Design and characterization of a bi-functional bybrid antibacterial peptide LLM against Pseudomonas aeruginosa
2022-02-04ZHANGZejinYAOHongjiXUEZhaoyiZHANYutongGUOYingjieWUMengLINKexinXIONGJingyanZHANGYong
ZHANG Ze-jin, YAO Hong-ji, XUE Zhao-yi, ZHAN Yu-tong, GUO Ying-jie, WU Meng,LIN Ke-xin, XIONG Jing-yan, ZHANG Yong✉
1. School of Basic Medicine and Life Science, Hainan Medical University, Haikou 571199, China
2. The First Clinical College of Hainan Medical University, Haikou 571199, China
Keywords:
ABSTRACT
1. Introduction
EThe drug resistance caused by traditional antibiotics using widespreadly has long been a difficult problem for clinical treatment in all around the world , which take a great threat to human health.Antibiotic resistance, especially Gram-negative bacteria, is severity and danger [1]. P.aeruginosa, belong to gram-negative bacteria,natural resistance to many antibiotics, is one of the major challenges of clinical infection. The treatment of P.aeruginosa infection also face the inflammatory storm from the release of endotoxin[2], which further induce multiple organ failure, such as sepsis [3], increasing the difficulty of clinical treatment. To get new potential drugs with anti-P.aeruginosa and endotoxin production reducing activity is one of the effective ways to treat P.aeruginosa infection.
LBP (86-99) and BPI (86-99) are two polypeptides with endotoxin neutralization by binding LPS (endotoxin) and blocking the physiological effect fromendotoxin, but their antibacterial activity against Gram-negative bacteria is unsatisfactory [4]. Myxinidin is a fish antimicrobial peptide against Gram-negative bacteria, which naturally exists in the mucus of hagfish surface. It is non-toxic and stable, and has antibacterial activity against P.aeruginosa, but has no endotoxin neutralization effect [5].The aim of this study is to use the GGGS-Linker to achieve one hybrid peptides owntwo different functions as anti-P.aeruginosa activity and endotoxin neutralizing effect. Our study will provide a new ideas and reference for the designing of new drugs for Gram-negative bacterial infection treatment.
2. Materials and Methods
2.1 Materials
2.1.1 Strains
P.aeruginosa CMCC10104 (P.eruginosa CMCC10104) and P.aeruginosa ATCC 9027 (P.aeruginosa ATCC 9027) were stored in our laboratory.
2.1.2 Main reagents and instruments
Nutrient broth medium, AGAR, sodium chloride, chromogenic matrix Limulus amebocyte lysate kit and other reagents were purchased from Beijing Solaibao Technology Co., LTD.Main instruments include: biosafety cabinet (Haier, HR900-IIA2),centrifuge, constant temperature incubator, multi-function microplate reader.
2.2 Methods
2.2.1 Design and synthesis of bifunctional heterozygous peptides
LBP (86-99) and BPI (86-99) could bind LPS, but their activity against P.aeruginosa was weak. Myxinidin is a fish antimicrobial peptide, which has anti-Gram-negative bacterial activity, cannot bind LPS, and does not neutralize endotoxin. In this study, the GGGS-Linker was used to achieve new hybrid peptides BLM and LLM (Table 1). The mother peptide and heterozygous peptide were synthesized by solid phase synthesis with purity>99%. The physicochemical parameters were analyzed by EMBOSS and ExPASy bioinformatics software.
2.2.2 Bacterial culture
P.aeruginosa CMCC10104 and P.aeruginosa ATCC 9027 single colonies were inoculated in MH medium and incubated at 37 ℃and 200 rpm for 18-24 h to obtain P.aeruginosa suspension. diluting as required for relevant experiments by MH medium. P.aeruginosa plate counting were cultured in MH solid medium.
2.2.3 Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) test
The MICs were determined according to our previous study [6] as follows: the carboxylbenzillin (CA) as a positive drug and ampicillin(Amp) as a negative drug were diluted twice with a maximum concentration of 256 μM. The MIC plate was prepared by adding 50 μL of serial peptides and antibiotics drug solution into a 96-well plate for each concentration. P.aeruginosa CMCC10104 and P.aeruginosa ATCC 9027 were cultured, diluted with MH medium and prepared into 2.0×105CFU/mL bacterial solution. 50 μL bacterial suspension was added to each well of MIC plate and incubated at 37 ℃ for 16-18 h.The lowest concentration of clarified culture wells was recorded as MIC. The number and concentration of P.aeruginosa in each well were determined by plate counting including MIC concentration and above. The MBC of the target was the lowest drug concentration corresponding to the culture well with the number of colonies lower than 99.9% of the initial inoculum size in each treatment well.
2.2.4 Time-kill curve assay
The method for determination of time-kill curve assay was summarized as follow: 2.0×105CFU/mL p.eruginosaat CC9027 solution was prepared as above, and 50 μL of bacterial solution was added to a 96-well plate. LLM solutions at final concentrations of 1×, 2×, and 4×MIC were added and incubated at 37 ℃, MH medium and 2×MIC CA as blank and positive controls. The samples were taken at 0, 1, 2, 4, 6 and 12 h time points, and serial dilutions of MH medium were plated to count visible colonies, and the time-kill curve was drawn.
2.2.5 Endotoxin neutralization experiments
The method of endotoxin neutralization experiments were referred to the previous study[6] as follows:
(1) Sample preparation: Preparation of 1.0×105CFU / mL P.aeruginosa ATCC 9027 bacterial solution. LLM with a final concentration of 4×MIC, 2×MIC CA and drug-free bacterial medium were prepared and seeded into 96-well cell culture plates with 6 replicates and incubated at 37 ℃ for 2 h.
(2) Bacterial endotoxin detection by limulus amebocyte lysate:3 replicates wells were selected for each treatment group, and the supernatant was taken, and the endotoxin level was measured by limulus amebocyte lysate. The detection methods as chromogenic matrix limulus lysate kit specification (T7571, Solibo).
(3) Detection of bacteria number of P.aeruginosa: 3 replicates wells were selected for each treatment group, and colony plate counts were performed to evaluate the antimicrobial activity of LLM.
2.2.6 Hemolytic assay
Hemolytic assay was determined by reference to our previous study[6]. A series of LLM solutions were obtained from 230 μM (800 μg/mL) to 0.1 μg / mL by doubling dilution. Take 5 mL whole blood from rabbits and collect red blood cells at 1500 rpm, 4 ℃ for 5 min.Erythrocyte precipitation was resuspended in 1mL sterile 0.9%NaCl solution, and the supernatant was removed by centrifugation as above. Repeat 3 times until colorless and transparent. A certain volume of normal saline was added to prepare 8% red blood cell suspension, and 100 μL LLM and red blood cell suspension were mixed into a 96-well plate. Sterile normal saline and 0.1%TritonX-100 were used as the control group. After incubation at 37 ℃ for 1h, the red blood cell solution in each well was transferred to a 1.5mL centrifuge tube at 1500rpm. After centrifugation at 4 ℃ for 5min, 100 μL of the supernatant was transferred to a new 96-well microplate, and the absorbance value at 540 nm was measured.The hemolysis was defined as 0% in the saline treatment group and 100% in the 0.1%TritonX-100. Hemolysis of each treatment group was calculated according to the following formula:
Hemolysis (%) =[(Abs540nmheterozygous peptide LLM-Abs540nmsaline)/(Abs540nm0.1%Triton X-100-Abs540nmsaline)]×100%
2.2.7 Statistical analysis methods
Graphpad Prism9.0 software was used for data processing and drawing. The t-test was used for comparison between different treatment groups. P < 0.05 was considered significant difference.
3. Results
3.1 Peptide design
In this study, BPI-Linker-Myx (BLM) and LBP-Linker-MyX1(LLM) antimicrobial peptides were designed using BPI, LBP and Myxinidin as the parent peptide and GGGS-Linker as the hinge. The theoretical molecular weight, positive charge number, isoelectric point, hydrophobic moment (μH) and hydrophobic (GRAVY)of related antimicrobial peptides were analyzed, and the specific parameters were shown in Table 1. Compared with the Myxinidin,the positive charges carried by BLM and LLM increased to +9 and +8, respectively, while GRAVY value decreased. In addition,the hydrophobic torque of BLM increased while the hydrophobic torque of LLM decreased. The mass spectrum identification show that, the molecular weight of BLM was 3371.2, which was close to the theoretical molecular weight of 3365 (Figure 1A) LLM mass spectrometry was 3459.6, which was also close to the theoretical molecular weight of 3454 (Figure 1B).
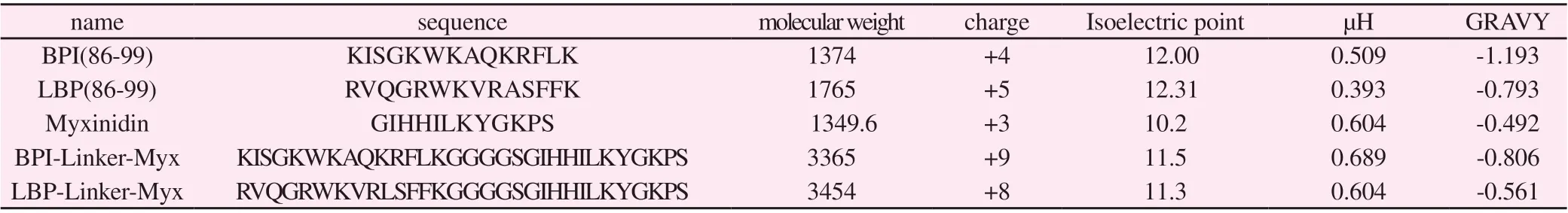
Tab1 Polypeptide sequence and physicochemical parameters
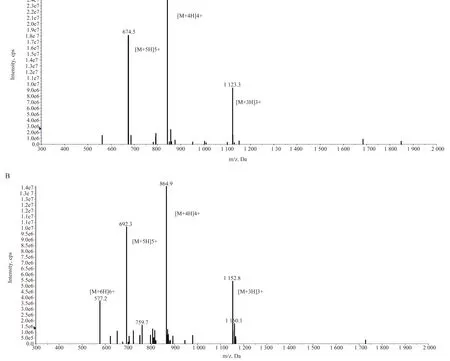
Fig1 Mass spectrometry
3.2 Antibacterial effect of BLM and LLM
P.aeruginosa CMCC10104 and P.aeruginosa ATCC 9027 were used as the target strainsin this study. The titers of CA and Amp against P.aeruginosa were determined as control. Maternal peptide BPI(86-99), LBP (86-99) and Myxinidin showed certain antibacterial activity against P.aeruginosa CMCC10104 and P.aeruginosa ATCC 9027 (Table 2). The MICs for P.aeruginosa CMCC10104 and P.aeruginosa ATCC 9027 were 32, 32 and 16 μM respectively. The BLM had no antibacterial activity against P.aeruginosa CMCC10104 and P.aeruginosa ATCC 9027 with MIC greater than 128μM. LLM showed strong anti-P.aeruginosa activity, with MICs of 2 - 4 μM against P.aeruginosa CMCC10104 and P.aeruginosa ATCC9027,respectively. Compared with the parent peptides, the antimicrobial activity was increased by 8-16 times (Table 2). The results of MBC determination are shown in Table 2. The MBC value of the parent peptide is 2 - 4 times of the MIC value, that is, MBC/MIC=2.However, the MBC value of LLM was the same as MIC value, that is, MBC/MIC=1, suggesting that LLM had stronger bactericidal activity. CA showed significant activity against P.aeruginosa,with MICs of 2 - 4 μM against p.aeruginosa CMCC10104 and p.aeruginosa ATCC9027, respectively, but with MBC/MIC values of 2 or 4 (Table 2). However, ampicillin was ineffective against P.aeruginosa, which was consistent with the theory (Table 2).

Tab2 The results of MIC and MBC (μmol/L)
3.3 Time-kill curve assays of LLM
Time-kill curve assays is an important parameter reflecting bactericidal characteristics of antibiotic. P.aeruginosa CMCC10104 and P.aeruginosa ATCC9027 were selected as the target bacteria.The initial inoculum levels of P.aeruginosa CMCC10104 and p.aeruginosa ATCC9027 were (4.46 ± 0.25) log10 CFU/mL and(4.20±0.09) log10 CFU/mL, respectively. After incubation at 37℃ for 12 h, the CK group without LLM intervention increased to (8.46±0.20) log10 CFU/mL and (8.34±0.07) log10CFU/mL,respectively (figure.2). After 1 h of LLM treatment with 1×LIC,2×MIC and 4×MIC, the number of colonies of p.aeruginosa CMCC10104 decreased by 2.7, 2.9 and 3.2 log10 CFU/mL,respectively (FIG. 2A). However, p.aeruginosa ATCC9027 reduced 3.0, 2.7 and 3.2 log10 CFU/ mL (FIG. 2B). After 12h of treatment, p.aeruginosa CMCC10104 and P.aeruginosa ATCC9027 decreased by 3.4 and 3.2 log10 CFU/mL, respectively (figure. 2A and B).Compared with CK group, the number of colonies in LLM group with 4×MIC for P.aeruginosa CMCC10104 and P.aeruginosa ATCC9027 decreased by 7.5 and 7.3 log10 CFU/mL, respectively.In conclusion, LLM showed strong and rapid bactericidal activity against P.aeruginosa, and no rebound was observed for 12 h (figure.2).
3.4 Endotoxic neutralization by LLM
P.aeruginosa ATCC9027 was selected as the test bacteria to evaluate the neutralizing effect of hybrid peptide LLM on endotoxin.As shown in figure.3, after 2 h of LLM treatment with 4×MIC, the number of P.aeruginosa ATCC9027 decreased significantly by 3.92 log10 CFU/mL, and CA with 2×MIC decreased by 2.82 Log10 CFU/mL. These results suggested that 4×MIC LLM exhibited stronger activity anti-P.aeruginosa ATCC9027 (figure. 3A). At the same time, the endotoxin level in the culture medium supernatant of P.aeruginosa was detected. The results showed that the endotoxin level in the normal group treated with ddH2O was 0.2 EU/ mL, while the endotoxin concentration in the group treated with 4×MIC LLM was significantly reduced to 0.09 EU/ mL (P <0.001). However, the level of endotoxin in 2×MIC CA treatment group was significantly higher to 0.24 EU/ mL (P <0.05) (figure. 3B). Compared with the normal group, the endotoxin level of the 2×MIC CA treatment group was also significantly increased (figure. 3B).
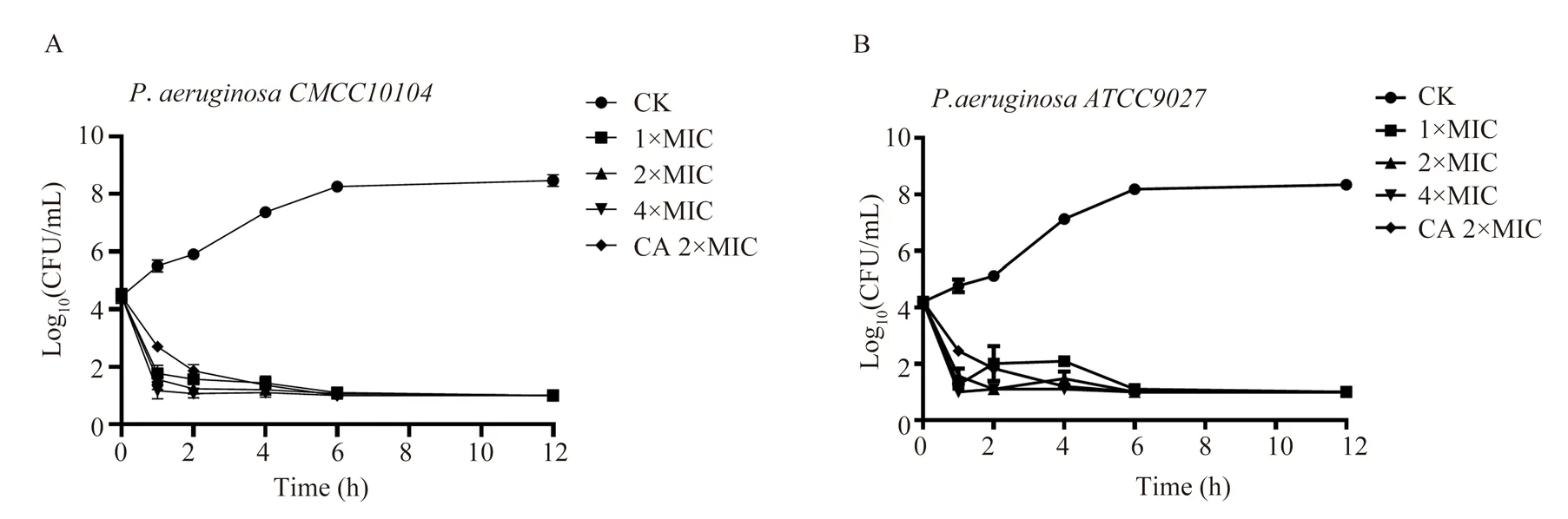
Fig2 Bactericidal kinetic curve of LLM
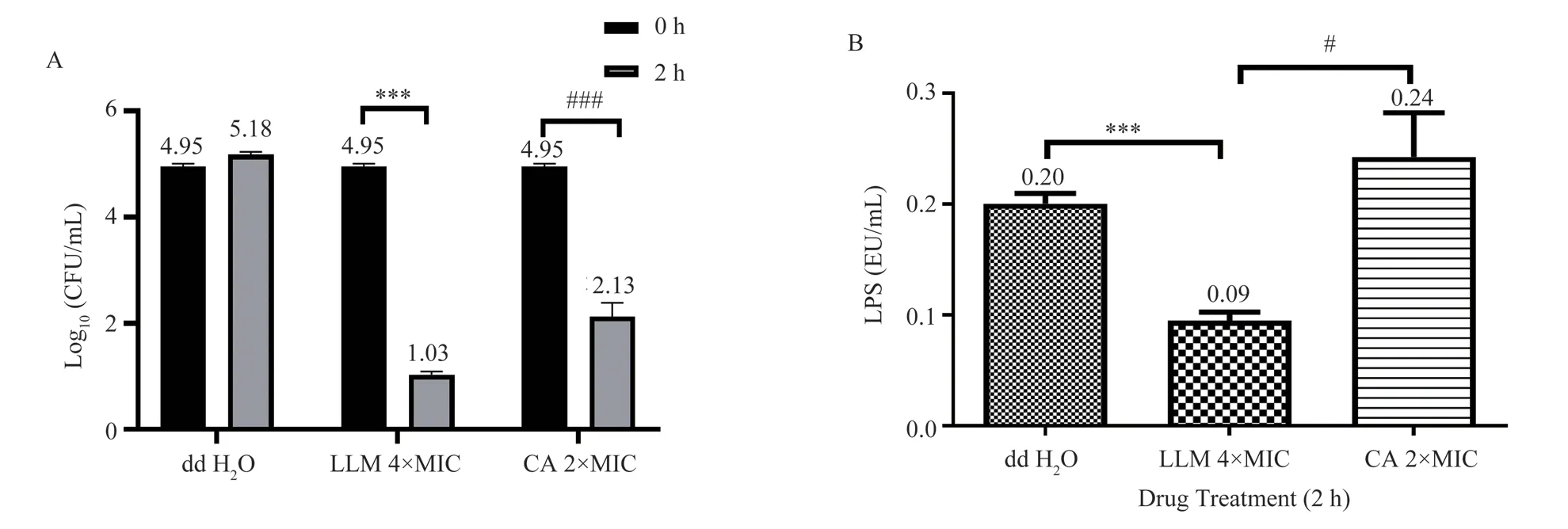
Fig3 The effects of LLM on anti-P.aeruginosa and neutralization endotoxin
3.5 Hemolysis of LLM
Erythrocyte toxicity of LLM was assessed by hemolysis. As shown in Figure 4, the hemolysis of 115μM (400 μg/mL) LLM was 0.5%(Figure 4) when incubated with rabbit erythrocytes, and almost no hemolysis was produced when the concentration was lower than 100μg/mL (28.75 μM), which was much higher than that of MBC.Therefore, LLM heterozygous peptide has no hemolytic toxicity to mammalian erythrocytes in the effective bactericidal concentration.
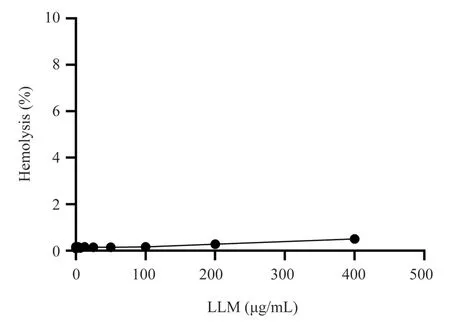
Fig4 Effect of LLM on hemolysis of red blood cells in rabbits
4. Discuss
Antibiotic resistance has become a global public health problem,posing a major threat to human health, especially the increasing number of super bacteria. Gram-negative bacteria because of specific cell wall and outer membrane structure usually exhibited more prominent clinical drug resistance [7-8]. Exploring new antimicrobial agents is one of the effective strategies to solve the problem of antibiotic resistance. Antibacterial peptide, has a wide source,strong antibacterial activity and less resistant polypeptide, is a new kind of anti-infection candidate drug against antibiotic resistance.P.aeruginosa is a gram-negative bacteria, which widely exists in human skin, medical apparatus and instruments, such as water, food and hospital or community air environment, has the strong ability of life [9]. P.aeruginosa belong to the conditional pathogenic bacteria,can infect all organs and parts, cause severe acute or chronic infection, and even cause death [10-11]. However, pseudomonas aeruginosa infection of antibiotic treatment process also faces the challenge of endotoxin release, which can easily induce endotoxemia and increase the difficulty of treatment [2,12-13]. In addition, due to P.aeruginosa resistant to many antibiotics due to its natural drug resistance and structural barrier, which also brings great challenges to the treatment of its clinical infection [2]. It is an effective strategy to explore new bifunctional anti-P.aeruginosa drug candidates with the effects of killing P.aeruginosa and neutralizing endotoxin to solve the above problems [14-15].
Antimicrobial peptides are potential target drug candidates for new antibiotic exploration, which have a variety of biological effects including sterilization, antiviral, anti-tumor and immunity enhancement [16]. Some antimicrobial peptides show neutralizing endotoxin activity by binding to LPS and reducing its activation on immune cells, and inhibiting the production of inflammatory cytokine [17-18]. Myxinidin is a fish antimicrobial peptide against Gram-negative bacterial, but cannot neutralize endotoxin [5]. Many studies have shown that different functional activities peptidescan be connected by polypeptides hinge or flexible linker to form one new multifunctional hybird peptides or proteins, which is an effective ways for drug design [19-21]. In this study, the tetrapeptide GGGSLinker was used to achieve LLM hybrid peptides with dual effects of killing and neutralizing endotoxin of P.aeruginosa, and its MICs was 4-16 times that of Myxinidin. LLM can reduce the LPS releasing from P.aeruginosa during killing, suggested that LLM may retain the independent structural characteristics of the parent peptide LBP (86-89) and its LPS binding ability, thus playing the role of neutralizing LPS. Thus, the strategy of designing multifunctional antimicrobial peptides with flexible linker or hinge is feasible.
The positive charges of antimicrobial peptides were closely related to their antibacterial activity. In general, the higher number of positive charges carried by antimicrobial peptides, may be the stronger interaction to the bacterial cell membrane (negative charge)[22, 23], thus show stronger membrane destruction and antimicrobial activity. Meanwhile, the balance between amphiphilicity and hydrophobicity of antimicrobial peptides is also an important factor affecting antibacterial activity [22]. In this study, we concluded that the enhanced anti-P.aeruginosa activity of LLM may be related to the increase of positive charges and the balance between hydrophilic and hydrophobic. The exact relationship and mechanism need to be further studied. Hemolysis is an important indicator for clinical drug safety evaluation [24]. We further evaluated the toxicity of LLM to erythrocytes and showed that LLM was not toxic to erythrocytes in the range of effective concentrations against P.aeruginosa.In conclusion, a novel antimicrobial peptide of LLM has been designed in this study, which has potent anti-P.aeruginosa and neutralizing LPS acitivity. LLM killed P.aeruginosa at MICs value with 2 - 4 μM, exhibited rapid bactericidal activity with no hemolysis, which has the potential value for P.aeruginosa infection treatment in future.
Author contribution description: Zhang Zejin and Zhang Yong were responsible for the design of this experiment, data processing and writing of the article; Zhang Zejin, Yao Hongji, Zhan Yutong,Xue Zhaoyi, Guo Yingjie, Wu Meng, Lin Kexin and Xiong Jingyan were responsible for the implementation of the experiment and data collection. None of the authors has a conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- In vitro study on the antiviral activity of 9 extracts of traditional Chinese herbal medicine against the human respiratory syncytial virus
- Study on gene regulation mechanism of Qiliqiangxin capsule on myocardial fibrosis in myocardial infarction rats
- Pharmacokinetics of Jiaotai pill self-microemulsion in insomnia rats
- Correlation between IL-33/sST2 signaling pathway and patients with essential hypertensive left ventricular hypertrophy
- Screening and comprehensive analysis of key genes in liver hepatocellular carcinoma based on bioinformatics
- Mechanism of Gan Dou Ling in improving liver fibrosis in Wilson disease based on network pharmacology and experimental verification
