genetics and Biology, 2003, 40(3): 234-241.
[27] GLASER A G, KIRSCH A I, ZELLER S, MENZ G, RHYNER C, CRAMERI R. Molecular and immunological characterization of Asp f 34, a novel major cell wall allergen of Aspergillus fumigatus[J]. Allergy, 2009, 64(8): 1144-1151.
[28] HE X J, LI X L, LI Y Z. Disruption of Cerevisin via Agrobacterium tumefaciens-mediated transformation affects microsclerotia formation and virulence of Verticillium dahlia[J]. Plant Pathology, 2015, 64(5): 1157-1167.
