Conductive polymer hydrogel-coated nanopipette sensor with tunable size
2022-02-01LinLiFengZhouandQiannanXue
Lin Li, Feng Zhou, and Qiannan Xue,2,a)
ABSTRACT Nanopipette-based sensors are one of the most effective tools for detecting nanoparticles,bioparticles,and biomolecules.Quantitative analysis of nanoparticles with different shapes and electrical charges is achieved through measurement of the blockage currents that occur when particles pass through the nanopore.However,typical nanopipette sensors fabricated using a conventional needle-pulling method have a typical pore-diameter limitation of around 100 nm.Herein,we report a novel conductive hydrogel-composited nanopipette sensor with a tunable inner-pore diameter.This is made by electrodepositing poly(3,4-ethylenedioxythiophene)polystyrene sulfonate onto the surface of a nanopipette with a prefabricated sacrificia copper layer.Because of the presence of copper ions,the conductive polymer can stably adhere to the tip of the nanopipette to form a nanopore;when nanoparticles pass through the conductive nanopore,more distinct blocking events are observed.The size of the nanopore can be changed simply by adjusting the electrodeposition time.In this way,suitable nanopores can be obtained for highly sensitive screening of a series of particles with diameters of the order of tens of nanometers.
KEYWORDS Nanopipette,Conductive polymer,Electrodeposition,Nanoparticles
I.INTRODUCTION
The word“nanopipette”usually refers to a borosilicate glass pipette with a pore diameter of less than 200 nm and a needle geometry.1Nanopipettes are usually used for biochemical sensing,2,3single-cell monitoring,4,5and scanning probe microscopy.6,7As an important type of nanopore sensor,nanopipettes can be used to test the material exchange in the deep parts of samples such as vesicles8and cells.9,10This is because of their probe-type structure,which allows for very flexibl testing in different scenarios.
There are two main strategies that are used to fabricate nanopipettes:pulling a heated capillary tube11,12and externally penetrating a nanocavity enclosed in the terminal of a capillary pipette.13–15Most nanoprobes are currently made from borosilicate glass or quartz.16Generally,borosilicate glass capillaries are used for the preparation of nanopipettes using a laser pipette puller.By changing various parameters of this puller(laser intensity,processing time,tensile force,etc.),17the same glass tube can be transformed into nanopores with different apertures at their tips.Borosilicate glass capillaries are soft and easy to fabricate,but it is difficul to use them to create a nanopipette with a pore diameter less than 100 nm.8
The preparation of nanopipettes using borosilicate capillaries is favored because of its simplicity and consistency,and the resulting nanopipettes can be further modifie to achieve various functions.For example,Ying et al.18used a nanopipette to probe into a cell to monitor its biochemical reactions.Nanopipettes can also be used to filte ions or detect molecules by attaching various molecules to their inner walls;these interact with ions or molecules passing through the nanopores.19A nanopore sensor can be used to distinguish the category of a measured object by detecting transient changes in conductance signals caused by the object passing through the pore.20Nanopipettes are generally suitable for the detection of larger biological particles such as exosomes,virus particles,and other particles above the 100 nm scale.21However,because of the excessive size of their apertures,the signals from nanopipettes are weak when attempting to identify particles with scales of the order of tens of nanometers.When facing the measured nanoparticles with dozens of nanometers,a simple fabrication method is highly desirable.In addition,if conductive materials can be used to form these nanopores,22this could be expected to result in the measured object causing larger transient changes in electrical signals,and this would be conducive to improving the detection resolution.
In this paper,a new method for preparing nanopores from conductive polymers is proposed,in which the pore size can be adjusted and controlled according to the size of the target particles.In this technique,poly(3,4-ethylenedioxythiophene)polystyrene sulfonate(PEDOT:PSS)gel is applied to the tip of a nanopipette as the framework,forming a conductive polymer nanopore by electrodeposition.This design allows nanoparticles of different sizes to pass through the nanopore while resulting in different transient current responses.This method has the following advantages.(i)Conductive polymers have good conductivity and better response sensitivity than quartz nanopores.(ii)PEDOT:PSS is electrodeposited on a copper layer,and the anode oxidizes this copper into copper ions for doping the PEDOT:PSS.This causes the conductive polymer to crosslink into a gel state and more firml adhere to the nanopipette around the nanopore.(iii)Using direct electrodeposition,the pore size can be changed by adjusting the deposition time.This allows a series of transient-current spectra to be obtained and more information to be collected for the identificatio of mixed particles.
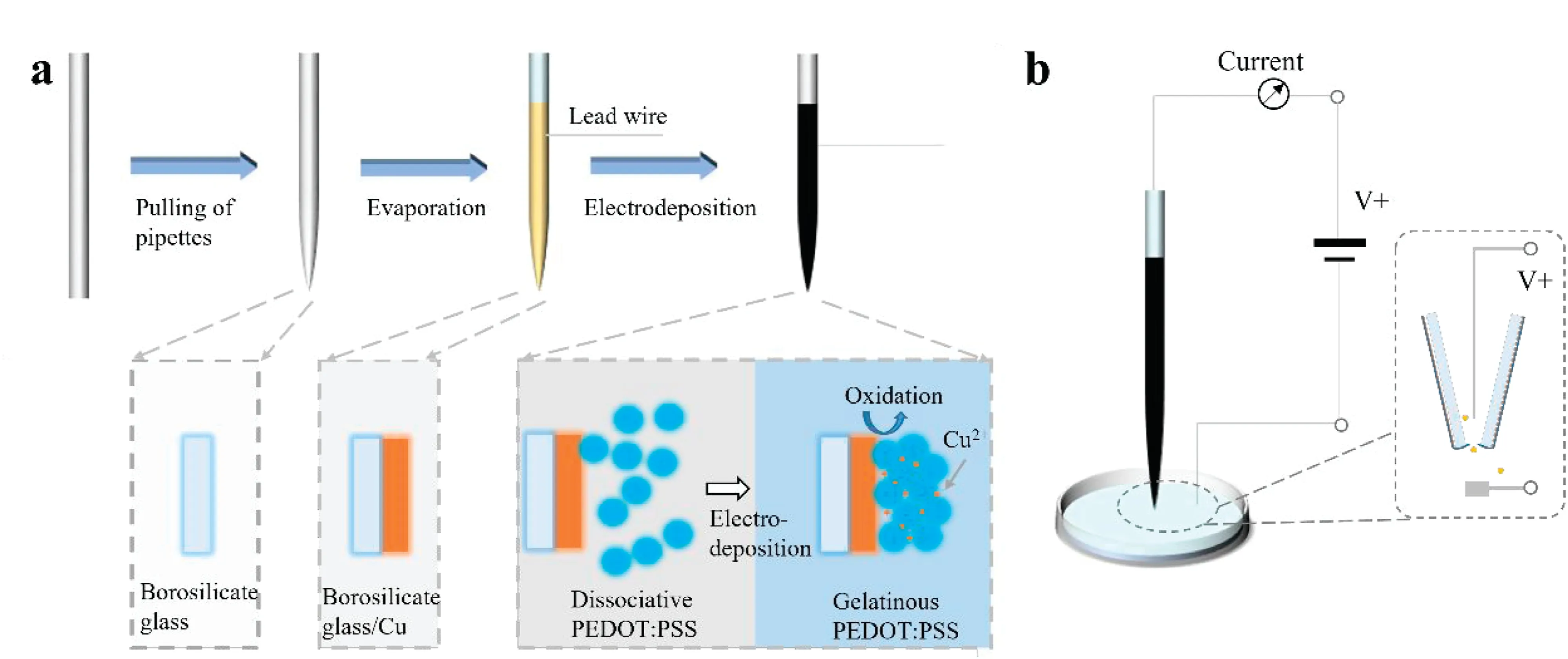
FIG.1.(a)Schematic ofthe process used to produce the PEDOT:PSS conductive polymer nanopore sensors in this study.(b)Detection principle ofthese sensors.
II.NANOPORE SENSOR FABRICATION
A.Preparation and processing of nanopores
A schematic of the process used to produce the nanopores in this study is shown in Fig.1(a).First,the nanopipettes are fabricated from borosilicate glass capillaries using a laser-based micropipette puller system(P-2000,Sutter Instrument Company).The pulling follows a two-step process(Line 1:Heat 350,Fil 3,Vel 30,Del 200,Pul 0;Line 2:Heat 340,Fil 2,Vel 27,Del 160,Pul 250).In the pulling parameters,Heat is related to the power of the laser;Fil is associated with the scanning mode of the laser;Vel is related to the pulling velocity during laser heating;Del is related to the duration of laser heating;and Pul is related to the tensile force after laser heating.Using these parameters,the pore diameter of the nanopore devices was found to be 138.4±3 nm;a scanning electron microscope(SEM)photograph of a nanopipette produced using these parameter values is shown in Fig.2(a).
Then,a layer of copper is evaporated onto the surface of the obtained nanopipettes using an electron beam evaporation method.The nanopipettes are then immersed in PEDOT:PSS solution(0.13 wt.%)for deposition.The copper layer is taken as the working electrode,a platinum electrode(10×10 mm2)is used as a counter electrode,and a calomel electrode is used as a reference electrode.A positive bias voltage is applied between the working electrode and the counter electrode,and the metallic copper layer of the working electrode is oxidized to form copper ions.These copper ions diffuse around the nanopipette and induce the PEDOT:PSS to become locally gelatinous.23As shown in the inset diagrams of Fig.1(a),the PEDOT:PSS is initially in a colloidal dispersion in the buffer.When the copper layer is oxidized to Cu2+,the electrostatic repulsion between the dispersed PEDOT:PSS particles disappears because of the introduction of copper ions,and they thus cross-link with each other and form a gel state.The instruments and materials required for this preparation process are listed in Table S3.
B.Optimization of nanopore processing parameters
We further optimized the parameters used to produce the nanopores.When the Pul parameters of lines 1 and 2 were 0 and 240,respectively,the pore diameter was found to be 189.4 nm[Fig.2(b)].When the Vel parameters of lines 1 and 2 were 27 and 24,respectively,the pore diameter was 109.3 nm[Fig.2(c)].When the Heat parameters of lines 1 and 2 were 340 and 330,respectively,the pore diameter was 219.5 nm[Fig.2(d)].When the Del parameters of lines 1 and 2 were 190 and 150,respectively,the pore diameter was 151.5 nm[Fig.2(e)].As shown in Fig.2(c),the nanopipette was not straight when the aperture was reduced to 109.3 nm.Therefore,we took the parameters used to prepare the nanopipette shown in Fig.2(a)as the optimum values.
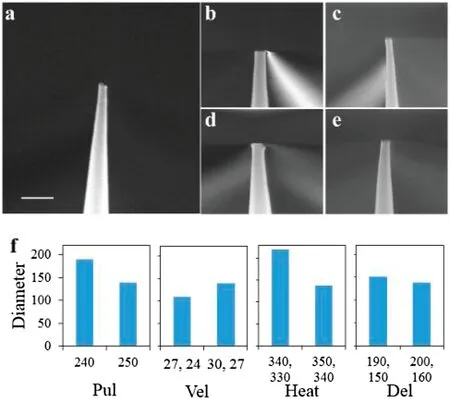
FIG.2.Nanopores produced using different processing parameters.(a)Nanopores obtained following the two-step process(scale bar is 500 nm).(b)–(e)Nanopores obtained after changing the processing parameters.(f)Comparison ofadjusted parameters and corresponding nanopore diameters(units:nm).
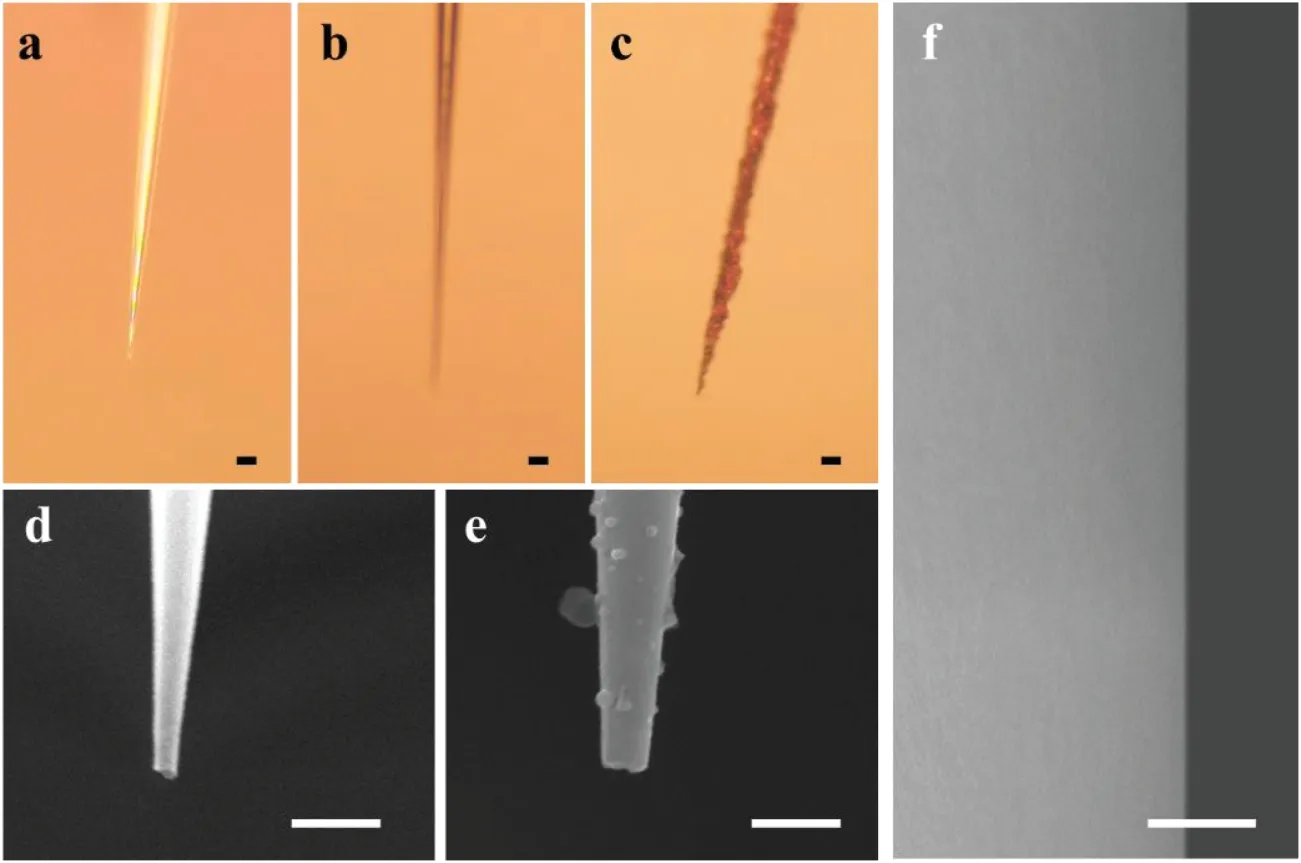
FIG.3.Optical microscope images of nanopipettes:(a)as initially prepared;(b)after copper deposition;(c)after formation of a PEDOT:PSS conductive polymer layer.SEM images of nanopipettes:(d)as initially prepared;(e)with a PEDOT:PSS conductive polymer layer.(f)SEM image showing enlarged section of PEDOT:PSS gel.The scale bars in(a)–(e)are 500 nm long;the scale bar in(f)is 200 nm long.
C.Characterization of nanopores
The morphology of the nanopipettes after each preparation step was observed with an optical microscope.It can be seen from Figs.3(a)and 3(b)that the metal copper layer was deposited on the outside of the nanopipette to form a good level of cover.Figure 3(c)demonstrates that the deposited PEDOT:PSS can be uniformly attached to the outer wall of a nanopipette with a diameter of only 100 nm.To demonstrate that the copper ions are conducive to the stable deposition of PEDOT:PSS,we prepared nanopipettes with evaporated gold layers under the same conditions.Figure S1 shows that in these conditions,it is difficul to form a perfect polymer layer on the gold surface.This is because copper ions are oxidized by applying a forward bias to the copper layer;the addition of copper ions causes the slightly negatively charged dispersed PEDOT:PSS particles near the copper layer to become crosslinked with each other,and a stable conductive polymer nanopore is formed at the tip of the nanopipette.If the copper is replaced with gold,it is difficul for the PEDOT:PSS to adhere to the outside of the nanoscale-diameter tubes because there is no metal cation involved.
We observed the nanopipettes before and after deposition by SEM.Figure 3(e)clearly shows that the PEDOT:PSS is wrapped over the outer walls of the nanopipettes,and the layer is relatively fla and uniform.Figure 3(f)shows a more uniform and flatte PEDOT:PSS gel polymer layer on a nanopipette.Compared with the control group with a gold layer,the sacrificia copper layer helps to obtain a stable conductive polymer layer at the tip of the nanopipette.
D.Experimental process
The PEDOT:PSS conductive polymer was electrodeposited on the outside of the tube wall using the nanopipette as the anode.The constant-current method was adopted,and the current was set to 30μA.The nanopores formed by the conductive polymer under four deposition conditions were compared.After the preparation of these four kinds of nanopore,we used a solution of 80 nm-diameter polystyrene spheres(0.1 nM,in 1M KCl,pH=7.4)as standard objects to test each in turn.As shown in Fig.1(b),the polystyrene nanoparticle solution was injected into the nanopipette,a silver probe was inserted into the nanopipette close to the nanopore,and the nanopipette was then put into a test cell with KCl solution(1M KCl,pH=7.4).An Ag/AgCl electrode was also placed in the test cell,and a bias voltage of 1 V was applied between the silver probe and the Ag/AgCl electrode.High-definitio current data were recorded using a patch clamp amplifie(HEKA Elektronik,GmbH).
III.RESULTS AND DISCUSSION
A.Optimization of electrodeposition time
We selected four kinds of nanopores prepared with different electrodeposition durations:0,180,360,and 600 s.As can be seen from Fig.4(a),as the deposition time increases,the average response current of the prepared conductive polymer nanopores when detecting nanoparticles decreases.It can be considered that under the test framework shown in Fig.1(b),the Ag probe and Ag/AgCl electrode are connected by ionic solution through the nanopores,forming an ionic conductivity,g.This conforms to the relationship g=Ig/Vr,where Vris the bias voltage applied between the silver probe and the control electrode and Igis the current obtained from the test.With increasing deposition time,the conductivity inside and outside the nanopores decreases,indicating that the pore size of the conductive polymer nanopores decreases gradually.
The pore diameter of the nanopores dtipcan be calculated using24
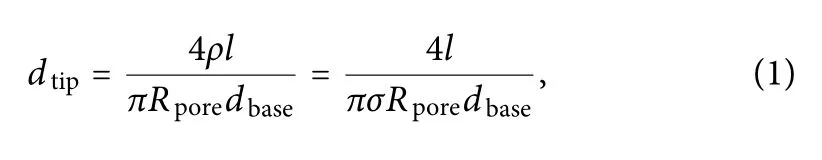
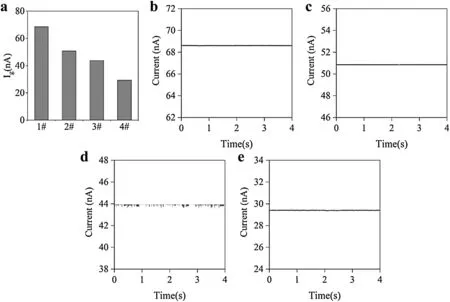
FIG.4.The current signals from the nanopipettes in response to 80 nm nanoparticles were tested with a patch clamp.(a)Average response currents of the nanopipettes under the four deposition times,0,180,360,and 600 s,labeled as 1#,2#,3#,and 4#,respectively;panels(b)–(e)show plots of the respective currents of the nanopipettes over time.
It is difficul to characterize the sizes of the pores using images,and the purpose of this study was to test nanoparticles with scales of the order of tens of nanometers using an electrical method.This is the reason that 80 nm-diameter polystyrene nanoparticles were used as the test object to observe the changes in transient current signals.Figures 4(b)–4(d)show the real-time current results from the four kinds of nanopores.When the nanopipette without any deposited conductive polymer was tested,no translocations were observed[Fig.4(b)].This shows that the nanoparticles were able to pass through the nanopore.Because the particle size is much smaller than the nanopore size,the particles will not cause single-particle translocation.
For a single particle to be observed using a nanopore,the ratio of the pore diameter to the particle diameter needs to be within a certain range.25When a nanopore prepared with a deposition time of 180 s was used to test the nanoparticles,only a very small transient current peak occurred[Fig.4(c)].This is because the nanopore size has been reduced,and it is possible for the nanoparticles to cause a blockage.However,since the pore size is still relatively larger than the particle size,few translocation events can be observed.When the nanopore prepared with a deposition time of 360 s was used to test the nanoparticles,the results showed regular transient current signals[Fig.4(d)].This is because in this case,the size of the nanoparticles is well matched with the pore size;therefore,when the nanoparticles pass through the nanopore one by one,there will be a parallel current with a peak value.When a nanopore prepared with a deposition time of 600 s was used to test the nanoparticles,there were no transient currents observed[Fig.4(e)].Under such deposition conditions,the size of the prepared nanopore is too small to allow 80 nm particles to pass through.These electrical test results are consistent with the calculated apertures,and this preliminarily demonstrates that the experimental assumptions are appropriate.Based on these results,a deposition time of 360 s was selected as the optimal preparation conditions for the modifie conductive polymer PEDOT:PSS.
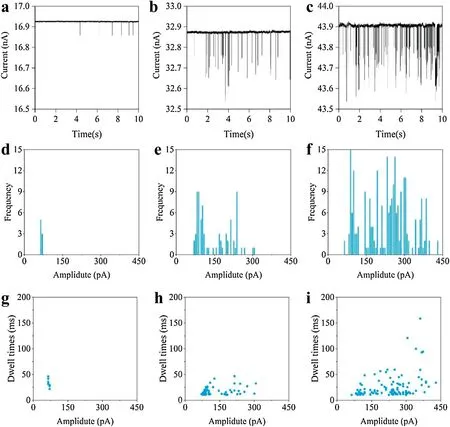
FIG.5.Transientcurrent signals under bias voltages of:(a)0.5 V;(b)0.75 V;(c)1 V.(d)–(f)Respective counts of currentpeaks with these three values of bias voltage.(g)–(i)Respective dwelltimes and currentpeaks for each single-particle translocation event.
B.Optimization of testing conditions for conducting polymer nanopores
Again using 80 nm-diameter polystyrene nanoparticles as the test object,the bias voltage was optimized to fin an appropriate value for examining the translocation events.The silver probe in the nanopore was used as the anode,and the silver chloride electrode in the solution was used as the cathode.Considering the upper limit of the system voltage,the bias voltage was set to test values of 0.5,0.75,and 1 V.Here,the nanopipette was prepared by drawing,evaporating a copper layer,and electrodeposition(constant current:30μA,360 s).Each nanoparticle passing through the conductive polymer nanopore will cause a single particle-translocation event,and this will result in a transient falling peak in the real-time current curve.
Figures 5(a)–5(c)show the transient-current signals under the three values of bias voltage.Correspondingly,Figs.5(d)–5(f)show counts of the numbers of current peaks.These results show that the current peaks increase significantl with increasing bias voltage:the number of current peaks is the highest under a bias voltage of 1 V.Figures 5(g)–5(i)show the dwell times under the three respective values of bias voltage;the longest average dwell times were observed with a bias voltage of 1 V.The tested polystyrene nanoparticles have negatively charged sulfonic-acid groups on their surfaces,so when they pass through a nanopore with a positive bias,the dwell time will increase with the bias voltage.The most translocation events occurred with a bias of 1 V,and they had the longest dwell times.
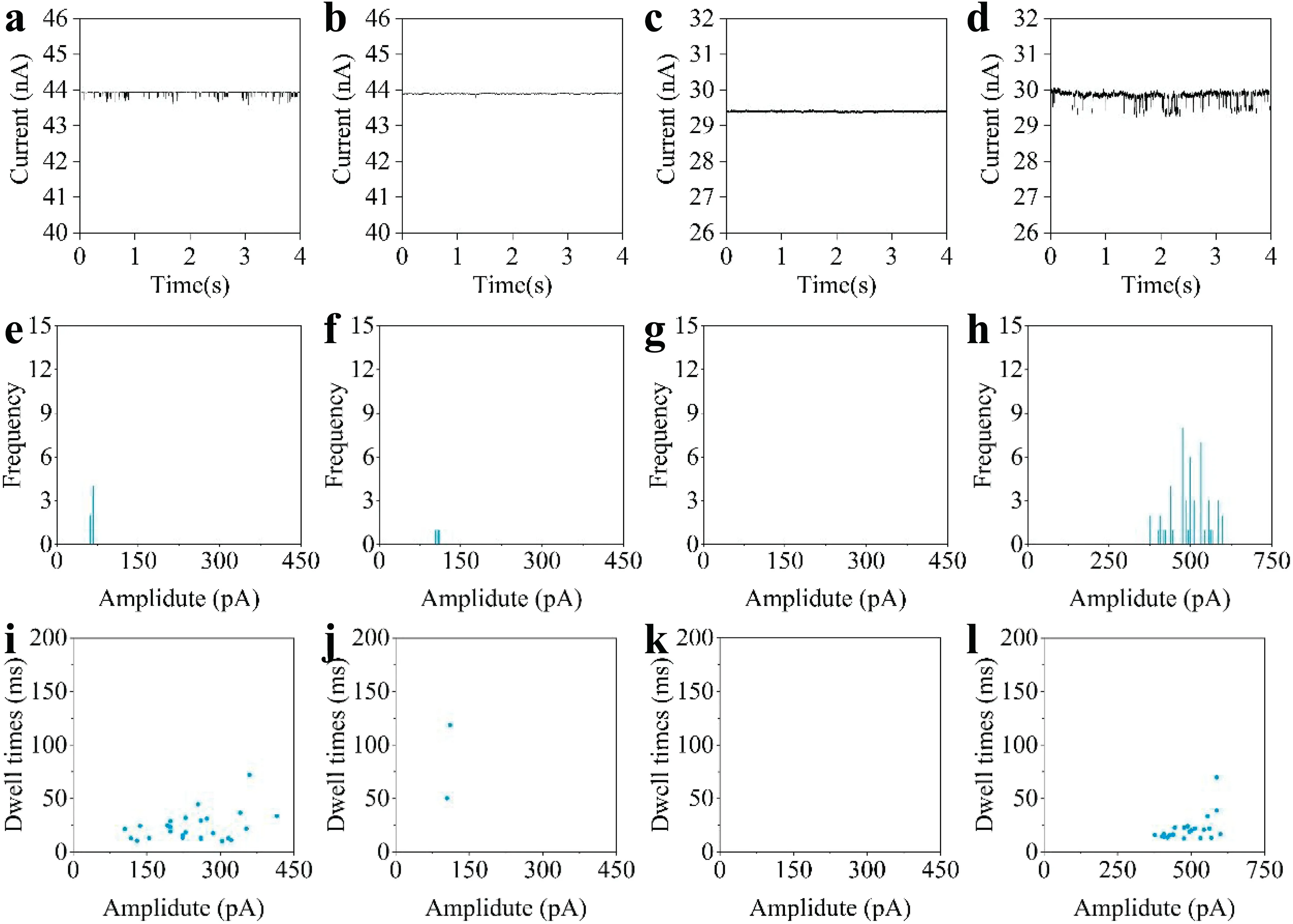
FIG.6.Real-time current curves of:(a)80 nm and(b)50 nm nanoparticles detected by nanopores deposited for 360 s;(c)80 nm and(d)50 nm nanoparticles detected by nanopores deposited for 600 s.(e)–(h)Counts ofcurrentpeaks in the four tests,corresponding to the current signals in(a)–(d),respectively.(i)–(l)Dwelltimes and current peaks for each single-particle translocation event in the four tests,corresponding to the currentcurves in(a)–(d),respectively.
C.Conductive polymer nanopores with adjustable pore size
Using the technique proposed in this paper,nanopores of different sizes can be prepared simply by adjusting the electrodeposition conditions.To demonstrate this,PEDOT:PSS conductive polymer layers were electrodeposited on the tips of nanopipettes with a copper sacrificia layer(constant current:30μA)using two different deposition durations:360 and 600 s.The results in Fig.4 indicate that the diameters of nanopores prepared with a 360 s deposition time should be slightly greater than 80 nm;with a 600 s deposition time,the nanopore diameter should be less than 80 nm.These two kinds of nanopore were used to test polystyrene nanoparticles with particle sizes of 80 and 50 nm to establish whether a differentiated response could be observed.From the real-time current curve for the nanopore prepared with a deposition time of 360 s,it can be seen that there are obvious current spikes in the test with 80 nm nanoparticles[Fig.6(a)],and there were 69 translocation events within a period of 4 s[Fig.6(e)].When the same nanopore was used to test 50 nm nanoparticles,it can be seen that the peaks become very small,and there are significantl fewer than with the 80 nmnanoparticles[Figs.6(e)and 6(f)].These results show that with a deposition time of 360 s,the diameter of the prepared nanopores is only slightly larger than 80 nm.When testing particles with 50 nm size,they cannot produce a response because of the large size difference.This demonstrates that the conductive polymer nanopores prepared under this condition are selective to 80 nm particles.
2016—2017年在襄阳市襄州区古驿镇张罗岗原种场、随州市随县农业科学研究所进行生产试验,表现分蘖力强、穗多、穗大、穗层整齐,抗倒性好,综合抗病性较好,每公顷产量分别为7680、7005kg/hm2。
Nanopores prepared with a deposition time of 600 s were also used for the testing of 80 and 50 nm-diameter nanoparticles.From Figs.6(c)and 6(d),it can be seen that these nanopores provide no response for the 80 nm nanoparticles.The test with 50 nm nanoparticles resulted in regular current-peak signals,and there were 51 translocation events within a period of 4 s[Fig.6(h)].Figures 6(i)–6(l)show the dwell times for the two kinds of nanopores tested with the two sizes of nanoparticle.It can be seen that the nanopore prepared with a deposition time of 600 s has a selective response to 50 nm-diameter nanoparticles.
The above results demonstrate that by simply changing the deposition time,the selective identificatio of nanoparticles of different sizes can be realized.These nanopore devices can work for more than 180 min under a 1 V bias voltage.The prepared nanopores respond sensitively and can identify single-particle translocation events stably and in real time.
IV.CONCLUSIONS
In this paper,a method for preparing nanopores based on electrodeposition of conductive polymers is proposed to meet the test requirements of nanoparticles with scales of the order of tens of nanometers,such as exosomes and virus particles.The nanopores prepared using conductive polymers have good conductivity,so they have better response sensitivity than nanopores prepared with borosilicate glass alone.To solve the problem of securely adhering the conducting polymers to the tips of the nanopipettes,copper ions were introduced,and this caused the polymers to become crosslinked into a gel state during electrodeposition.Stable attachment to the nanopipette tip and the formation of conductive polymer nanopores was successfully achieved.The size of the nanopores can be changed by simply changing the duration of electrodeposition,and this was demonstrated by obtaining a series of transient-current spectra with two kinds of nanoparticles.This method provides a new approach for identificatio of multiple particles at scales of the order of tens of nanometers.
SUPPLEMENTARY MATERIAL
See the supplementary material for more details about the deposition of PEDOT:PSS on the gold-plated nanopipette and the instruments and materials used in the experiments.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflict to disclose.
Author Contributions
L.L.and F.Z.contributed equally to this work.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financia support from the National Natural Science Foundation of China(Grant No.62174119),the National Key R&D Program of China(Grant No.2021YFC3002202),the 111 Project(Grant No.B07014),and the Scientifi Research Transformation Foundation of Wenzhou Safety(Emergency)Institute of Tianjin University.The authors gratefully acknowledge Wenlan Guo,Quanning Li,Chongling Sun,Chen Sun,Xuejiao Chen,and Bohua Liu for their help.
DATA AVAILABILITY
The data that support the finding of this study are available within the article and its supplementary material.
猜你喜欢
杂志排行
纳米技术与精密工程的其它文章
- Controllable blood–brain barrier(BBB)regulation based on gigahertz acoustic streaming
- A new approach for accurate determination of particle sizes in microfluidic impedance cytometry
- Multi-temperature modeling of femtosecond laser pulse on metallic nanoparticles accounting for the temperature dependences of the parameters
- Demands and technical developments of clinical flow cytometry with emphasis in quantitative,spectral,and imaging capabilities