Simultaneous enantioseparation and simulation studies of atenolol,metoprolol and propranolol on Chiralpak®IG column using supercritical fluid chromatography
2022-01-19PranavPandyaPriyankaShahPranavShrivastav
Pranav A.Pandya,Priyanka A.Shah,Pranav S.Shrivastav
Department of Chemistry,School of Sciences,Gujarat University,Ahmedabad,380009,India
Keywords:
Enantioseparation
Supercritical fluid chromatography
β-blockers
Chiralpak®IG column
Molecular docking
Binding energy
Peer review under responsibility of Xi’an Jiaotong University.
A B S T R A C T
Enantioseparation of three β-blockers,i.e.,atenolol,metoprolol and propranolol,was studied on amylose tris(3-chloro-5-methylphenylcarbamate)immobilized chiral stationary phase using supercritical fluid chromatography(SFC).The effect of organic modifiers(methanol,isopropanol and their mixture),column temperature and back pressure on chiral separation of β-blockers was evaluated.Optimum chromatographic separation with respect to resolution,retention,and analysis time was achieved using a mixture of CO2and 0.1% isopropyl amine in isopropanol:methanol(50:50,V/V),in 75:25(V/V)ratio.Under the optimized conditions,the resolution factors(Rs)and separation factors(α)were greater than 3.0 and 1.5,respectively.Further,with increase in temperature(25-45°C)and pressure(100-150 bars)there was corresponding decrease in retention factors(k),α and Rs.However,a reverse trend(α and Rs)was observed for atenolol with increase in temperature.The thermodynamic data from van't Hoff plots revealed that the enantioseparation was enthalpy driven for metoprolol and propranolol while entropy driven for atenolol.To understand the mechanism of chiral recognition and the elution behavior of the enantiomers,molecular docking studies were performed.The binding energies obtained from simulation studies were in good agreement with the elution order found experimentally and also with the free energy values.The method was validated in the concentration range of 0.5-10μg/mL for all the enantiomers.The limit of detection and limit of quantitation ranged from 0.126 to 0.137μg/mL and 0.376-0.414μg/mL,respectively.The method was used successfully to analyze these drugs in pharmaceutical preparations.
1.Introduction
Separation of enantiomers to obtain pure chiral drugs is a subject of intense research and is now gaining priority,especially in the pharmaceutical industry[1-3].There are several reports that show marked differences in the pharmacodynamics and pharmacokinetics of enantiomers of the drug,wherein one of the enantiomers has the desired pharmacologic effect,while the other is either inactive or is associated with undesirable side effects[1,4].This difference in the pharmacokinetics is mainly due to stereoselective drugs binding(generally with plasma proteins),absorption,clearance,and excretion.Thus,there is a constant need to develop methods both analytical and preparative that have high resolution power and good efficiency for chiral purity testing and pharmacokinetic studies[4,5].
β-adrenoceptor antagonists or β-blockers are used for the treatment of several cardiovascular diseases,including hypertension,ischemic heart disease,and migraines.They are mainly administered and marketed as racemic mixtures.However,the pharmacological activity resides with the S-enantiomer due to its greater stereoselective affinity towards β-receptors.The R-enantiomers are either pharmacologically inactive or toxic[6-8].Atenolol is a second-generation β-blocker used in the treatment of hypertension,angina pectoris,and acute myocardial infarction[9].Metoprolol is a β1 selective adrenergic blocker used in the management of ischemic heart disease,heart failure and hypertension[8].Propranolol,a nonselective β-blocker,is used to prevent migraines,and for the treatment of hypertension and anxiety[7].
Several analytical techniques have been used for enantiomeric separation of these drugs,namely,thin layer chromatography[10],surface enhanced Raman scattering[11],counter current chromatography[12],electrochromatography[13,14],capillary electrophoresis[15-18],high performance liquid chromatography(HPLC)[9,19-23],liquid chromatography-tandem mass spectrometry(LCMS/MS)[24-29],and supercritical fluid chromatography(SFC)[30-33].Among different chiral stationary phases(CSPs),polysaccharide(cellulose or amylose)based phases are the most widely used with these techniques[20,21,23,32,33].Nikolai et al.[24]have quantified atenolol,metoprolol and propranolol by LC-MS/MS using Chirobiotic V vancomycin-based chiral column within 20 min.Recently,Li et al.[23]reported enantiomeric separation of six βblockers on Chiralpak IB column using HPLC and used molecular docking technique to understand the mechanism of chiral recognition.However,there are very few SFC based methods that report enantioseparation of the drugs studied in the present work.Svan et al.[32]employed Chiralpak IB column for the chiral separation of atenolol,metoprolol,propranolol and the zwitterionic metoprolol acid using SFC-MS/MS.
As evident from the literature,chiral HPLC is the technique of choice for separations of β-blockers.However,due to some inherent limitations of HPLC such as higher solvent consumption and long analysis time,SFC presents an alternative approach using environment friendly mobile phases.It employs relatively less toxic and non-polar CO2as the basic component of the mobile phase,and utilizes a majority of the CSP used in HPLC.Further,the advantage of SFC over HPLC is that column efficiency does not decrease with an increase in flow rate at the same rate as seen in HPLC.Thus,one can operate SFC at a higher linear velocity relative to HPLC,resulting in shorter analysis time[34,35].In one such report,much better enantioresolution and shorter analysis time were found with SFC compared to HPLC on Chiral Art Cellulose-SB column for these drugs[33].Thus far,there are no reports on the use of Chiralpak®IG column with amylose tris(3-chloro-5-methylphenylcarbamate)immobilized chiral stationary phase for enantioseparation of atenolol,metoprolol and propranolol.
Thus,the objectives of the present work were 1)to optimize conditions for simultaneous enantioseparation of atenolol,metoprolol and propranolol on Chiralpak®IG column in a single analysis and 2)to study the thermodynamic aspects of chiral separation for understanding the mechanism of chiral recognition.Type of polar modifier in the mobile phase greatly influences the interaction of the analyte with the stationary phase and thereby the resolution of chiral substances.It can alter the solvent strength and mobile phase density,can compete with the analytes for adsorption sites,and might induce some changes in the stationary phase structure[36].Further,molecular docking studies were performed to understand the binding energy required to interact with the CSP and correlation with the experimentally evaluated thermodynamic parameters.The validated method was also used to analyze the drugs in their tablet formulations.
2.Experimental
2.1.Chemicals and reagents
Reference standards of rac-atenolol(≥98%),rac-propranolol hydrochloride(≥99%),rac-metoprolol tartrate(≥99%)were procured from Sigma Aldrich Chemicals Pvt.Ltd.(Bangalore,India),while S(-)-atenolol,R(+)-atenolol,S(-)-metoprolol,R(+)-metoprolol,S(-)-propranolol,R(+)-propranolol enantiomers of purity≥98.0% were purchased from Toronto Research Chemicals Inc.(Ontario,Canada).HPLC grade methanol,isopropanol and isopropylamine(99%)were acquired from Sigma Aldrich Chemicals Pvt.Ltd.(Bangalore,India).Liquid carbon dioxide(CO2,99.9%)was procured from SICGIL Industrial Gases Limited(Baroda,India).
2.2.Instrumental and chromatographic conditions
Chromatographic analysis for the β-blockers was carried out on a Waters SFC Investigator system(Milford,MA,USA)equipped with a fluid delivery module,an autosampler with partial loop volume injection system,a backpressure regulator,column oven and photodiode array(PDA)detector.The ChromScope v1.2.1 software was used for data handling.All six enantiomers were separated on Chiralpak®IG column(250 mm × 4.6 mm,5μm)packed with amylose tris(3-chloro-5-methylphenylcarbamate)immobilized with silica gel.The temperature of the column oven was 40°C.The mobile phase was a mixture of CO2and 0.1% isopropyl amine in isopropanol:methanol(50:50,V/V),in 75:25(V/V)ratio and was pumped at a constant flow rate of 4.0 mL/min.The injection volume was 10μL and detection wavelength was set at 220 nm.The backpressure of the system was 100 bars.The sample cooler temperature was kept at 10°C.For optical rotation measurement,MCP 5100 Modular Circular Polarimeter,from Anton Paar India Pvt.Ltd.(Haryana,India)was used with sodium source,wavelength 589 nm.
2.3.Preparation of stock solutions and calibrators
Separate standard stock solutions(2500μg/mL)of rac-atenolol,rac-propranolol hydrochloride,rac-metoprolol tartrate,S(-)-atenolol,R(+)-atenolol,S(-)-metoprolol,R(+)-metoprolol,S(-)-propranolol,and R(+)-propranolol were prepared in methanol.Their working solutions(500μg/mL)were prepared in methanol using their respective standard stock solutions.For construction of linear curves,calibration standards(CSs)of enantiomers were prepared from their working solutions to obtain solutions with the following concentrations,0.50,1.00,2.00,3.00,4.00,6.00,8.00 and 10.0μg/mL,respectively.Similarly,quality control(QC)samples were prepared at 1.50 μg/mL(low),5.00 μg/mL(medium),and 9.00 μg/mL(high),respectively.
2.4.Assay of tablet formulation
In order to evaluate the content of pharmaceutical formulations,20 tablets each of Betaloc®50 mg(metoprolol tartrate from Astra Zeneca Pharma India Ltd.,Ahmedabad,India),Betacap®10 mg(propranolol hydrochloride from Sun Pharmaceutical Industries Ltd.,Mumbai,India)and Betacard®50 mg(atenolol from Torrent Pharmaceuticals Ltd.,Ahmedabad,India)were weighed and ground to fine powder.An amount equivalent to 50 mg metoprolol,10 mg propranolol and 50 mg atenolol was taken into separate 50 mL volumetric flask containing 25 mL methanol.Thereafter,the solutions were sonicated for 30 min and then made up to volume with methanol.Their working solutions(10μg/mL)were prepared by diluting the stock solution with methanol.For analysis,10μL was applied to the column in six replicates.Peak area for all the enantiomers was determined at 220 nm and the amount of each enantiomer present in the tablets was estimated from their regression equations.
2.5.Molecular docking study
Molecular docking of the enantiomers was done with an Intel dual CPU(2.00 GHz)on Windows 10 operating system.To sketch the structures of the enantiomers and the amylose derivatized CSP,Marwin Sketch software was utilized[37].The structures were sparked to 3D and saved in a PDB file.The structure of amylose derivatized CSP was docked using Auto Dock Tools(ADT)4.2 by handing over Gasteiger charges,integrating nonpolar hydrogen atoms,and saving in PDBQT file format.The docking permitted all the rotatable bonds of the ligands as a rotatable and rigid receptor[38].The isomers were edited and saved in the PDBQT format using the same tool.The lattice box size used was 70 Å × 80 Å × 70 Å with spacing of 0.375 Å.Auto Dock Vina software was then applied to acquire the binding energy/affinity between the receptor, amylose tris(3-chloro-5-methylphenylcarbamate)and the enantiomer.The output file was then opened in Discovery Studio Visualizer(Dassault systems Biovia Corporation)for virtual screening,molecular docking,to study the binding site and to estimate the interaction and the bond length between stationary phase and the enantiomer.
3.Results and discussion
3.1.Effect of organic modifier
Initially,the effect of organic modifier(methanol,isopropanol and their mixture)was studied on Chiralpak®IG column,having an immobilized amylose tris(3-chloro-5-methylphenylcarbamate)stationary phase.The experiments were performed using CO2with 20% organic modifier containing 0.1% isopropylamine at 40°C and 100 bars back pressure.The basic additive,isopropylamine,provided good resolution,peak shape and sufficient response for the isomers as compared to diethylamine or triethylamine which is commonly used in SFC.All three organic modifiers afforded complete separation of enantiomer pairs individually;however,it was not possible to separate simultaneously all six enantiomers in a single run within an optimum analysis time in methanol and isopropanol,respectively.Though these drugs are not available in combination,it is advantageous to have one single method rather than three separate methods/elution conditions to analyze these drugs.
As shown in Fig.1,the S(-)isomers of metoprolol and propranolol co-eluted in isopropanol and methanol,and R(+)isomers of atenolol and propranolol in isopropanol.Nevertheless,all the enantiomers were baseline resolved in methanol-isopropanol(50:50,V/V)mixture.Besides,the elution order of enantiomers remained unchanged(S(-)ahead of R(+)isomer)for all the modifiers.This was confirmed by collecting the fractions and measuring their optical rotation and also from their individual reference standards.Furthermore,there was greater retention of enantiomers with methanol compared to isopropanol and their mixture.On the other hand,the retention was relatively less with isopropanol,especially for the S(-)isomers of metoprolol and propranolol(Table S1).However,based on the criterion of separation factor(α≥1.5)and resolution factor(Rs≥1.5),together with simultaneous separation of all six enantiomers,a mixture of methanol:isopropanol(50:50,V/V)was considered in the entire work.

Fig.1.SFC chromatograms showing effect of organic modifiers(A)isopropanol,(B)methanol,and(C)isopropanol:methanol(50:50,V/V)on the separation of enantiomers;(D)chemical structures of enantiomers.Column:Chiralpak® IG;mobile phase:CO2:0.1% isopropyl amine in organic modifier(80:20,V/V);temperature:40 °C;back pressure:100 bars;detection wavelength:220 nm;flow rate:4 mL/min.
The effects of different proportions(15,20,25,30 and 35)of methanol:isopropanol(50:50)on retention time,retention factors(k),α and Rsof the enantiomers are given in Table S2.The retention of enantiomers decreased with increase in organic modifier content,which is due to the increase in the solvating power of the CO2based mobile phase.Fig.2A shows the variation in ln k values with percentage of methanol:isopropanol(50:50,V/V)in the mobile phase.As evident,there was greater retention of R(+)isomer than that of S(-)isomer for all the three β-blockers.A similar downward trend was observed with the separation factors(α)as shown in Fig.2B.However,for atenolol which is more polar than the other βblockers,this decrease was more prominent and was affected more with increase in organic modifier content in the mobile phase.
3.2.Effect of back pressure
It is well known that the solvation ability of supercritical CO2increases with increase in back pressure,and thus helps in rapid elution of the analytes from the column[39].The effect of back pressure on enantioseparation was studied at 100,125 and 150 bars.The retention of the enantiomers decreased with increase in back pressure for all the β-blockers(Table S3).A similar trend was observed with the resolution factors,with no major change in separation factors.Further,the decrease in retention of S-isomers was much less than that of the R-isomers with increase in back pressure.The Rsvalues decreased from 5.68 to 5.01,6.83-6.02 and 3.48-3.26 for the enantiomers of metoprolol,propranolol and atenolol,respectively at 40°C.This trend can be related to greater solvation ability of supercritical CO2with increasing back pressure leading to faster elution of the enantiomers.
3.3.Effect of temperature on the enantioseparation
Temperature plays a significant role in enantiomeric separations as reported in several studies[39,40].It can produce changes in retention time,selectivity,and resolution.The effect of temperature was studied in the sub and supercritical region from 25°C to 45°C in 5°C increments(Table S3).Similar to the back pressure effect,with increase in temperature the retention of the enantiomers decreased for all three drugs at 100 bars as shown in Fig.3.However,it is worth noting that there was reversal in the elution order in the case of R(+)-metoprolol and R(+)-atenolol in the sub critical regionat 25°C and 30°C.Under typical supercritical conditions,the retention should increase with increase in temperature as the fluid density decreases,which results in decrease in the fluid elution strength[39].However,to explain the observed behavior,at higher temperature the solubility of the enantiomers increased in the mobile phase due to decrease in cohesiveness of the fluid,leading to decreased retention.This behavior is typically observed in HPLC separations.

Table 1Thermodynamic parameters for enantiomers on Chiralpak®IG column under different back pressures.
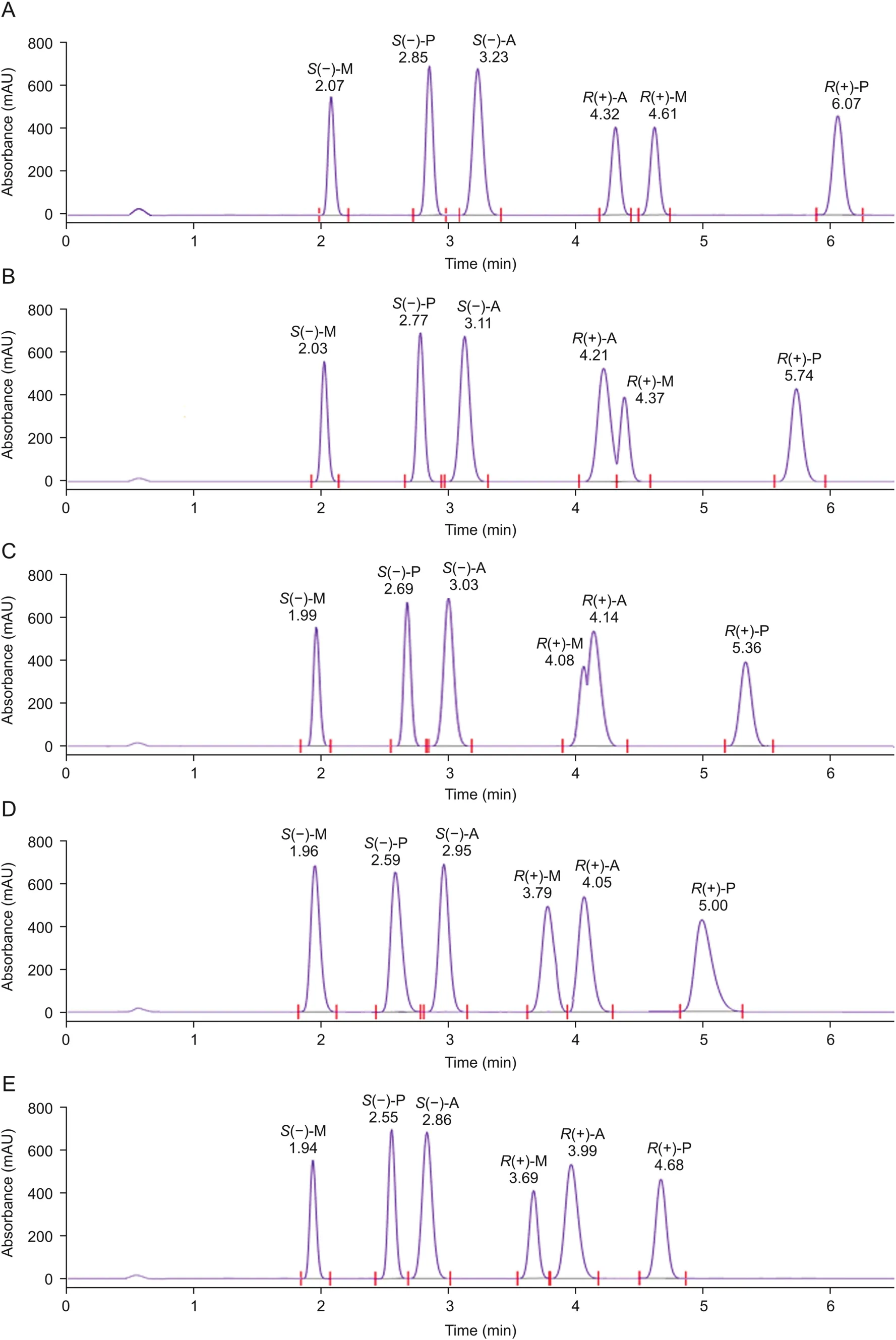
Fig.3.SFC chromatograms showing effect of temperature(A)25 °C,(B)30 °C,(C)35 °C,(D)40 °C,and(E)45 °C on the enantioseparation of the drugs.Column:Chiralpak® IG;mobile phase:a mixture of CO2and 0.1% isopropyl amine in isopropanol:methanol(50:50,V/V),in 75:25(V/V)ratio;back pressure:100 bars;detection wavelength:220 nm;flow rate:4 mL/min.
The relation between the retention factor(k)and the temperature is expressed by the van't Hoff equation,
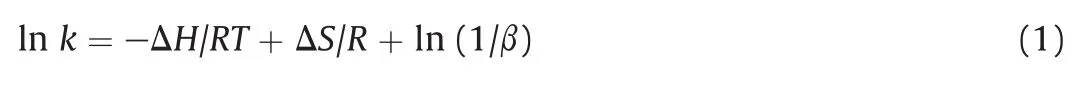
where ΔH and ΔS are the standard molar enthalpy and molar entropy for transfer of analyte from the mobile phase to the stationary phase,respectively.β represents the phase ratio and R is the ideal gas constant(8.314 J/mol K).The plots of logarithm of retention factors,ln k versus temperature(1000/T)at different back pressures are shown in Fig.4.These plots were almost linear for all the enantiomers,which indicates no significant change in the phase ratio due to change in the density at different temperatures.The standard molar free energy(ΔG),ΔH and ΔS values at different back pressures for the isomers are summarized in Table 1.The ΔG values were negative for all the enantiomers at different pressures and also at different temperatures(Table S4).Likewise,ΔH and ΔS values were also negative,which indicates that the transfer of enantiomers from the mobile phase to the stationary phase was enthalpy driven.

Fig.4.van't Hoff plots of retention factors(ln k)of atenolol,metoprolol and propranolol enantiomers versus temperature(1000/T)at different back pressures(A)100 bars,(B)125 bars,and(C)150 bars.Column:Chiralpak®IG;mobile phase:a mixture of CO2and 0.1% isopropyl amine in isopropanol:methanol(50:50,V/V),in 75:25(V/V)ratio;detection wavelength:220 nm;flow rate:4 mL/min.
Table 2Thermodynamic parameters from van't Hoff plots of separation factors versus temperature.
From Fig.5,it can be observed that the selectivity increases with decrease in temperature for metoprolol and propranolol at constant pressure,whereas a slight decrease in selectivity was found for atenolol.The relationship between the separation factor and temperature can be expressed as


Fig.5.Changes in the separation factors(lnα)of enantiomers with temperature(1/T)at three different back pressures(A)100 bars,(B)125 bars,and(C)150 bars.Column:Chiralpak®IG;mobile phase:a mixture of CO2and 0.1% isopropyl amine in isopropanol:methanol(50:50,V/V),in 75:25(V/V)ratio;detection wavelength:220 nm;flow rate:4 mL/min.ln α1,ln α2,and ln α3 represent separation factors between enantiomers of metoprolol,propranolol,and atenolol,respectively.
where,ΔΔH and ΔΔS represent differential enthalpy and entropy,respectively.The plot of ln(α)versus 1/T is linear if the enantioselective separation does not change over the temperature range studied.The plots were linear for all enantiomers,which indicates that a temperature value(isoelution temperature,Tiso)exists at which the isomers co-elute.The separation is enthalpy driven below Tisoand the separation factors can increase with decrease in temperature.Above Tiso,the chiral separation is entropy driven and the separation factors are expected to increase with increase in temperature[40].From regression lines(Fig.5),the values of ΔΔH,ΔΔS and Tisowere computed and are presented in Table 2.The values of ΔΔH and ΔΔS were negative for metoprolol and propranolol while the reverse was found for atenolol at different pressures.Further,with increase in back pressure there was an increase in the absolute values of ΔΔH and ΔΔS.The absolute values of ΔΔH/RT were greater than ΔΔS/RT for metoprolol and propranolol,which suggests that the separation process was enthalpy controlled.The Tisovalues were above the working range of temperature for metoprolol and propranolol and can be improved by decreasing the temperature.On the other hand,the Tisovalues were below the temperature range studied for atenolol and thus the enantioseparation was entropy driven.This thermodynamic data were analyzed in terms of enthalpy-entropy compensation.The plot of ΔΔH versus ΔΔS was a straight line,which shows enthalpyentropy compensation for enantioselectivity(Fig.S1).

Table 3Molecular docking results for enantiomers of β-blockers.
3.4.Molecular docking studies with chiral stationary phases
To study the elution pattern and understand the chiral recognition mechanism,molecular docking was performed using Auto Dock Tools(ADT)4.2 software.This tool facilitates prediction of most favored orientation of small molecules to interact with the stationary phase.The binding energies and the bond lengths of different interaction modes can be estimated with reasonable accuracy[22,23].Further,it can help in understanding the elution behavior of the enantiomers based on binding energies.The binding energies are a result of different intermolecular interactions such as H-bonding and Van der Waals,π-π interactions and dipoledipole interactions.More negative values reflect greater stability of enantiomer-CSP binding.The 3D interaction of the enantiomers with the chiral stationary phase is shown in Fig.6.The CSP has>C=O,-NH-and a phenyl ring with alkyl and chloro groups,while the enantiomers have carbamoyl group(only in atenolol),-OH,-O-,>C=O,secondary amine,isopropyl groups and aromatic ring systems(phenyl and naphthyl).As such they can interact via H-bonding, π-π interactions,and hydrophobic interactions.The binding energy,ligand efficiency,number and type of intermolecular interactions with bond lengths are presented in Table 3.The binding energy(kJ/mol)for the enantiomers followed the order:S(-)-metoprolol(-3.35)>S(-)-propranolol(-3.95)>S(-)-atenolol(-4.37)>R(+)-metoprolol(-4.88)>R(+)-atenolol(-5.00)>R(+)-propranolol(-5.31).It can be inferred that R(+)-propranolol formed the strongest interaction with the CSP,while S(-)-metoprolol the weakest.Additionally,the ΔG values(kJ/mol)at 100 bars pressure and 25°C for the transfer of enantiomer from the mobile phase to the stationary phase also had a similar trend:S(-)-metoprolol(-1.290)>S(-)-propranolol(-2.464)>S(-)-atenolol(-2.864)>R(+)-metoprolol (-3.989)>R(+)-atenolol (-4.120)>R(+)-propranolol(-4.800)(Table S4).These observation are in good agreement with the elution trend observed experimentally,S(-)-metoprolol(1.96 min)>S(-)-propranolol(2.59 min)>S(-)-atenolol(2.95 min)>R(+)-metoprolol(3.79 min)>R(+)-atenolol(4.05 min)>R(+)-propranolol(5.00 min)(Table S2).To further relate the binding energy with enantioselectivity,the difference in the binding energies of the enantiomers ΔER-S(kJ/mol)was also evaluated.The absolute ΔER-Svalues were 1.53,1.36 and 0.63 kJ/mol for metoprolol,propranolol and atenolol,respectively.The largest difference of 1.53 kJ/mol for metoprolol indicates relatively easier separation of the enantiomers than propranolol or atenolol.These values can have direct correlation with the separation factor of the drug enantiomers,metoprolol(2.53 kJ/mol)>propranolol(2.33 kJ/mol)>atenolol(1.51 kJ/mol)(Table S2),which were found experimentally.
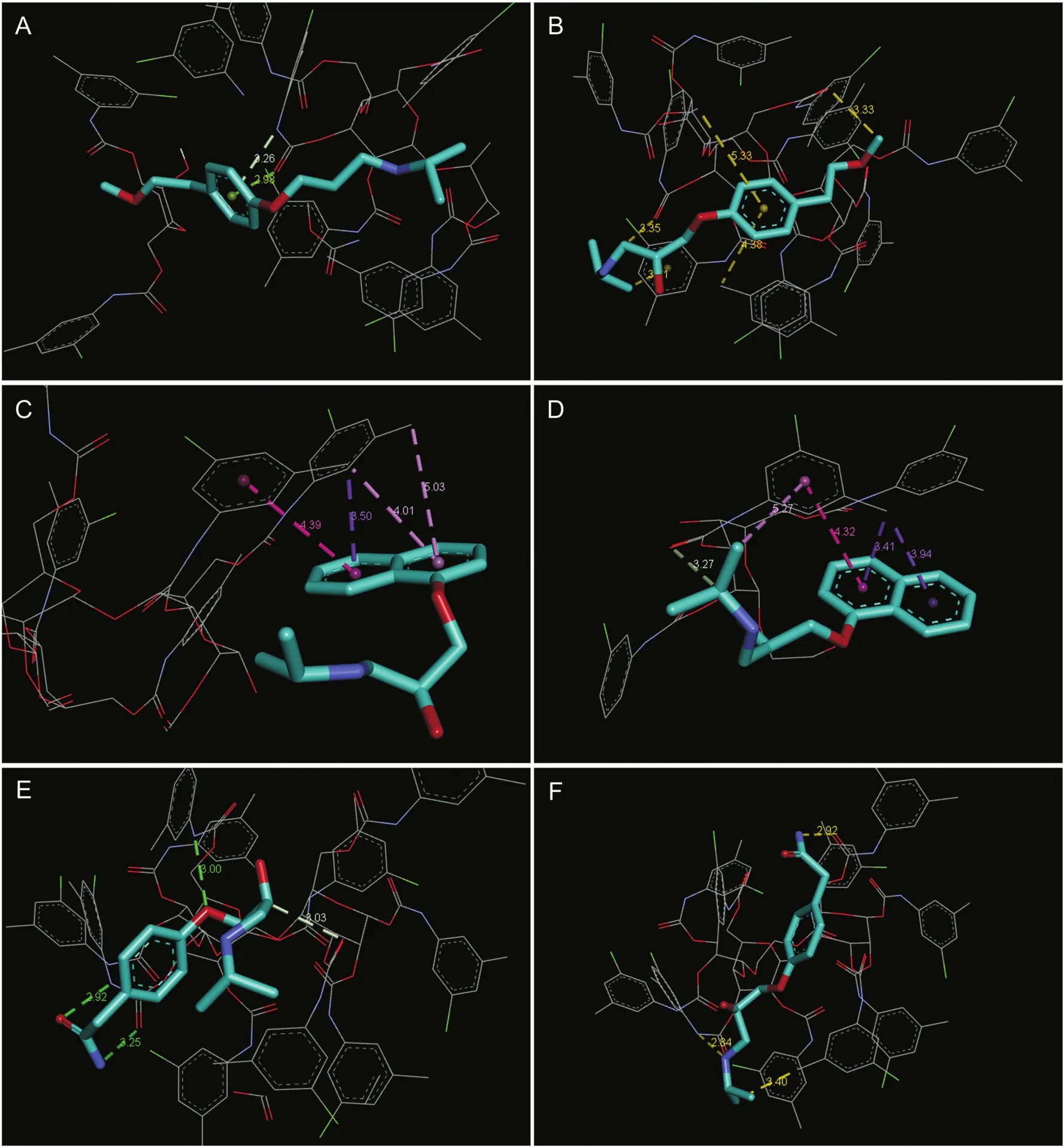
Fig.6.3D interaction modes of enantiomers:(A)S(-)-metoprolol,(B)R(+)-metoprolol,(C)S(-)-propranolol,(D)R(+)-propranolol,(E)S(-)-atenolol,and(F)R(+)-atenolol with Chiralpak®IG chiral stationary phase immobilized with amylose tris(3-chloro-5-methylphenylcarbamate.
Further,chiral recognition mechanism can be understood from the 3D docking figures of enantiomers(Fig.6).Appropriate fit of the enantiomers in the structure of CSP is paramount for chiral separation.The detailed information about each enantiomer is summarized in Table 3.As is apparent,hydrogen bonding and hydrophobic interactions were mainly responsible for enantioseparation.R(+)-propranolol,the highly retained enantiomer under the optimized experimental conditions,was bound with CSP through four hydrophobic interactions(bond lengths/distance 3.41-5.27 Å)and one hydrogen bond.Hydrophobic interactions were mainly generated from π-alkyl,π-sigma and π-π stacking between the π-electrons of naphthyl ring in the enantiomer and the alkyl group or the π-electrons of the phenyl ring.On the other hand,S(-)-metoprolol which was the least retained had only twopoint interaction with the CSP,via π-donor,H-bond and π-lone pair interaction.However,R(+)-metoprolol had greater retention due to two hydrogen bonds(between the hydrogen of-CO-CH3and CO of the-CO-NH-group)and three hydrophobic interactions involving π-alkyl and π-sigma bonding.Conventional hydrogen bonding was primarily responsible for the separation of atenolol enantiomers.The H-donor/acceptor was either the carbamoyl group of the enantiomer or carbamate group of the CSP(bond lengths/distance 2.84-3.25 Å).
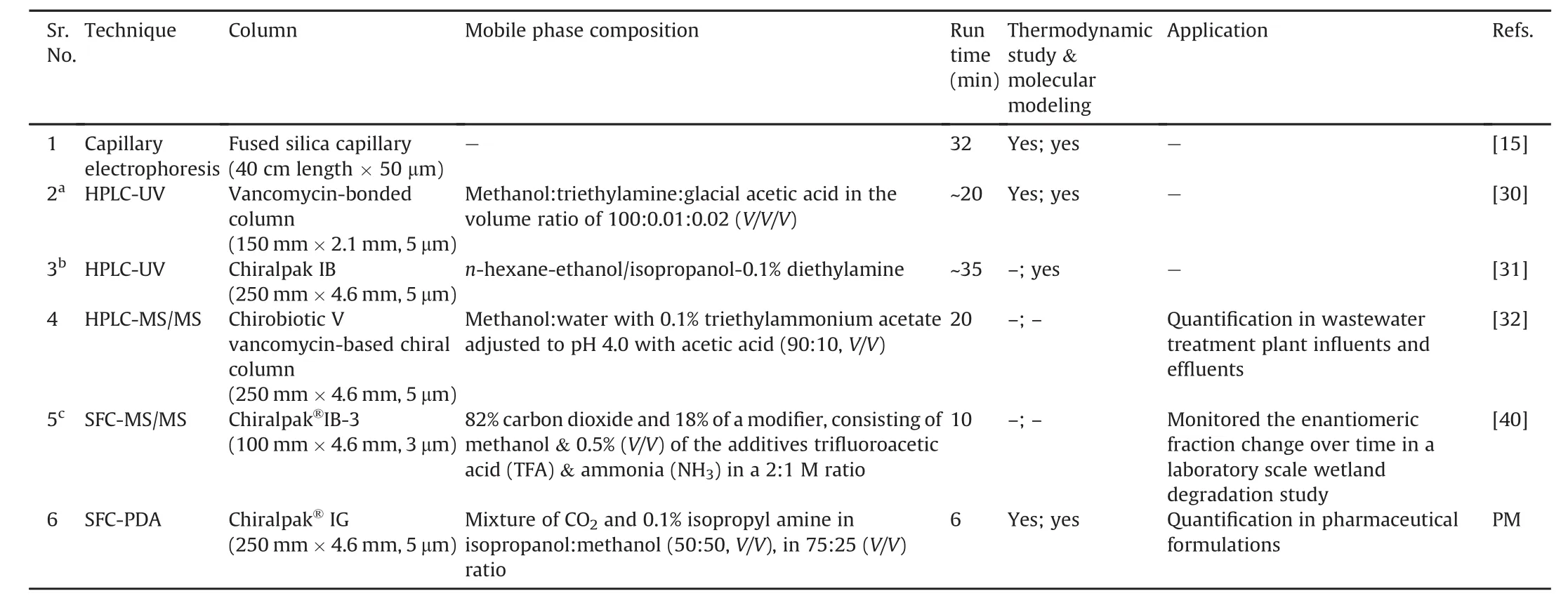
Table 4Comparison of chromatographic methods developed for simultaneous separation of atenolol,metoprolol and propranolol.
Though it is difficult to comprehend the enantioseparation and retention behavior solely based on molecular docking,nevertheless,the higher retention of R(+)enantiomers for the three β blockers can be associated with a greater number of interactions with the CSP compared to the S(-)counterparts.Furthermore,comparison with reported work on these drugs using different chiral stationary phases shows some similarities as well as some variations.The work of Li et al.[22,23]on vancomycin-bonded and Chiralpak IB columns using HPLC showed S-enantiomers eluted first for some analytes which confers with the present work using Chiralpak®IG,while it was reverse with a similar column using SFC-MS/MS[32].Additionally,the difference in the binding energies of the enantiomers as evaluated from simulation studies was the smallest for atenolol among the three β-blockers[23],which is comparable with the results obtained in the present work.
3.5.Comparison with reported work
Currently,the methods which deal with the simultaneous enantioseparation and determination of atenolol,metoprolol and propranolol include capillary electrophoresis[15],HPLC[22,23],LC-MS/MS[24],and SFC-MS/MS[32].The salient features of these methods are summarized in Table 4.However,all reported methods require separation time ranging from 10 to 35 min.In contrast,the present method allowed separation of all six enantiomers within 6.0 min.Besides,a majority of reported procedures entailed large quantities of organic solvents for separation except one report using SFC-MS/MS[32].Additionally,only two methods have discussed thermodynamic considerations,as well as molecular docking study to understand the interactions of the analytes with the chiral stationary phase[15,22].Li et al.[23]investigated the chiral recognition mechanisms by molecular docking technique.Nevertheless,this was the first report on use of SFC technique for chiral separation which involves thermodynamics of drug interaction with the stationary phase and simulation study to understand the retention behavior of enantiomers.Furthermore,all the enantiomers were separated under identical elution conditions.In comparison to the existing procedures,the current method led to faster and more efficient separations while reducing development and validation time for chiral separation of these drugs.

Table 5Linear range and chromatographic characteristics of enantiomers of metoprolol,propranolol and atenolol.
3.6.Method validation results
The method was validated for linearity,limit of detection(LOD=3.3σ/S,where σ is the standard deviation of the intercept and S the slope of the calibration lines),limit of quantitation(LOQ=10σ/S),specificity,intra-day and inter-day accuracy and precision and recovery following ICH guidelines[41].For quantitative studies,a mixture of CO2and 0.1% isopropyl amine in isopropanol:methanol(50:50,V/V),in 75:25(V/V)ratio was employed as the mobile phase.Although adequate resolution(Rs>1.5)of the enantiomers was possible with 5%-20% organic modifier,25% was considered based on optimum analysis time,response,resolution and selectivity.The calibration curves were generated by plotting the peak area against the concentration of the enantiomers.The linearity(0.5-10 μg/mL,r2≥0.9995)was established from five calibration lines by least square linear regression for each isomer.
The LOD and LOQ of the method were 0.126/0.381,0.130/0.394,0.128/0.389,0.124/0.376,0.137/0.414 and 0.132/0.401μg/mL for S(-)-metoprolol,R(+)-metoprolol,S(-)-propranolol,R(+)-propranolol,S(-)-atenolol and R(+)-atenolol,respectively.The method specificity was determined by comparing the retention time of the standards and real samples(pharmaceutical formulations).The results showed good correlation in the measurement of retention time for all the enantiomers(% CV,0.51-1.12).The detailed chromatographic characteristics are summarized in Table 5.
The results for intra-day and inter-day precision and accuracy of the method for all the enantiomers at three QC levels are summarized in Table S5.The intra-day and inter-day precision(%CV)ranged 1.2%-2.9% and 1.0%-2.9%,respectively.The accuracy(recovery)of the method was determined at 80%,100%,and 120% of the claimed value by standard addition technique.The results showed good accuracy in the range of 98.63%-100.92%(Table S6).
3.7.Analysis of pharmaceuticals
The developed method was used to analyze these drugs in their commercial dosage forms.Fig.S2 shows the chromatograms of enantioseparation of metoprolol,propranolol and atenolol from their tablet formulations.The results obtained showed acceptable accuracy and precision of the assay(Table S7).Moreover,there was no interference from the excipients present in the formulations.Further,the enantiomeric purity(or optical purity)of the separated analytes was also determined,S(-)-atenolol:99.5%,[α]25D=-24.2°(c=1.0,ethanol);R(+)-atenolol:99.6%,[α]25D=+24.4°(c=1.0,ethanol);S(-)-metoprolol:99.4%,[α]25D=-30.2°(c=1.0,ethanol);R(+)-metoprolol:99.2%,[α]25D=+29.9°(c=1.0,ethanol);S(-)-propranolol:99.3%,[α]25D=-21.9°(c=1.0,ethanol);and R(+)-propranolol:99.5%,[α]25D=+22.3°(c=1.0,ethanol).
To show the significance of the developed method,a statistical comparison of the results was made with reported methods[19,21]using t-test and F-test.The t and F values obtained were less than the tabulated values at four degrees of freedom,suggesting no significant difference between the two methods for any of the drugs.
4.Conclusions
Herein,we have described a new SFC method for enantioseparation of atenolol,metoprolol and propranolol on a chiral stationary phase using a single elution protocol.The influence of organic modifier and its proportion produced a greater effect on selectivity than the column temperature and back pressure.Although methanol and isopropanol were able to separate the enantiomers individually,a mixture of methanol and isopropanol provided the best conditions for their simultaneous separation in a single run with adequate resolution,selectivity and chromatographic efficiency.The thermodynamic data showed enthalpy driven separation for metoprolol and propranolol and entropy driven for atenolol.Molecular docking study substantiated the elution order of the enantiomers observed experimentally and also the mechanism for chiral recognition.Further,hydrogen bonding and hydrophobic interactions played a major role in enantioselectivity of the studied drugs.Finally,the SFC method was effectively applied to analyze commercially available formulations of these drugs.
Declaration of competing interest
All authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank Department of Chemistry,Gujarat University,for supporting this work.One of the authors,Ms.Priyanka A.Shah,gratefully acknowledges Human Resource Development Group-Council of Scientific&Industrial Research(CSIR),New Delhi,for Research Associate Fellowship(File No.:09/070(0058)2K18 EMR-I).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.12.005.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Effect of Shengmai Yin on the DNA methylation status of nasopharyngeal carcinoma cell and its radioresistant strains
- Spectroscopic studies of the interaction between phosphorus heterocycles and cytochrome P450
- Impaired tricarboxylic acid cycle flux and mitochondrial aerobic respiration during isoproterenol induced myocardial ischemia is rescued by bilobalide
- Evaluation of the gastrointestinal anti-motility effect of Anacardium occidentale stem bark extract:A mechanistic study of antidiarrheal activity
- Synergistic effects of methyl 2-cyano-3,11-dioxo-18beta-olean-1,-12-dien-30-oate and erlotinib on erlotinib-resistant non-small cell lung cancer cells
- A living cell-based fluorescent reporter for high-throughput screening of anti-tumor drugs
