Evaluation of the gastrointestinal anti-motility effect of Anacardium occidentale stem bark extract:A mechanistic study of antidiarrheal activity
2022-01-19BlessingOmolsoFrnisOluwoleOlugengOuknmiJuliusAesnwoAhmeIsholKyoeAewole
Blessing O.Omolso,Frnis S.Oluwole,Olugeng A.Ouknmi,Julius K.Aesnwo,Ahme A.Ishol,Kyoe E.Aewole
aDepartment of Physiology,Faculty of Basic Medical Sciences,University of Medical Sciences,Ondo City,340001,Ondo State,Nigeria
bDepartment of Physiology,College of Medicine,University of Ibadan,Ibadan,200001,Nigeria
cDepartment of Chemistry,Obafemi Awolowo University,Ile-Ife,230001,Osun State,Nigeria
dCentral Research Laboratories Limited,University Road Ilorin,Kwara State,240001,Nigeria
eDepartment of Biochemistry,Faculty of Basic Medical Sciences,University of Medical Sciences,Ondo City,340001,Ondo State,Nigeria
Keywords:
Anacardium occidentale
Antidiarrheal
Muscarinic receptor
Gastric emptying
Gut motility
GC-MS analysis
Peer review under responsibility of Xi’an Jiaotong University.
A B S T R A C T
Diarrhea is a prevalent gastrointestinal problem associated with fatal implications.It is a huge public health concern that requires better alternatives to current drugs.This study investigated the mechanisms involved in the antidiarrheal activity of Anacardium occidentale(Ao)stem bark extract,a plant commonly used in the management of diarrhea in Nigeria.Methanolic stem bark extract of the plant was partitioned into three fractions:hexane fraction,ethyl acetate fraction(AoEF)and methanol fraction.In vitro studies on the effect of these fractions on guinea pig ileum(GPI)strips,as well as the modulatory effect of AoEF on standard agonists-and antagonists-induced GPI contraction and relaxation,revealed AoEF as the most active fraction.In vivo studies to assess the effect of AoEF on the dopaminergic,muscarinic,and serotonergic pathways were carried out using gastric emptying(GE)and gastrointestinal transit(GT)as experimental end points.AoEF was subjected to GC-MS analysis,while the identified compounds were docked with the muscarinic acetylcholine receptor M3(CHRM3)using AutodockVina.Results indicated that AoEF inhibited GE and GT via inhibition of CHRM3.In addition,GC-MS analysis revealed the presence of 24 compounds in AoEF,while docking indicated that octadecanoic acid 2-(2-hydroxylethoxy)ethyl ester exhibited the highest binding affinity to CHRM3.This study indicated that the antidiarrheal activity of Ao is through its antimotility effect via the inhibition of the muscarinic pathway.And since none of the identified compounds exhibited higher binding affinity to CHRM3 relative to loperamide,the antimotility activity of these phytoconstituents may be via synergism.
1.Introduction
Diarrhea is a prevalent gastrointestinal problem associated with fatal implications and outcomes[1].It is thus a huge public health concern,especially in developing countries where it contributes a significant percentage to the causes of death in children,particularly those around the age of 5 years[2,3].Studies have shown that various factors,including nutritional intolerance,infections,therapeutics,and intestinal disorders,may trigger this disease condition by upsetting gut motility and fluid transport processes,resulting in an intestinal disorder clinically marked as diarrhea[4-7].An overview of the pathogenesis of diarrhea in relation to gut motility has indicated the involvement of muscarinic acetylcholine receptors M3(CHRM3)[8],serotonin receptor antagonists(5-HT3)[9],and histamine receptors(H1)[10]via their respective agonists.Consequently,these proteins have become targets for new anti-diarrhea drug therapies.
Anacardium occidentale(Ao),popularly known as cashew,is one of the most commonly used herbs in the management of diarrhea in African Traditional Medicine,especially in Nigeria[11].Studies have reported the antidiarrheal properties of various parts of this plant[12,13],including the leaves[14],the gum[15],the stem bark[1]and the kernel[13].The bark decoction is particularly used in the folkloric management of severe diarrhea.Although studies have revealed the potential of Ao stem bark extract as an alternative herbal remedy against diarrhea[1],its mechanism of action and the active components have not been clearly defined.Thus,this study was designed to investigate the antimotility mechanism of action of the fractions obtained from the methanolic stem bark extract and to identify the compounds responsible for the observed antidiarrheal activity,for the possible development of antidiarrheal drug agents.Furthermore,because of the complementary role of experimental work and computational studies in the understanding of bioactivity and mechanism of action of potential drug agents[4,16],in vivo,in vitro and in silico strategies were adopted in this study.
2.Materials and methods
2.1.Plant material
The stem bark of Ao was collected at Abeokuta,Ogun State,Nigeria,and identified and authenticated at the Herbarium of the Department of Botany,University of Ibadan,where a voucher specimen(UIH-22599)was deposited for reference.
2.2.Preparation of Ao fractions
Fractions were prepared as described by Leila et al.[17].Briefly,500 g of Ao was pulverised and mixed with methanol to form a paste.The paste was impregnated with dry stem bark sample to remove excess methanol.The resulting dry sample was packed into a column and eluted with consecutive liquid/liquid partition of 500 mL of hexane,ethyl acetate,and methanol.The resulting respective fractions,i.e.,hexane fraction(AoHF),ethyl acetate fraction(AoEF),and methanol fraction(AoMF),were collected,concentrated using a rotary evaporator and kept at 4°C.
2.3.Experimental animals
Swiss albino mice(25-35 g)and guinea pigs(450-550 g)used for this study were purchased from the central animal holding facility of the University of Ibadan,Oyo State,Nigeria.The animals were housed in woody wire-meshed cages in the animal holding facility of the Department of Physiology,University of Ibadan,Oyo State,Nigeria,and allowed free access to animal chow(Vital feed®,Lagos State,Nigeria)and clean water ad libitum.The animals were given humane care as approved by the Animal Ethics Committee of the University of Ibadan,in line with the Guide for the Care and Use of Laboratory Animals(8th edition,National Academic Press)[18].
2.4.In vivo experimental design
2.4.1.The effect of AoEF interactions with metoclopramide,serotonin,and carbachol on gastric emptying(GE)and gastrointestinal transit(GT)
The method described by Suchitra et al.[19]was used for this study.This method is based on the fact that pathways leading to increase in smooth muscle activity will be selectively stimulated by the components of the fraction.Cholinergic pathway activation was by carbachol,while serotonergic and dopaminergic pathways were by serotonin and metoclopramide,respectively.Thirty-five male Swiss mice were randomly assigned to seven groups of five mice per group.Groups 1,2,3,and 4 received AoEF(400 mg/kg p.o),carbachol(10 mg/kg p.o),serotonin(10 mg/kg p.o),and metoclopramide(30 mg/kg p.o,0.1 mL/10 g of mice),respectively.Animals in groups 5,6 and 7 received AoEF(400 mg/kg p.o)30 min before they were treated with carbachol(10 mg/kg p.o),serotonin(10 mg/kg p.o),and metoclopramide(30 mg/kg p.o,0.1 mL/10 g of mice),respectively.Thereafter,all groups received carboxyl methylcellulose semisolid solution(CMS,0.5 mL/animal,p.o.).After 15 min,animals were sacrificed by slight decapitation.The abdomens were opened for access to the stomachs and intestines.The stomachs were separated from the intestines at the pyloric junction and used for the assessment of GE,while the intestines were used for the assessment of GT.
2.4.2.Assessment of GE
The stomachs were homogenized in 7 mL of distilled water and then centrifuged at 3,000 r/min for 15 min;1 mL of NaOH(0.025 M)was added to 1 mL of the supernatant and mixed thoroughly,and the absorbance of the resulting mixture was read at 560 nm,using a spectrophotometer(Shimadzu Corporation,Kyoto,Japan).GE was estimated using the following formula:
GE(%)=100-(E×100/C)
Where E is the absorbance of supernatant of the homogenized stomach;C is the absorbance of supernatant of the homogenized stomach of control animals(control animals were sacrificed at 0 min following administration of CMS).
2.4.3.Assessment of GT
For the assessment of GT,the total length of the small intestine and the distances traveled by the marker were measured.GT was calculated using the formula:
GT(%)=P/L×100
Where P is the distance traveled by phenol red;L is the total length of the small intestine.
2.5.In vitro studies
2.5.1.Preparation of isolated guinea pig ileum(GPI)strip
Guinea pigs were given free access to water,but deprived of food for 18 h before the experiment.The animals were sacrificed by cervical dislocation,their abdomens were opened,and about 2 cm strips of ilea were harvested for further study.The ilea were immersed in a tissue bath containing Tyrode solution up to the 15 mL mark.The solution was aerated and kept at 37°C.The mounted tissues were suspended by a silk on a force transducer connected to a microdynanomometer(model 7050,Ugobasile,Milan,Italy)set at 2 mV sensitivity.The resulting responses from microdynanomometer were recorded on a graph paper.
2.5.2.Protocol for tissue responses of the fractions
Specific volumes(0.1,0.2,0.4,and 0.8 mL)of varying concentrations(1,10,and 100 mg/kg)of the fraction were added noncumulatively to ileum strip in a bath containing 15 mL of Tyrode solution.After tissue equilibration,tissue response was recorded to cover 2 cm unit of the microdyanomometer graph.Once tracing reached the 2 cm mark,specific volumes of the fraction were introduced into the bath and the tracing allowed to cover 2 cm of the graph.Thereafter,the tissue was washed and allowed to rest for 30-45 min before the effect of the next volume of the fraction was tested.At each point of recording the tissue response of a known volume of fraction,the normal response of the ileum was recorded.Six independent experiments were carried out.
2.5.3.Interaction of AoEF with acetylcholine,histamine,and serotonin
Graded concentrations(0.0033-0.267μg/mL)of standard agonists(histamine,acetylcholine,and serotonin)were interacted with 2.7 mg/mL of AoEF non-cumulatively using the earlier prepared GPI strips.After the GPI strip had equilibrated,the dose response of each of the agonists was estimated by adding 0.1,0.2,0.4,and 0.8 mL of 0.05 mg/mL of AoEF non-cumulatively.The dose responses of the agonists were repeated non-cumulatively with pre-incubation with 0.4 mL of 100 mg/mL of AoEF for 5 min before the addition of each dose of the agonists.The dose responses of agonists alone or agonist with AoEF were measured and plotted against log concentrations to obtain log-concentration-response curves.The EC50values were determined using GraphPad Prism 5.01 software(GraphPad Software Inc.,San Diego,CA,USA);the values were compared and possible interactions were deduced.
2.5.4.Interactions of AoEF with hexamethonium,atropine,and N(omega)-nitro-L-arginine methyl ester(L-NAME)
AoEF(2.7 mg/mL)was interacted with standard antagonists(hexamethonium(5×10-3and 1×10-3M),L-NAME(100×10-6M and 200×10-6M),and atropine(10-5M and 10-4M)),using prepared GPI.After the GPI strips had equilibrated,the responses of AoEF(0.4 mL of 100 mg/mL)were recorded.Thereafter,GPI strips were pre-incubated with specific concentrations of the antagonists before they were exposed to AoEF(0.4 mL of 100 mg/mL).The time of pre-incubation for hexamethonium,L-NAME,and atropine was 10,30,and 5 min,respectively.In each case,the heights of relaxation(mm)of the normal tonus of GPI strip by AoEF alone and in the presence of the antagonists were compared and possible interactions were deduced.
2.6.GC-MS analysis
Since results indicated that AoEF was the most active fraction,it thus becomes rational to identify its active components.As such,this fraction was subjected to GC-MS analysis,using GC-MS QP2010 Plus(Shimadzu Corporation,Kyoto,Japan)gas chromatography(0.25 mm,60 m,XTI-5)coupled with Mass Spectroscopy(Shimadzu Corporation,Kyoto,Japan)at an ionization voltage of 70 eV following a procedure described earlier[20].
2.7.In silico studies
2.7.1.Protein preparation
The crystal structure of CHRM3(with PubChem database identity,Protein Data Bank(PDB)ID:5ZHP)was retrieved from the PDB(www.rcsb.org)and prepared using AutoDock v4.2 program(Scripps Research Institute,San Diego,CA,USA)into the dockable pdbqt format in preparation for molecular docking.
2.7.2.Ligand preparation
The structures of loperamide and the 24 compounds identified in the AoEF(in structure-data file format)were retrieved from the PubChem database(www.pubchem.ncbi.nlm.nih.gov)and converted to mol2 chemical format using Open Babel[21].Also,the simplified molecular input line entry system of octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester and octadecanoic acid,2-hydroxylmethyl ester were converted to mol2 chemical format using Open Babel.The protein and ligand molecules were then converted to the dockable pdbqt format using AutoDock tools.
2.7.3.Molecular docking
Docking of the ligands to the target protein was done using AutoDock Vina[22].Pdbqt format of the protein and ligands was dragged into their respective columns and the software was run.The binding affinities of the compounds to the target protein were recorded and ranked by their affinity scores.To compare in silico performance,the molecular interactions between the protein and the compound with the highest binding affinity besides the standard inhibitor(loperamide)were viewed with BIOVIA Discovery Studio Visualizer,2016(version 16.1).Also,the binding pocket of the best scoring ligand was viewed and compared with that of loperamide using receptor cavity method in BIOVIA Discovery Studio Visualizer,2016(version 16.1).
2.8.Statistical analysis
Data are presented as mean±standard error of mean for all the in vivo studies.Comparisons between groups were made using the one-way analysis of variance(ANOVA),followed by Dunnett's posthoc test,and differences at P<0.05 were considered statistically significant.Graphs were generated using Graph Pad Prism 5.01(GraphPad Software Inc.,San Diego,CA,USA).
3.Results
3.1.Effect of AoEF interactions with carbachol,serotonin,and metoclopramide on GE and GT in mice
The results obtained on the effects of AoEF interactions with carbachol,serotonin,and metoclopramide on GE in mice are presented in Fig.1A.GE was significantly(P<0.05)increased by carbachol(74.8%±5.8%),serotonin(78.9%±0.6%),and metoclopramide(82.5%±5.7%)relative to control animals(58.1%±0.3%).Pretreatment with AoEF significantly inhibited the action of carbachol on GE(74.8%±5.8%vs.26.1%±3.1%),but with no significant effect on the action of serotonin and metoclopramide on GE.The results obtained on the effect of AoEF interaction with carbachol,serotonin,and metoclopramide on GT in mice are shown in Fig.1B.GT was significantly(P<0.05)increased by carbachol(10 mg/kg),serotonin(10 mg/kg),and metoclopramide(30 mg/kg)by 74.3%±2.6%,59.3%±2.5% and 56.9%±5.5%,respectively,relative to control animals(40.16%±1.5%).However,pretreatment with AoEF(400 mg/kg)significantly(P<0.05)inhibited carbacholinduced GT,with no effect on serotonin-and metoclopramideinduced GT.

Fig.1.Effect of AoEF on(A)gastric emptying and(B)gastrointestinal motility.aValues that were significantly different from control;bValues that were significantly different from carbachol;c,dValues that were not significant compared to serotonin and metocloppramide,respectively(P<0.05).
3.2.Effects of AoEF on DPI strip
The three fractions elicited negligible activity at the low concentrations of 0.0067-0.053 mg/mL.However,at higher concentrations(0.067-5.3 mg/mL),the fractions modified GPI strip tonus activity by reversibly relaxing the ileum strips in a concentrationdependent fashion(Fig.2),with AoEF having the lowest IC50value relative to the other 2 fractions,AoMF and AoHF(Table 1).
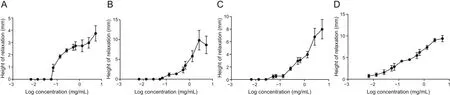
Fig.2.Log concentration-response curve showing relaxation of guinea pig ileum strip by graded concentration of(A)Anacardium occidentale hexane fraction(AoHF),(B)Anacardium occidentale ethyl acetate fraction(AoEF),(C)Anacardium occidentale methanolic fraction(AoMF),and(D)Anacardium occidentale methanolic extract.

Table 1IC50values of different fractions of the stem bark of Anacardium occidentale(Ao).

Table 2GC-MS analysis of the ethyl acetate fraction of Ao stem bark methanolic extract.

Table 3Binding affinities of compounds isolated from AoEF for muscarinic acetylcholine receptor M3(CHRM3).
3.3.Effect of AoEF on histamine-,serotonin-,and acetylcholineinduced GPI strip contraction
Contractions of isolated GPI strips by histamine,serotonin,and acetylcholine were all reduced by pre-incubation with AoEF(Figs.3A-C).This observation was demonstrated by the logconcentration-response curves,which was shifted to the right in parallel fashions by AoEF.The EC50value of histamine(deduced from curve)was raised from 0.14 to 0.26 mg/mL,that of serotonin was raised from0.06 to 0.08 mg/mL,while that of acetylcholine was raised from 0.12 to 0.53 mg/mL.
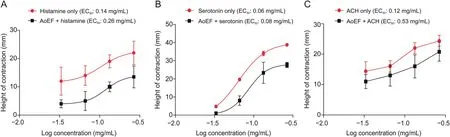
Fig.3.Non-cumulative log concentration-response curve showing relaxation in(A)histamine-induced,(B)serotonin-induced,and(C)acetylcholine(ACH)-induced contraction of guinea pig ileum strip by AoEF.
3.4.Effect of AoEF on L-NAME-,hexamethonium-,and atropineinduced GPI strip relaxation
AoEF non-significantly(P>0.05)increased the relaxant effects of L-NAME and hexamethonium(Figs.4A and B),while significantly(P<0.05)increased the relaxant effect of atropine(Fig.4C).

Fig.4.Effect of AoEF on(A)N(omega)-nitro-L-arginine methyl ester(L-NAME)-induced,(B)hexamethonium(HEX)-induced,and(C)atropine-induced relaxation of guinea pig ileum strip.
3.5.Identification of components in AoEF by GC-MS
GC-MS analysis of AoEF revealed the presence of 24 components,with oleic-acid ethyl ester and 11-octadecenoic acid methyl ester with percentage compositions of 13.90% and 10.18%,respectively,being the predominant components(Table 2).These results are also demonstrated on the chromatogram which shows the peaks of these compounds(Fig.5).
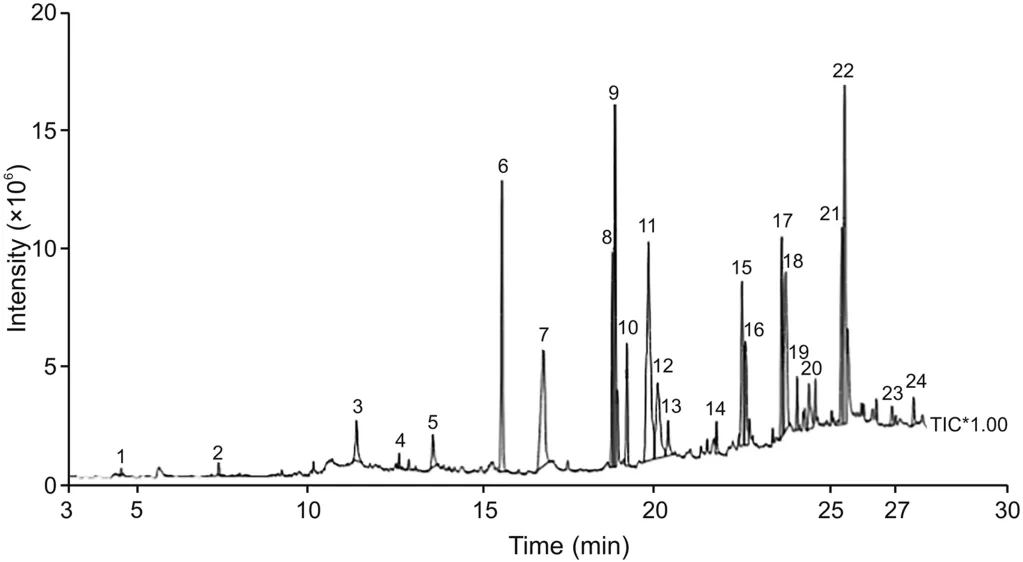
Fig.5.Chromatogram showing the peak of compounds in the AoEF.
3.6.Result of in silico studies
Since results from the in vivo and in vitro studies suggested that the anti-motility mechanism of Ao extract is via its inhibitory effect on the muscarinic receptor,the binding affinities of the 24 compounds to CHRM3 were evaluated,using loperamide as the standard drug.From the result obtained,it was revealed that octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester had the highest binding affinity to CHRM3 amongst the 24 compounds(Table 3),displaying a binding energy of-12.3 kcal/mol(in bold type in Table 3)compared to-16.7 kcal/mol for loperamide.Comparison of the binding profiles of loperamide and octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester to CHRM3 revealed interaction with amino acids at the same binding pocket(Fig.6A).Hydrophobic interactions were predominant between PHE124,TRP143,and TYR529 of CHRM3 and loperamide(Fig.6B).Octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester interacted with the receptor via hydrogen bonds with ARG179 and THR180,hydrophobic interaction with PHE163,and electrostatic interaction with ASP164(Fig.6C).

Fig.6.Binding of loperamide and octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester to CHRM3,showing(A)receptor binding site occupied by the two compounds,(B)interaction between CHRM3 and loperamide and(C)interaction between CHRM3 and octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester.
4.Discussion
The use of herbal extracts in the management of diarrhea is a traditional and regular practice in most African communities,where a large proportion of the population still relies heavily on herbal concoctions for the management and treatment of a number of diseases despite brilliant progress and discoveries in medical sciences[23].From the in vitro study,the fractions reversibly relaxed the spontaneous activity of GPI smooth muscle in a concentration-dependent manner.In the guinea pig small intestine,inhibitory transmission to the smooth muscle depends on slow inhibitory junction potentials that are regulated by nitric oxide(NO)[24].The relaxant effect of Ao fractions may be through increased release of NO.From the log-concentration-response curves obtained for the relaxant effect of Ao fractions,the IC50value of AoEF was the least.This indicates that AoEF is the most potent among the three fractions[25].Results also revealed that pretreatment with AoEF inhibited the mechanical response evoked by acetylcholine,histamine,and serotonin.This finding was further supported by the shift of the log-concentration response curves to the right(Fig.3).Furthermore,it is well known that when these agonists are dropped into a bathing medium containing GPI strip,they evoke contractile responses[26],and that the action of acetylcholine is via CHRM2 and CHRM3,while 5-HT binds to specific receptors,such as the 5-HT3and 5-HT4receptors to initiate gut motility,and that the dominant effect of histamine on GPI motility is contraction,mediated via H1receptor[27].The observed relaxant effect of the extract suggests that the relaxant effect is via antagonizing the muscarinic,histaminergic,and serotonin receptors in the ileum.However,considering the fact that AoEF shows no selectivity between contractile agents being able to reduce contraction by different agonists,there is a possibility that the mechanism of action of AoEF may be beyond the receptor,and may be through indirect mechanism,such as release of transmitter from nerve terminals,or non-specific mechanism,such as release of NO.And since NO has been confirmed as a messenger in the gastrointestinal tract,the inhibitory component of the gut peristalsis is thought to be mediated by NO,either singly or along with other cotransmitters[28].It has also been reported that the actions of certain biological agents that relax the gut are through interactions with NO release[29].Thus the use of L-NAME(inhibitor of the nitric oxide synthase)is an established protocol in demonstrating the involvement of NO in the inhibitory and relaxant effect of drugs[30].In the present study,the involvement of NO may not be considered as a possible mechanism in the inhibitory effect of the fraction,since interaction of L-NAME with the fraction did not alter its relaxant effect.Furthermore,interference with transmitter release from nerve terminal is also not a valid mechanism,since pretreatment with hexamethonium,a neuronal ganglion blocker,did not reduce the relaxant effect of the fraction.
In vivo study indicated the inability of the extract to alter the effects of metoclopramide and serotonin,indicating that dopaminergic and 5-HT pathways are not target mechanisms for the inhibitory effect of the fraction.However,the ability of the fraction to block both GE and propulsive movement activated by carbachol(a muscarinic agonist like acetylcholine)suggests that the fraction antagonizes the action of carbachol on the muscarinic receptors.The likelihood that the antimotility mechanism of the extract is via its inhibitory action on the muscarinic receptor is further supported by the significant increase in the relaxant effect of the fraction observed with pretreatment with atropine.
From the in silico docking study,results revealed that all the 24 Ao-derived compounds displayed lower binding affinity to the muscarinic receptor relative to loperamide,the standard antidiarrhea drug.However,occupation of the same binding pocket as loperamide on the muscarinic receptor by octadecanoic acid,2-(2-hydroxylethoxy)ethyl ester indicates that this ligand may have a similar effect on this receptor as loperamide,although this might not be remarkable,since the amino acid interaction of this ligand at this particular binding pocket is not similar to that of the standard drug(Figs.6B and C).Although information on the anti-motility properties of these compounds is still scanty,it has been reported that oleic acid,one of the constituent compounds,slows down gastrointestinal transit and reduces diarrhea in humans and experimental animals[31].Plant-derived preparations have been known to contain a pool of components with desirable complementary pharmacological properties,and their use in the treatment of various diseases via synergism is an area of active interest[32,33].Therefore,the possibility of synergism between the component compounds of the fraction with the receptor cannot be ruled out,since none of the identified compounds solely exhibited a higher binding affinity to the target protein,relative to loperamide.
5.Conclusions
It can,therefore,be concluded that the relaxant and the antimotility effects of the fraction are possibly mediated through the blockage of muscarinic receptors,and that synergism between the components of the plant may not be ruled out for the observed anti-motility property of the plant.Thus,further studies should be considered to isolate the chemical compounds identified for further evaluation of their anti-motility and safety properties for possible application in the treatment and management of diarrhea.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Effect of Shengmai Yin on the DNA methylation status of nasopharyngeal carcinoma cell and its radioresistant strains
- Simultaneous enantioseparation and simulation studies of atenolol,metoprolol and propranolol on Chiralpak®IG column using supercritical fluid chromatography
- Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide
- Synergistic effects of methyl 2-cyano-3,11-dioxo-18beta-olean-1,-12-dien-30-oate and erlotinib on erlotinib-resistant non-small cell lung cancer cells
- A living cell-based fluorescent reporter for high-throughput screening of anti-tumor drugs
