In vitro study on the antiviral activity of 9 extracts of traditional Chinese herbal medicine against the human respiratory syncytial virus
2022-02-04KeyLaboratoryofTropicalTranslationalMedicineofMinistryofEducationHainanMedicalUniversityHaikou571199China
1. Key Laboratory of Tropical Translational Medicine of Ministry of Education, Hainan Medical University, Haikou 571199, China
2. Hainan Provincial Key Laboratory of R&D on Tropic Plants, Hainan Medical University, Haikou 571199, China
3. Haikou Key Laboratory of Li Nationality Medicine, Hainan Medical University, Haikou 571199, China
4. School of Pharmacy, Hainan Medical University, Haikou 571199, China
5. Hainan Medical University-The University of Hong Kong Joint Laboratory of Tropical Infectious Diseases, Hainan Medical University, Haikou 571199, China
Keywords:
ABSTRACT
1. Introduction
Human respiratory syncytial virus (HRSV) is a non-segmented single-stranded negative-stranded RNA virus with an enveloped structure, belonging to the order of single-stranded negative-stranded virus, Paramyxoviridae, Pneumovirus subfamily, Pneumovirus genus . The virus was first isolated from chimpanzees by Morris et al. in 1956, and has since been isolated from infants with severe lower respiratory tract disease [1]. HRSV is one of the most common viral pathogens causing acute lower respiratory infection (ALRI)in infants and young children [2]. Today, HRSV remains a common cause of bronchiolitis in children under one year of age, especially in premature infants [3]. According to Zhang et al.'s statistical analysis of the detection results from 135 reported literatures, among the pathogen detection results of 489 641 patients with acute respiratory tract infection, the infection rate of HRSV was the highest, as high as 18.7%, which was higher than that of influenza virus and parainfluenza virus. The infection rate of common respiratory pathogens such as rhinovirus and rhinovirus, most of which are infants and young children [4]. Currently, only palivizumab and ribavirin are approved for the prevention or treatment of HRSV infection. Palivizumab is a humanized monoclonal antibody that has been shown to significantly reduce disease severity in highrisk children, but it can only be used to prevent HRSV infection in high-risk children, not to treat HRSV infection. It is expensive and cannot meet the needs of clinical applications [5]. Ribavirin is a broad-spectrum antiviral drug, but it has large toxic and side effects,and is generally only recommended for severe infants in high-risk groups of HRSV infection [6]. Although cardiac glycosides, digoxin and cyclopiazonic acid have been reported to have anti-HRSV infection activity and some drugs have been in clinical trials, but at present no other anti-infective and therapeutic drugs are available [7,8]. Traditional Chinese medicine antiviral has the advantages of less toxic and side effects, high efficiency, and economic benefits, and has received extensive attention from researchers at home and abroad[9, 10]. Evodia lepta, Clausena lansium, Clerodendrum cyrtophyllum,Callicarpa nudiflora, Nauclea officinalis, Elaeagnus gonyanthes and Zanthoxylumarmatum are traditional Chinese medicines with heatclearing, detoxifying and anti-inflammatory effects, which are commonly used in the treatment of clinical inflammation-related infectious diseases [11-13].
In this experiment, a total of 9 traditional Chinese medicine extracts (the water extract of Evodia lepta, Clausena lansium,Clerodendrum cyrtophyllum, Callicarpa nudiflora, Nauclea officinalis and Elaeagnus gonyanthes, the alcohol extract of Nauclea officinalis,Elaeagnus gonyanthes and Zanthoxylumarmatum) were evaluated for their anti-HRSV effects in vitro, in order to provide a basis for the development of new anti-HRSV drugs.
2. Materials and methods
2.1 Materials
2.1.1 Cells and viruses
Hep-2 (ATCC CCL-23) cells and human respiratory syncytial virus type A (HRSV-A, ATCC VR-26) were donated by the Pasteur Institute, Shanghai, Chinese Academy of Sciences, and kept in our laboratory.
2.1.2 Source of medicinal materials
(1) Evodia lepta was collected from Limus Mountain, Qiongzhong,Hainan, and was identified as the root of Evodia lepta (Spreng.)Merr., a plant of the Rutaceae family, Evodia lepta (Spreng.) Merr.,by Professor Tian Jianping of Hainan Medical College.
(2) Clausena lansium was collected in Tunchang, Hainan, and identified as the leaves of Clausena lansium (Lour.) Skeels, a genus of Rutaceae, by Professor Tian Jianping of Hainan Medical College.
(3) Clerodendrum cyrtophyllum was collected in Yongxing,Haikou, and was identified as the aerial part of the Verbena plant Clerodendrum cyrtophyllum Turcz. by Professor Tian Jianping of Hainan Medical College.
(4) Callicarpa nudiflora was collected in Wuzhi Mountain, Hainan,and was identified as the leaves of the Verbena plant Callicarpa nudiflora Hook.et Arn. by Professor Tian Jianping of Hainan Medical College.
(5) Nauclea officinalis was collected in Qiongzhong, Hainan, and was identified by Professor Tian Jianping of Hainan Medical College as the stem and branch of the Rubiaceae Nauclea officinalis Pierre.ex Pitard.
(6) Elaeagnus gonyanthes was collected from Wuzhi Mountain in Hainan and identified by Professor Tian Jianping of Hainan Medical College as the root of Elaeagnus gonyanthes Benth.
(7) Zanthoxylumarmatum was collected in Nanning, Guangxi, and was identified as the root of Zanthoxylumarmatum DC., a Rutaceae plant, by Professor Tian Jianping of Hainan Medical College.
2.1.3 Sample preparation
(1) Water extract of Evodia lepta: take 100 g of Evodia lepta medicinal material, add more than 8 times the amount of water for 2 extractions, combine the 2 extracts, and then rotate to 100 mL after suction filtration. Measure 50 mL of the extract and evaporate it to dryness at 80 ℃ to obtain 4.9 g of the extract. The calculated content of the extract is 98 mg/mL.
(2) Water extract of Clausena lansium: take 100 g of Clausena lansium medicinal material, add more than 8 times the amount of water for 2 extractions, combine the 2 extracts, and then rotate to 100 mL after suction filtration. Measure 50 mL of the extract and evaporate to dryness at 80 ℃ to obtain 11.9 g of the extract, and the calculated content of the extract is 238 mg/mL.
(3) Water extract of Clerodendrum cyrtophyllum: Take 100 g of Clerodendrum cyrtophyllum medicinal material, add more than 8 times the amount of water for 2 extractions, combine the 2 extracts,and then rotate to 100 mL after suction filtration. Measure 50 mL of the extract and evaporate to dryness at 80 ℃ to obtain 12.7 g of the extract. The calculated content of the extract is 254 mg/mL.
(4) Water extract of Callicarpa nudiflora: Take 100 g of Callicarpa nudiflora, add more than 8 times the amount of water for 2 extractions, combine the 2 extracts, filter and rotate to 100 mL liters.Measure 50 mL of the extract and evaporate to dryness at 80 ℃ to obtain 2.5 g of the extract. The calculated content of the extract is 50 mg/mL.
(5) Water extract of Nauclea officinalis: Take 100 g of Nauclea officinalis medicinal material, add more than 8 times of water for 2 extractions, combine the 2 extracts, and then rotate to 100 mL after suction filtration. Measure 50 mL of the extract and evaporate to dryness at 80 ℃ to obtain 3.2 g of the extract. The calculated content of the extract is 64 mg/mL.
(6) Alcoholic extract of Nauclea officinalis: take 20 g of Nauclea officinalis medicinal material, add 8 times the amount of 95% ethanol to extract twice, combine the two extracts, and dry under reduced pressure to obtain 0.557 g of extract, and accurately weigh the alcoholic extract of Nauclea officinalis 32 mg, add 1 mL of DMSO to dissolve, and obtain a bile sample with a content of 32 mg/mL.
(7) Alcoholic extract of Elaeagnus gonyanthes: Take 50 g of Elaeagnus gonyanthes medicinal material, add 8 times the amount of 95% ethanol to extract twice, combine the two extracts, and dry under reduced pressure to obtain 2.369 g of extract, which is precisely weighed. Take 30 mg of Elaeagnus gonyanthes extract, add 1 mL of DMSO to dissolve, and obtain the alcohol extract sample of Elaeagnus gonyanthes with a content of 30 mg/mL.
(8) Water extract of Elaeagnus gonyanthes; take 50 g of Elaeagnus gonyanthes medicinal material, add more than 8 times the amount of water for 2 extractions, combine the 2 extracts, and then rotate to 100 mL after suction filtration. Measure 50 mL of the extract and evaporate to dryness at 80 ℃ to obtain 1.929 g of the extract. The calculated content of the extract is 38.6 mg/mL.
(9) Alcoholic extract of Zanthoxylumarmatum: take 50 g of medicinal materials, add 8 times the amount of 95% ethanol to extract twice, combine the two extracts, and dry under reduced pressure to obtain 4.7 g of extract, and accurately weigh the extract of Zanthoxylumarmatum 20 mg, add 1 mL of DMSO to dissolve,and obtain a sample of Zanthoxylumarmatum with a content of 20 mg/mL.
(10) Epigallocatechin gallate (EGCG) was purchased from Chengdu Purifa Technology Development Co., Ltd. as a positive control.
2.1.4 Main reagents and instrumentsDMEM medium, fetal bovine serum (FBS), trypsin (0.25% EDTATrypsin), antibiotics (penicillin and streptomycin), and PBS buffer were purchased from Gibco BRL Company, and CCK-8 reagent was purchased from Dojindo Dongren Chemical Science and Technology(Shanghai) Co., Ltd., the biological safety cabinet was purchased from Sujing Antai Company, the carbon dioxide incubator was purchased from Esco Company, the biological inverted microscope(XDS-1B) was purchased from Chongqing Optical Instrument Factory, and the microplate reader (SpectraMax i3x) was purchased from Molecular Devices Corporation.
2.2 Methods
2.2.1 Cell Culture
The passaged cells Hep-2 were cultured in T25 cell culture flasks and cultured in DMEM medium containing 10% FBS, and the cell growth was observed every day. When the growth reaches 90% of the culture flask, discard the old culture medium, digest the cells with trypsin (0.25% EDTA-Trypsin), remove the trypsin, and dilute with new DMEM medium containing 10% FBS and 1% antibiotics.Transfer to a new culture flask or cell culture plate and culture in a 37 ℃, 5% CO2 incubator.
2.2.2 Virus culture
Passage Hep-2 cells one day in advance. When the cells reach 80% ~90% of the culture flask, discard the cell culture medium, rinse with PBS buffer and add 200 μL virus stock solution and 800 μL DMEM medium containing 2% FBS , placed in a 37 C, 5% CO2incubator for 1.5 h, shaking the culture flask every 15 min to fully infect the cells with the virus. Supplement with 2% FBS medium to 5 mL.After culturing for 2-3 d, when 80% ~ 90% cytopathic changes were observed, the culture medium was aspirated, centrifuged, and the supernatant was aliquoted, quick-frozen in liquid nitrogen for 10 min, and then stored in a -80 ℃ refrigerator.
2.2.3 Determination of virus titer TCID50
Hep-2 cells were diluted to 1 × 104cells/mL in DMEM medium containing 2% FBS. The virus was diluted according to a gradient of 10-1, 10-2, 10-3, 10-4, 10-5, 10-6, 10-7, 10-8, and the diluted virus solution was mixed with the diluted cells in equal volumes. Add to 96-well plate, 100 μL per well, 6 wells per gradient. For cell control, only 100 μL of medium containing 5×103cells was added to each well.The cells were cultured in a 37 ℃, 5% CO2incubator, and the degree of cytopathic changes was observed every day. After 5 days of culture, the cell viability was measured according to the instructions of the CCK-8 kit and the cell survival rate was calculated. If the cell survival rate in each well is less than or equal to 50%, it is positive for virus infection, and if it is more than 50%, it is negative for virus infection. According to the Reed-Muench method, the half-infectious dose of virus ( TCID50 ) is calculated. The titer of virus HRSV-A used in this experiment was 6.32×105TCID50/mL.
2.2.4 Toxicity experiments of drugs on cells
Spread Hep-2 cells into a 96-well plate at 1×104cells per well,incubate at 37 ℃, 5% CO2incubator for 24 h until the cells grow into a monolayer, and use DMEM medium containing 2% FBS to press the drug. 2-fold dilution to a series of different concentrations,discard the old culture medium in the wells, add a series of drug dilutions of different concentrations, 100 μL per well, cell control wells and blank control wells without drug, add 100 μL containing 2% DMEM medium with FBS was placed in a 37 ℃, 5% CO2incubator for 48 h. Three replicate wells were set for each drug concentration. The cytopathic conditions of the drug-treated group were observed under a microscope, and the cell viability was determined by the CCK-8 method. 10 μL of CCK-8 reagent was added to each well according to the instructions, and incubated at 37 ℃ in a 5% CO2incubator for 1.5 h, and the microplate reader was measured at 450 nm. OD value. Calculate the cell viability (%)of each concentration of the drug = [(ODsample-ODblankcontrol)/(ODcellcontrol-ODblankcontrol)]×100%, use the nonlinear regression method of GraphPad Prism 7.04 software to calculate the half of the drug Toxicity concentration (CC50) [14].
2.2.5 Inhibitory effect of drugs on HRSV-A
Spread Hep-2 cells into a 96-well plate at 1×104cells per well,incubate at 37 ℃, 5% CO2for 24 h until the cells grow into a monolayer, and dilute the drug with DMEM medium containing 2% FBS. to the desired concentration. Dilute the virus to a certain concentration (calculated at MOI=0.5 per well) with DMEM medium containing 2% FBS. Mix the diluted drugs of different concentrations with the virus diluent in an equal volume of 1:1,and mix them evenly. Discard the old culture medium in the wells,add the drug and virus mixture, 100 μL per well. Cell control wells and blank control wells were not added with drugs and viruses,100 μL of DMEM medium containing 2% FBS was added, and cultured in a 37 ℃, 5% CO2incubator for 48h. Three replicate wells were set for each drug concentration. Add 10 μL of CCK-8 reagent to each well according to the instructions, incubate at 37 ℃in a 5% CO2incubator for 1.5 h, and measure the OD value with a microplate reader at 450 nm. Calculate the cell viability (%) of each concentration of the drug = [(ODsample-ODblank)/(ODcellcontrol-ODblankcontrol)]×100%, and use the nonlinear regression method of GraphPad Prism 7.04 software to calculate the half effective concentration of the drug (EC50) [14]. Therapeutic index (TI) = drug median toxic concentration (CC50) / drug median effective concentration (EC50)
3. Results
3.1 Toxicity experiments of drugs on cells
In order to evaluate the toxic effect of the drug on cells, the drug was diluted to a series of concentrations in a 2-fold gradient and added to Hep-2 cells respectively. After 48 hours of culture, the cell viability was detected by the CCK-8 method, and the CC50was calculated. The results are shown in Figure 1, with the increase of the concentration of each drug, the cell viability showed a significant decreasing trend. The CC50of the Evodia lepta water extract, Clausena lansium water extract, Clerodendrum cyrtophyllum water extract, Callicarpa nudiflora water extract, Nauclea officinalis water extract, Nauclea officinalis alcohol extract, Elaeagnus gonyanthes alcohol extract, Elaeagnus gonyanthes water extract and Zanthoxylumarmatum alcohol extract were 1.85 mg/mL, >2.38 mg/mL, 0.93 mg/mL, 0.65 mg/mL, 0.85 mg/mL, 1.00 mg/mL,0.32 mg/mL, 2.84 mg/mL and 0.10 mg/mL, respectively (Figure 1 A-I). Among them, the water extract of Clausena lansium has the weakest toxic effect on cells, and the cell survival rate can still reach more than 95% at the maximum concentration of 2.38 mg/mL in this experiment, and the CC50is > 2.38 mg/mL (Figure 1 B).Among the drugs tested in this experiment, the alcoholic extract of Zanthoxylumarmatum was the most toxic to cells, with a CC50 of 0.1 mg/mL (Figure 1I). The CC50 of the positive control EGCG was 0.04 mg/mL (Figure 1J).
3.2 Inhibitory effect of drugs on HRSV-A
According to the results of cytotoxicity experiments, drug concentrations with cell viability greater than 50% were selected for antiviral experiments, and the cell viability and half effective concentration (EC50) of different concentrations of each drug were calculated. The results are shown in Figure 2 and Figure 3, except that the alcohol extract of Zanthoxylumarmatum has no obvious inhibitory effect on HRSV-A (Figure 2 I), and no EC50results, the other 8 tested drugs (Evodia lepta water extract, Clausena lansium water extract, Clerodendrum cyrtophyllum water extract, Callicarpa nudiflora water extract, Nauclea officinalis water extract, Nauclea officinalis alcohol extract, Elaeagnus gonyanthes alcohol extract and Elaeagnus gonyanthes water extract ) has different degrees of inhibition, and all are dose-dependent, with EC50of 0.30 mg/mL,0.70 mg/mL, 0.05 mg/mL, 0.03 mg/mL, 0.11 mg/mL, 0.53 mg/mL,>2 mg/mL and 0.05 mg/mL, respectively (Figure 2 A-H). Among them, the most inhibitory effect on HRSV-A is the water extract of Callicarpa nudiflora, with an EC50of only 0.03 mg/mL (Figure 2D),followed by the water extract of Clerodendrum cyrtophyllum and Elaeagnus gonyanthes, with EC50both were 0.05 mg/mL (Figure 2 C and H). The EC50of the positive control EGCG was 0.002 mg/mL(Figure 2 J).

Fig1 Cytotoxic effect of different concentrations of drugs on Hep-2 cells

Fig2 Anti-HRSV effect of different concentrations of drugs
Virus HRSV-A infection of cells Hep-2 will lead to cell fusion to form multinucleated syncytia, and cytopathic conditions can be observed under a light microscope. The results are shown in Figure 3, large pieces of syncytia appeared in the virus-infected control group, and most of the cells fell off and floated (Figure 3 B). There was no obvious syncytia in the treatment groups of the water extract of Callicarpa nudiflora and the water extract of Clerodendrum cyrtophyllum (Figure 3 C and D), and the cell morphology was close to that of the normal cell control group (Figure 3 A). There were very few syncytia in the water extract-treated group of Elaeagnus gonyanthes, but the morphology of most cells was still close to that of the normal cell control group (Figure 3A).
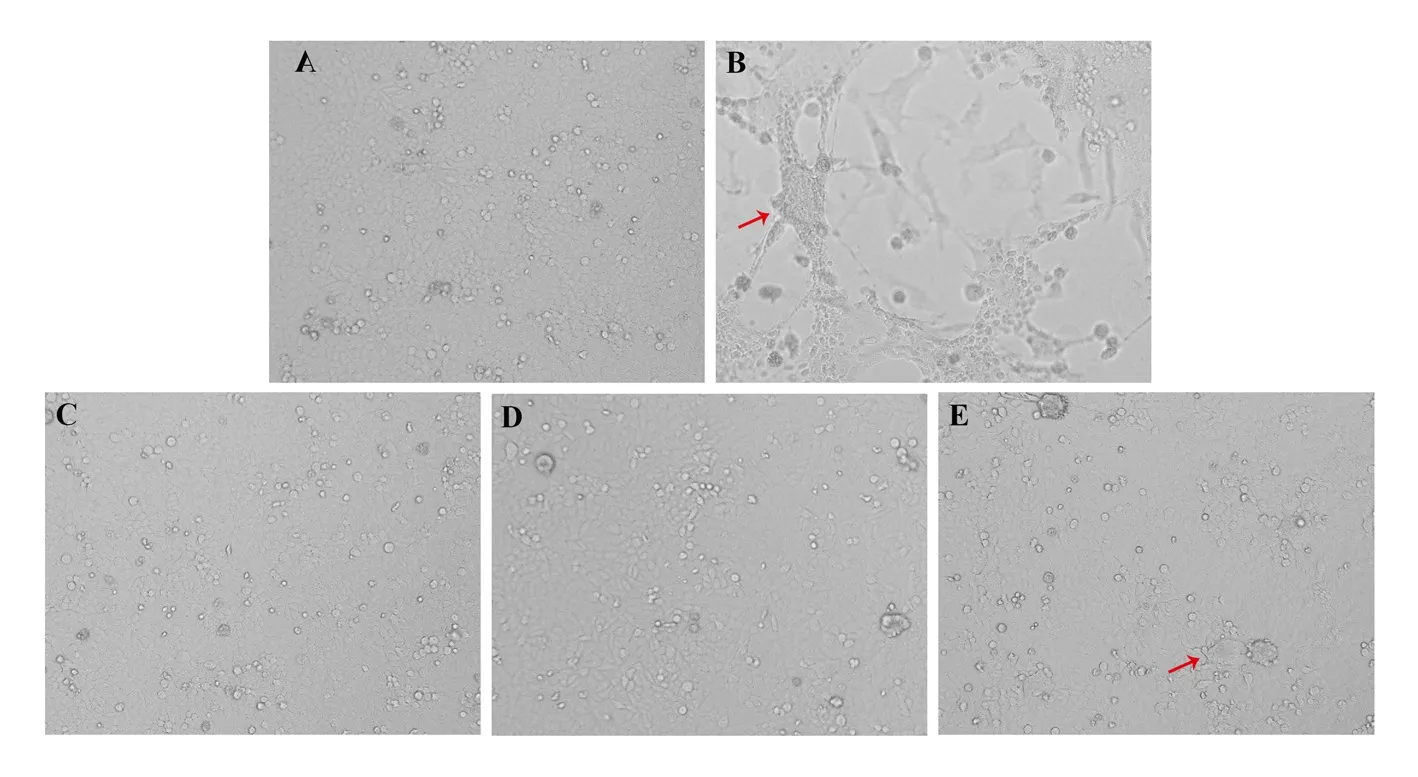
Fig3 Morphology of Hep-2 cells treated with drugs (200 ×)
The therapeutic index (TI) of each drug was calculated, and the results were shown in Table 1. The highest TI was the water extract of Elaeagnus gonyanthes, which reached 56.80, followed by the water extract of Callicarpa nudiflora and Clerodendrum cyrtophyllum were 21.67 and 18.60, respectively, and the TI of the positive control EGCG was 20.00.

Tab1 Therapeutic index (TI) of drugs
4. Discussion
Respiratory syncytial virus (RSV) is one of the most common viral pathogens causing acute lower respiratory tract infection in infants and young children, posing a serious threat to the health of infants and young children. So far, only palivizumab and ribavirin are clinically approved for the prevention or treatment of respiratory syncytial virus infection, and the effect is not good. For a long time,traditional Chinese medicine has played an important role in the treatment of viral infectious diseases, and has the advantages of obvious anti-virus, less toxic and side effects, and rich sources[15].At present, a variety of traditional Chinese medicines have been reported to have anti-HRSV activity, and the compounds mainly include Jinxin Oral Liquid, Jinzhen Oral Liquid, Dingchuan Decoction, No. The single traditional Chinese medicines mainly include Scutellaria baicalensis extract, epigallocatechin gallate(EGCG), Potentilla discolor and total flavonoids of chrysanthemum[16-20].
Chinese medicine water extraction and alcohol extraction are the two most commonly used extraction methods. Among them,water extraction mainly obtains the hydrophilic components in the medicinal materials, and 95% ethanol extraction can obtain the lipophilic components in the medicinal materials. Evodia lepta,Clausena lansium, Clerodendrum cyrtophyllum and Callicarpa nudiflora are mainly flavonoids and phenolic glycosides[21-24],which have good water solubility, so the method of water extraction was selected in this experiment; while Zanthoxylumarmatum is mainly lignans compounds [25], the water solubility is poor, so the method of 95% ethanol extraction is adopted in this paper; for the less-researched Nauclea officinalis and Elaeagnus gonyanthes,two extraction methods were used to observe and compare in the experiment. The results of this study show that, The Clerodendrum cyrtophyllum water extract, the water extract of Callicarpa nudiflora and the water extract of Elaeagnus gonyanthes have good anti-HRSV-A effect. Among them, the water extract of Callicarpa nudiflora has the strongest anti-HRSV-A activity with the lowest EC50 of 0.03 mg/mL. Therapeutic index (TI) is usually a reference index for evaluating the safety of drugs. Our study found that the TI value of the water extract of Callicarpa nudiflora was comparable to that of the positive control EGCG, but the CC50of the aqueous extract of Callicarpa nudiflora was much higher than that of EGCG.CC50, suggesting that the water extract of Callicarpa nudiflora may have lower toxicity. In addition, the TI value (56.80) of the water extract of Elaeagnus gonyanthes is the highest relative to the water extract of Clerodendrum cyrtophyllum, the water extract of Callicarpa nudiflora and EGCG, which suggests that the safety of Elaeagnus gonyanthes may be better than that of Clerodendrum cyrtophyllum water. Extract, Callicarpa nudiflora water extract and EGGG, It also has potential development and application value.
Callicarpa nudiflora is a commonly used traditional ethnic medicinal material in Hainan. It is mainly used for hemostasis and anti-inflammatory in clinical practice. In addition, studies have shown that Callicarpa nudiflora also has antibacterial, antiviral,wound healing, antioxidative and antitumor properties. function [11,26]. It has been reported that Callicarpa nudiflora has anti-herpes simplex virus and EV-71 virus activity [27, 28], but there is no report on its anti-HRSV, this study found that Callicarpa nudiflora water extract has strong anti-HRSV-A active. Elaeagnus gonyanthes is one of the traditional medicinal materials commonly used in the Li nationality area of Hainan. It is mainly used for the treatment of rheumatoid arthritis, leg pain, puffer fish poisoning, bronchial asthma, chronic bronchitis and other diseases [12]. There is no relevant report on its antiviral effect. We evaluated the cytotoxicity and antiviral effects of the alcoholic and aqueous extracts of Elaeagnus gonyanthes, respectively, by an in vitro cell infection model, The results showed that the antiviral effect of Elaeagnus gonyanthes alcohol extract was not ideal and its cytotoxicity was stronger, while Elaeagnus gonyanthes water extract had strong anti-HRSV activity and good therapeutic index, suggesting that the antiviral component of Elaeagnus gonyanthes Mainly water-soluble components.
In the current research on anti-HRSV traditional Chinese medicine,compound prescriptions have been studied more deeply, and they have obtained good curative effects and have broad application prospects [16], Therefore, in future research, it is possible to further evaluate the combined antiviral activity of drugs with better anti-HRSV effect; at the same time, further research and exploration of their antiviral active ingredients and pharmacological mechanisms are needed.
Author contributions: Hu Xiao-yuan and Zhang Xu-guang are responsible for experimental implementation, data processing and paper writing; Li Yong-hui is responsible for medicinal material collection, sample preparation and experimental guidance; Zhang Jun-qing and Yin Feif-ei are responsible for experimental design,experimental guidance and paper revisions. All authors have no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Study on gene regulation mechanism of Qiliqiangxin capsule on myocardial fibrosis in myocardial infarction rats
- Pharmacokinetics of Jiaotai pill self-microemulsion in insomnia rats
- Correlation between IL-33/sST2 signaling pathway and patients with essential hypertensive left ventricular hypertrophy
- Design and characterization of a bi-functional bybrid antibacterial peptide LLM against Pseudomonas aeruginosa
- Screening and comprehensive analysis of key genes in liver hepatocellular carcinoma based on bioinformatics
- Mechanism of Gan Dou Ling in improving liver fibrosis in Wilson disease based on network pharmacology and experimental verification
