Clinical efficacy of integrated Chinese and western medicine in the treatment of the corona virus disease 2019(COVID-19):A meta-analysis
2022-01-13XueFengJiangYiGuiLaiHuiJieFanYeJianHuFangMinChenFangHuaYangQiangWangYingChangFu
Xue-Feng Jiang ,Yi-Gui Lai ,Hui-Jie Fan ,Ye-Jian Hu ,Fang-Min Chen ,Fang-Hua Yang ,Qiang Wang,Ying-Chang Fu*
1Department of Traditional Chinese Medicine,Yangjiang People's Hospital,Yangjiang 529500,China.2College of traditional Chinese Medicine,Southern Medical University,Guangzhou 510515,China.
Abstract:Objective:To evaluate the clinical efficacy of integrated Chinese and western medicine(ICWM)in the treatment of COVID-19.Methods:Literature retrieval was performed in PubMed,Cochrane Library,EMBASE,Web of science,CNKI,VIP and Wanfang database from inception to November 1,2020.The data extraction and quality assessment were completed by two independent reviewers.Meta-analysis was performed using Review Manager 5.3.Results:30 clinical trials were included with 3379 participants.The meta-analysis showed that there were significant differences in the total effective rate (RR=1.24,95%CI [1.15,1.35]; P<0.01),the conversion rate from mild or moderate to severe (RR=0.32,95%CI [0.24,0.44]; P<0.01),the mortality(RR=0.52,95%CI[0.30,0.93]; P<0.05),the disappearance time of fever(MD=-1.60,95%CI [-1.99,-1.21]; P<0.01) and cough (MD=-1.43,95%CI [-1.96,-0.90]; P<0.01) and fatigue (MD=-1.44,95%CI [-2.33,-0.55]; P<0.01),the rate of CT improvement (RR=1.29,95%CI [1.13,1.47]; P<0.01) and the rate of adverse reactions (RR=0.52,95%CI [0.31,0.87]; P<0.05) between ICWM and western medicine (WM) alone.While there were no differences between two groups in the cure rate (RR=1.22,95%CI [0.98,1.52]; P>0.05) and the disappearance rate of fatigue (RR=1.18,95%CI [0.97,1.45]; P>0.05).Conclusion:Meta-analysis results indicated that ICWM in the treatment of COVID-19 can improve clinical efficacy and safety.However,high-quality,large-sample,multi-center RCTs are required to confirm the efficacy of traditional Chinese medicine intervention COVID-19.
Key words:COVID-19;efficacy;integrated Chinese and western medicine;meta-analysis
Introduction
The new coronavirus pneumonia (COVID-19) is an emerging infectious disease,caused by severe acute respiratory syndrome coronavirus 2(SARS CoV-2) [1].As of January 18,2021,88,336 cases of COVID-19 have been diagnosed,82,400 cured and 4,635 died in China,while a total of 95,555,281 cases and 2,041,289 deaths have reported globally [2].The rapid spread of COVID-19 around the world has become a global emergency,which severely damaged the world economy and people's health.
People with COVID-19 mainly have fever,dry cough and fatigue symptoms.Severe patients may develop dyspnea,acute respiratory distress syndrome,etc.If treatment is not timely,it is easy to cause death [3].At present,there is no special antiviral drug for novel coronavirus.The WM treats this disease with symptomatic and supportive treatment,such as general treatment,antivirus and anti-infection [4].Since the outbreak of COVID-19 in January 2020,the Chinese government had established the principle ofICWMtreatment,and the traditional Chinese medicine (TCM) has been fully and deeply involved in the management of the epidemic in China.As of August 18,2020,Chinese health authorities had released eight versions of COVID-19 diagnosis and treatment guidelines,starting with the third version that includes TCM prescriptions and drugs .TCM adopts the method of syndrome differentiation and treatment,which can effectively relieve symptoms,improve the cure rate,reduce the disease death rate,and promote the body recovery of people in the convalescence period [6].According to the white paper issued by the Chinese government,92% of all confirmed cases received TCM treatment,which proved effective [7].
During the epidemic,Wuhan doctors used Chinese herbal medicine,such as Qingfei Paidu Decoction,as supplements to WM,which proved to be hlpeful in speeding up the recovery of patients [8].Many Chinese patent medicines such as Lianhua Qingwen,Huoxiang Zhengqi have been used in clinical practice and achieved good curative effect [9].Along with the widespread clinical application,a large number of clinical trials have been registered and carried out to evaluate the effectiveness and safety of TCM interventions.According to data provided by Chinese Clinical Trial Registry,as of August 27,2020,a total of 165 TCM clinical trials had been registered,accounting for 22.3% (165/741) of all COVID-19 trials in Chinese Clinical Trial Registry.However,TCM treatment is characterized by"individualization",so it is difficult to establish standard treatment rules,which leads to the low quality of TCM clinical efficacy evidence[10].Therefore,it is very necessary to carry out strict and objective quality evaluation for different types of clinical studies,and the effectiveness analysis results obtained on this basis are more convincing.Based on the published clinical study of TCM treatment of COVID-19,this research conducted a systematic assessment and meta-analysis,in order to more intuitive understanding of the cooperation ofICWMtreatment the curative effect of COVID-19,and evaluate the clinical trials of traditional Chinese medicine quality and found evidence of a potential problem,the purpose is to provide reference for the design of clinical trials of traditional Chinese medicine and the follow-up work.
Materials and methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[11].
1.1 Literature search strategy
Two researchers conducted a systematic literature search through databases (PubMed,The Cochrane Library,EMBASE,Web of science,Chinese National Knowledge Infrastructure Database (CNKI),Chinese Scientific Journals Database(VIP) andWanfang database) from the start of each database up to November 1,2020.The language of publication is limited to Chinese or English.The following search terms will be used:(“coronavirus disease 2019”OR“new coronavirus”OR“novel coronavirus”OR“COVID-2019”OR“SARS CoV-2”OR“2019 novel coronavirus”OR“Novel Coronavirus Pneumonia”OR“NCP”OR“2019-nCoV”OR“Severe acute respiratory syndrome coronavirus 2”)AND (“ integrated Chinese and western medicine”OR“integrated traditional Chinese and western medicine”OR“integrative Chinese and western medicine”OR“integrative traditional Chinese and western medicine”OR“ Chinese medicine”OR“Traditional Chinese medicine”OR“herbal medicine”OR“oriental medicine”).
1.2 Inclusion and Exclusion Criteria
The inclusion criteria were as follows:(1) Research objects:the patient was diagnosed as novel coronavirus pneumonia,regardless of age,gender,or severity of the condition;(2) Type of study:randomized controlled trials (RCTs) or clinical retrospective studies(CRSs),no blinding restriction was used;(3) Intervention measures:the control group was treated with conventional therapy,and the treatment group was treated with Chinese medicine combined with conventional therapy,There are no limits to dosage,usage,and course of treatment;(4) Outcome measures:the main outcome measures were effective rate,cure rate,conversion rate from mild or moderate to severe,mortality.The secondary indicators included main symptoms (fever,cough,fatigue) disappearance time or rate,blood test results (white blood cell count,percentage of lymphocytes,c-reactive protein),improvement by CT (scan inflammatory absorption shown in chest X-ray),negative conversion ratio of novel coronavirus nucleic acid,hospital stay,and incidence and severity of adverse events.
Trials were excluded if they did not meet the criteria above and included the following:(1) The research could not find the outcome measurements;(2) Reviews,non-clinical studies,and case observations;(3) There are repeated reports and incomplete information;(4) Non-Chinese and English Literature.
1.3 Data extraction and quality Assessment
The data collected by twoindependent investigators using standardized data collection form.Collect the following data:(1) Basic information including first author,publication year,location,and type of study;(2) The information about patients:Sample size,age,disease stage;(3) Intervention method:details of the interventions and controls (regimens),course of treatment;(4) Outcomes:outcome measures,study results,and adverse events.The authors included in the study were contacted for unreported data or missing data.
Two reviewers independently completed the quality assessment.The Cochrane Collaboration’s Risk of Bias Assessment tool was used to assess the methodological quality of RCTs,and the Newcastle-Ottawa Scale (NOS) was used to assess the quality of CRSs [12,13].Each RCT was assessed for low risk,high risk,or unclear risk associated with sequence generation,allocation concealment,blinding of outcome assessors,incomplete outcome data,selective outcome reporting,and other sources of bias.Each RCT was assessed using three levels:‘Low risk’ (adequate and correct description of methods or procedures),‘High risk’ (incorrect description of methods or procedures) or‘Unclear risk’ (no description of methods and procedures) relating to the following items:(1) Selection bias (random sequence generation and allocation concealment);(2) Performance bias (blinding of participants and personnel);(3) Detection bias (blinding of outcome assessment);(4) Attrition bias (incomplete outcome data);(5)Reporting bias (selective reporting);(6) Other bias (other sources of bias).The NOS assesses the quality of CRSs with eight questions in three broad categories:(1) Patient selection;(2) Comparability of study groups;(3) Assessment of the outcome.The total score is 9,the higher the score,the better the quality of the study.
1.4 Statistical Analysis
Review Manager 5.3(The Nordic Cochrane Centre,The Cochrane Collaboration,Copenhagen,Denmark)was used to conduct the meta-analysis.Heterogeneity among studies was estimated using the Cochran’s Q statistic and I2tests.P<0.10 or I2>50% were defined to have heterogeneity.The random effects model was used when there was significant statistical heterogeneity;otherwise the fixed effects model was used.The risk ratios (RRs) or odds ratios (ORs) with 95%confidence intervals (CIs) were calculated for dichotomous data,while the mean differences (MDs) with 95% CIs were calculated for continuous data.
Results
1.5 Search results
A total of 1952 articles were identified from the online database,and 1116 articles were left after removing duplication.After screening titles and abstracts,705 articles were excluded,because they did not Covid-19 or clinical researches or non-ICWM.We reviewed the remaining 131 studies in depth.102 articles were excluded as reviews and case reports,without complete data or data duplication.Finally,29 eligible articles [14-42] were identified in the meta-analysis(Figure 1).
3.2 Study characteristics and quality assessment
There were 30 clinical trials including 17 RCTs[15-18,22,25,26,28,29,31,32,34,35,37,40,41]and 13 CRSs[14,19-21,23,24,27,30,33,36,38,39,42] that compared the effects of ICWM to WM in the treatment of COVID-19.All studies were published online in Chinese or English between February 6,2020 and September 23,2020.Samples sizes ranged from 11 to 430 participants.In these clinical trials,a total of 3379 participants (1848 in the experimental group and 1531 in the control group) aged above 18 years were recruited from public hospitals in China.The subjects'conditions were classified as mild,moderate,severe,or critical (Table 1).12 RCTs reported random sequence generation methods[15,16,18,22,25,26,28,31,32,35,41] of the 17 trials.None of the studies mentioned allocation concealment and blinding,and 17 of them were reported the unclear risk of bias.No reporting bias was detected,as all stated study outcomes were reported by the included studies.The risk of bias assessment for the included RCTs was shown in Figure 2.In addition,the NOS scale evaluation of CRSs was listed in Table 1.
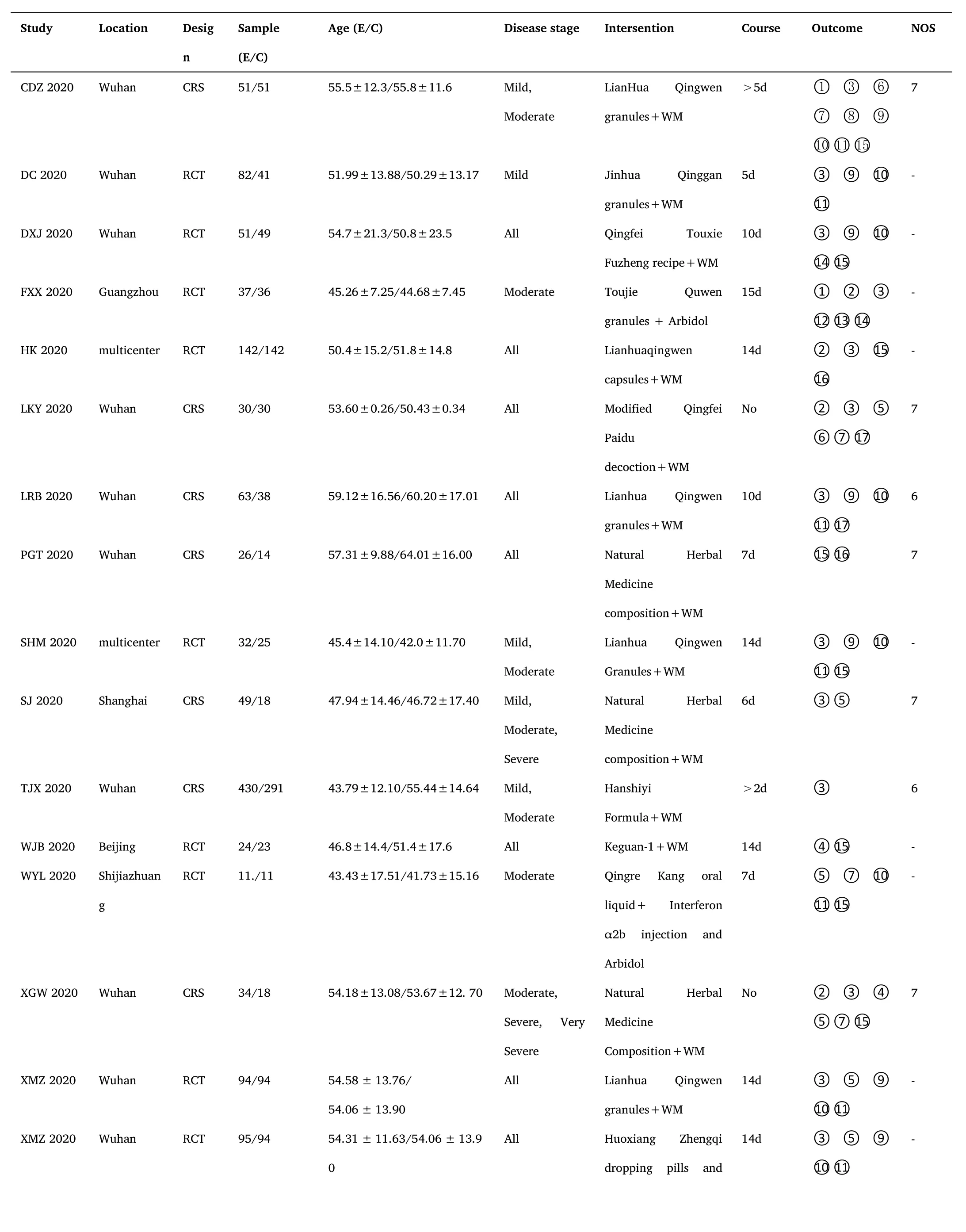
Table 1 Characteristics of included studies
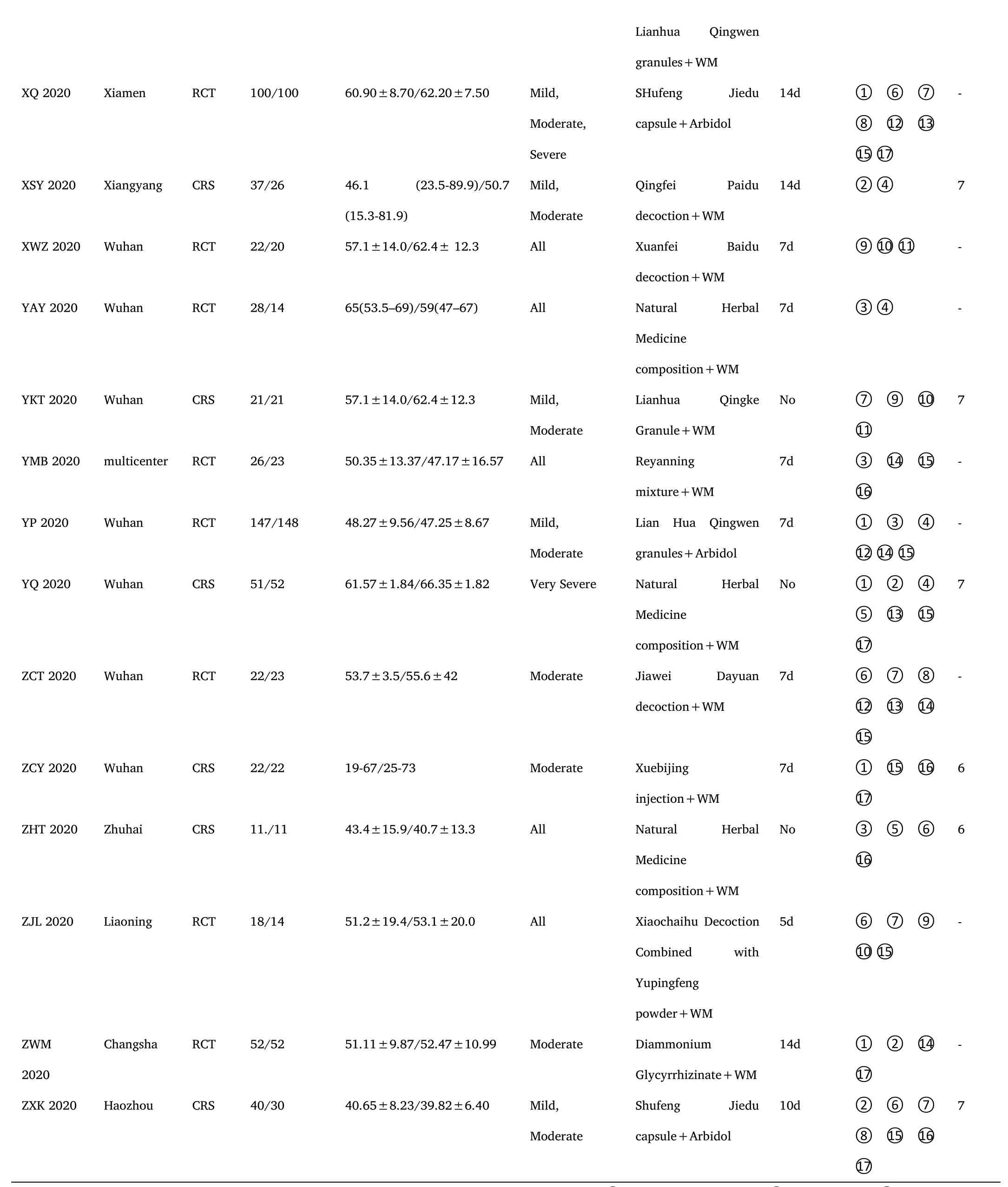
Note.RCT:Randomized controlled trial;CRS:Clinical retrospective study;WM:Western medicine;①:The total effective rate;②:The cure rate;③:The conversion rate from mild or moderate to severe;③ :Mortality;⑤:The hospital stay;⑥:The disappearance time of ever;⑦:The disappearance time of cough;⑧:The disappearance time of fatigue;⑨:The disappearance rate of fever;⑩The disappearance rate of cough;⑪:The disappearance rate of fatigue;⑫:White blood cell count;⑬:Percentage of lymphocytes;⑭:C-reactive protein;⑮:The rate of CT improvement;⑯:The negative conversion ratio of novel coronavirus nucleic acid;⑰:Adverse events.
3.3 Evaluation of clinical curative effects
In the 30 included trials,7 studies[14,17,29,35,36,38,41] assessed the total effective rate of the treatment of COVID-19.The heterogeneity test results indicated that there was no statistical heterogeneity between studies (Chi²=1.33,df=6 (P=0.97);I²=0%).The fixed effects model was applied to calculate the combined risk ratio and 95%CI (RR=1.24,95%CI [1.15,1.35];P<0.01),which meant thatICWMin the treatment of COVID-19 could significantly improve the total effective rate compared with WM (Figure 3).8 studies [17-19,27,36,37,41,42] compared the cure rate of the COVID-19.The results of random effect model demonstrated that there was no significant difference in the cure rate between theICWMand WM alone (RR=1.22,95%CI [0.98,1.52];P>0.05) (Figure 4).Besides,ICWMcould reduce the conversion rate from mild or moderate to severe (RR=0.32,95%CI [0.24,0.44];P<0.01) [14-20,22-24,27,28,32,34,35,39] (Figure 5) and mortality (RR=0.52,95%CI [0.30,0.93];P<0.05) [25,27,30,32,35,36],the difference was statistically significant (Figure 6).At the same time,compared with WM treatment,ICWMtreatment can shorten the hospital stay(MD=-2.63,95%CI [-2.78,-2.48];P<0.01) [19,23,26-28,36,39](Figure 7).
3.4 Evaluation of the disappearance time of symptoms (fever,cough,fatigue)
A total of 10 studies reported the disappearance time of clinical symptoms (fever,cough,fatigue) in patients with COVID-19,of which 9 studies [14,19,26,27,29,33,37,40,42] showed that the fever disappeared in theICWMgroup in a shorter time than that in the WM group (MD=-1.60,95%CI [-1.99,-1.21];P<0.01).The disappearance time of cough was recorded in 7 studies [14,19,29,37,39,40,42],and the results showed that the cough resolution time of COVID-19 withICWMwas significantly less than that of the WM treatment group(MD=-1.43,95%CI [-1.96,-0.90];P<0.01).4 studies [14,29,37,42]compared the disappearance time of fatigue of theICWMand WM on the clinical symptoms.TheICWMcan effectively relieve the symptoms of fatigue (MD=-1.44,95%CI [-2.33,-0.55];P<0.01) (Figure 8).The results showed that theICWMcan reduce the symptoms disappearance time than WM.
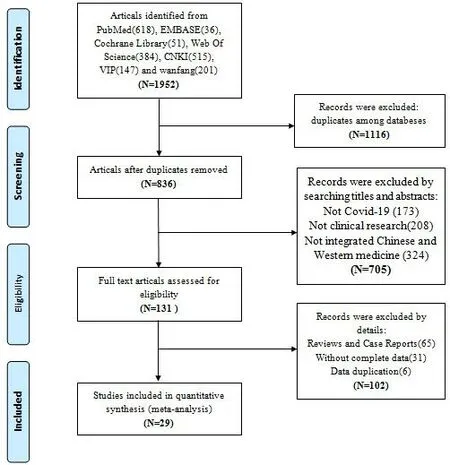
Figure 1 Flow diagram of the eligible studies

Figure 2 Risk of bias graph of randomized controlled trials
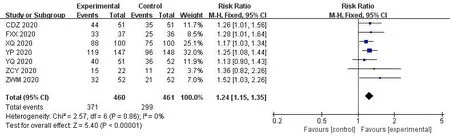
Figure 3 Forest plot of the total effective rate
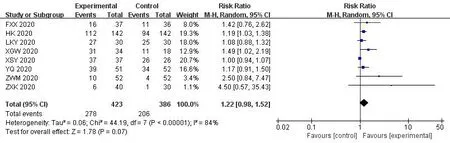
Figure 4 Forest plot of the cure rate
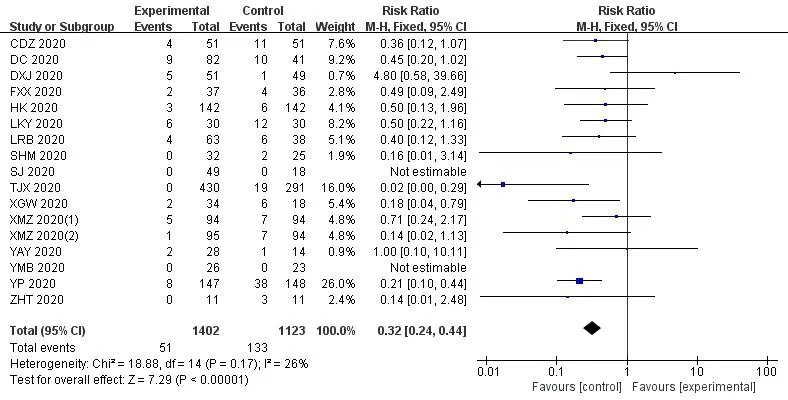
Figure 5 Forest plot of the conversion rate from mild or moderate to severe
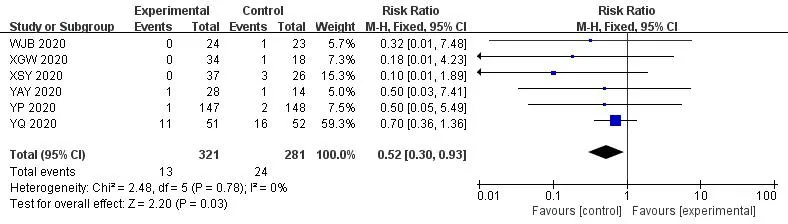
Figure 6 Forest plot of the mortality
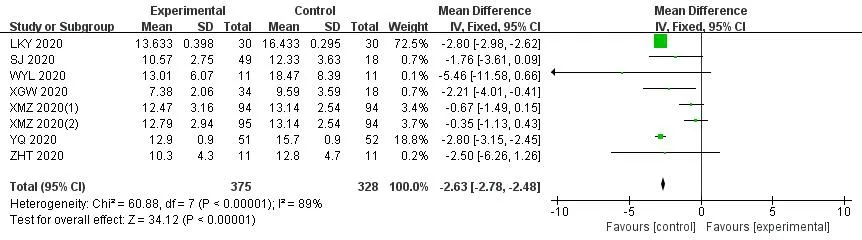
Figure 7 Forest plot of the hospital stay
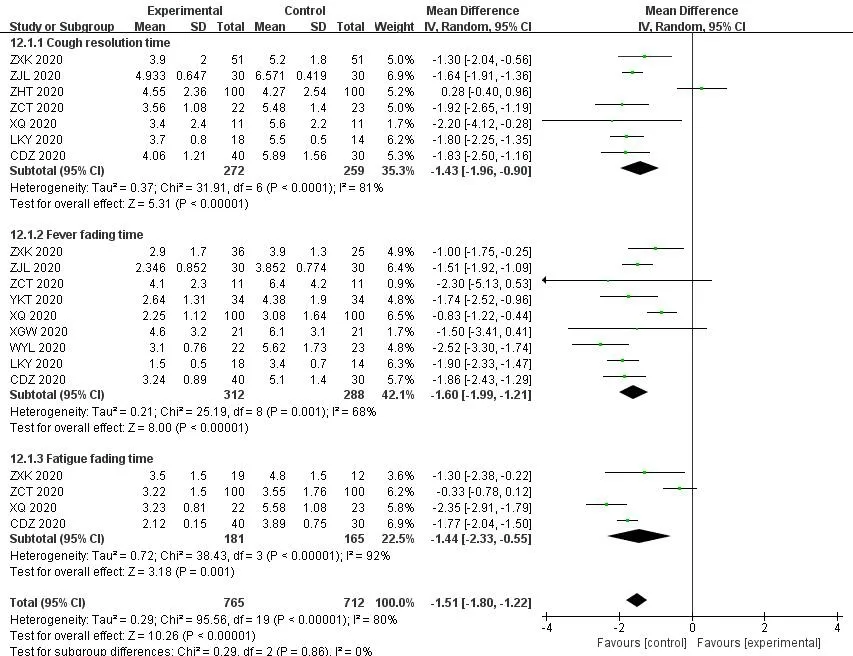
Figure 8 Forest plot of the disappearance time of symptoms (fever,cough,fatigue)
3.5 Evaluation of the disappearance rate of symptoms (fever,cough,fatigue)
As shown in Figure 9,we compared the disappearance rate of theICWMand WM on the clinical symptoms.Meta-analysis showed that the combination of theICWMcan improve the disappearance rate of fever (RR=1.18,95%CI [1.02,1.37];P<0.05) [14-16,20,22,28,31,33,40].11 studies[14-16,20,22,26,28,31,33,40]analyzed the the disappearance rate of fever in patients with COVID-19.The results showed thatICWM could improve thedisappearance rate of cough and sputum production (RR=1.34,95%CI [1.07,1.69];P<0.05).However,The combined therapy did not have a positive effect on the disappearance rate of fatigue (RR=1.18,95%CI [0.97,1.45];P>0.05)[14,15,20,22,26,28,31,40].
3.6 Evaluation of blood test results (white blood cell count,percentage of lymphocytes,c-reactive protein)
8 studies examined the changes in the complete blood count through routine blood tests after the intervention.Meta-analysis showed that compared with WM group,theICWMcan significantly increase white blood cell counts (MD=0.40,95%CI [0.32,0.47];P<0.01) [17,29,35,37].4 studies[17,29,36,37]also showed combined therapy have a beneficial effect on for lymphocyte percentage (MD=1.46,95%CI[1.03,1.89];P<0.01).Meanwhile,theICWMtreatment could Reduce the patient's c-reactive protein levels (MD=-2.67,95%CI [-3.08,-2.26];P<0.01) [16,17,34,36,37,41] (Figure 10-12).
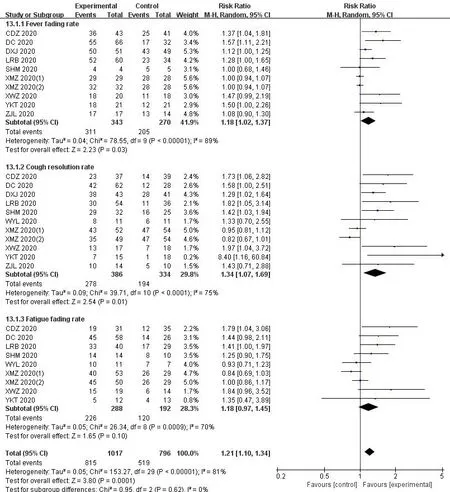
Figure 9 Forest plot of the disappearance rate of symptoms (fever,cough,fatigue)
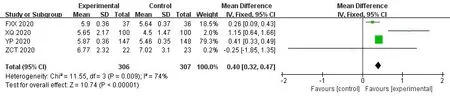
Figure 10 Forest plot of the white blood cell count

Figure 11 Forest plot of the percentage of lymphocytes
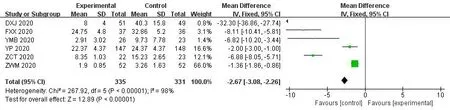
Figure 12 Forest plot of the c-reactive protein levels
3.7 Evaluation of the rate of CT improvement
A total of 16 studies [14,16,18,21,22,25-27,29,34-38,40,42]were included to report theICWMtreatment on CT improvement rate of COVID-19 patients.Meta-analysis results of fixed effect model showed that the rate of CT improvement of patients with COVID-19 treated by integrated traditional medicine was better than that treated by WM (RR=1.29,95%CI [1.13,1.47];P<0.01) (Figure 13).
3.8 Evaluation of the negative conversion ratio of novel coronavirus nucleic acid
The negative conversion ratio of novel coronavirus nucleic acid was evaluated in 6 studies[18,21,34,38,39,42].The results showed that theICWMcan improve the transformation of nucleic acid of novel coronavirus into negative,and the efficacy was better than that of WM(RR=1.15,95%CI [1.02,130];P<0.05) (Figure 14).
3.9 Incidence and severity of adverse events
In most of the studies,patients in either group had no serious drug-related adverse reactions during treatment,and only 7 studies[19,20,29,36,38,41,42] reported nausea,vomiting,and diarrhea and other adverse events.Meta-analysis results showed thatICWMcould reduce adverse reactions (RR=0.52,95%CI [0.31,0.87];P<0.05) (Figure 15).

Figure 13 Forest plot of the rate of CT improvement

Figure 14 Forest plot of the negative conversion ratio of novel coronavirus nucleic acid
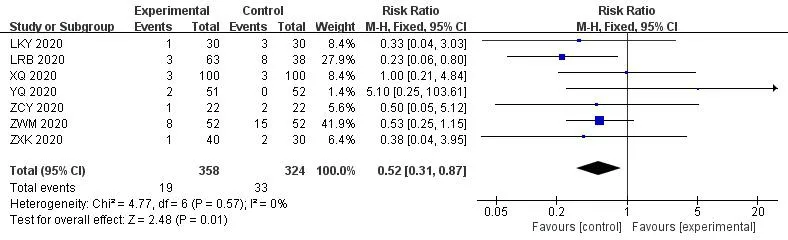
Figure 15 Forest plot of the adverse events
3.10 Subgroup analysis and sensitivity analysis
As shown in Figure 16,we performed a subgroup analysis based on the type of Chinese medicine.Compared with WM,Chinese medicine compound drugs could improve the rate of CT improvement of patients with COVID-19 (RR=1.47,95%CI [1.23,1.75];P<0.05),which was superior to Chinese patent medicine (RR=1.19,95%CI[1.02,1.38];P<0.05),Chinese medicine injection (RR=1.40,95%CI[1.04,1.89];P<0.05),but there is no difference with Chinese herbal compound combined with Chinese patent medicine (RR=1.32,95%CI[0.93,1.88];P>0.05).Natural herbal medicine composition commonly used in the treatment of novel coronavirus include novel coronavirus include Keguan-1,Hanshiyi Formula,Qingfei Paidu decoction,etc.Due to the small sample size,the results were biased to some extent.
The sensitivity analysis of the CT improvement rate of the included studies was conducted by the method of one-by-one elimination.There was no significant change in the results of the Meta-analysis,suggesting that the results were relatively robust.
3.11 Publication bias analysis
Funnel plots was used to assess the publication bias in the rate of CT improvement and symptoms resolution rates.Funnel plots showed evident asymmetry,and there may be publication bias in our study,which may affect our analysis results(Figure 17).
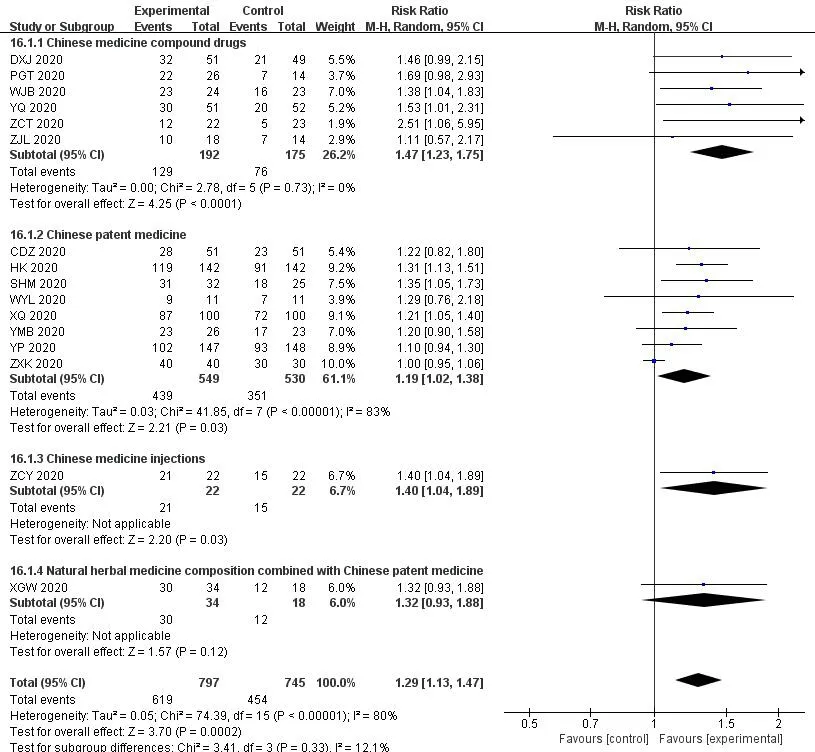
Figure 16 Subgroup analysis of the type of Chinese medicine
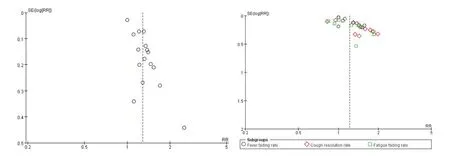
Figure 17 Funnel plot for publication bias.A:The rate of CT improvement;B:The rate of symptoms resolution
3.12 The composition of TCM in treatment regimens.
Table 2 listed the Chinese medicine composition used to treat COVID-19.In some studies,different TCM compounds were given according to patients' constitution types.There were some studies where the TCM interventions are fixed natural herbal medicine composition or Chinese patent medicine.I systematically counted the traditional Chinese medicine compounds in each study.The basic composition was jinyinhua (Lonicera japonica),lianqiao (Forsythia),mahuang (Ephedra),xingren (Almond),chaihu (Bupleurum),fuling(Poria),huoxiang (Agastache),banlangen (Radix Isatidis),bohe (Mint),qinghao (Artemisia apiacea),taizishen (Radix pseudostellariae),banxia(Pinellia ternata),chenpi (Tangerine peel),zisuye (Perilla leaf),beimu(Fritillary),lugen (Reed rhizome),huangqin (Scutellaria baicalensis),sangye (Folium mori),juhua (Chrysanthemum),huzhuang (Polygonum cuspidatum),peilan (Eupatorium fortunei),cangzhu (Atractylodes lancea),xuanshen (Radix scrophulariae),shigao (Gypsum fibrosum),gancao (Licorice).In Chinese medicine,these herbs together have the effect of clearing distemper and detoxifying and freeing lung and reducing heat.
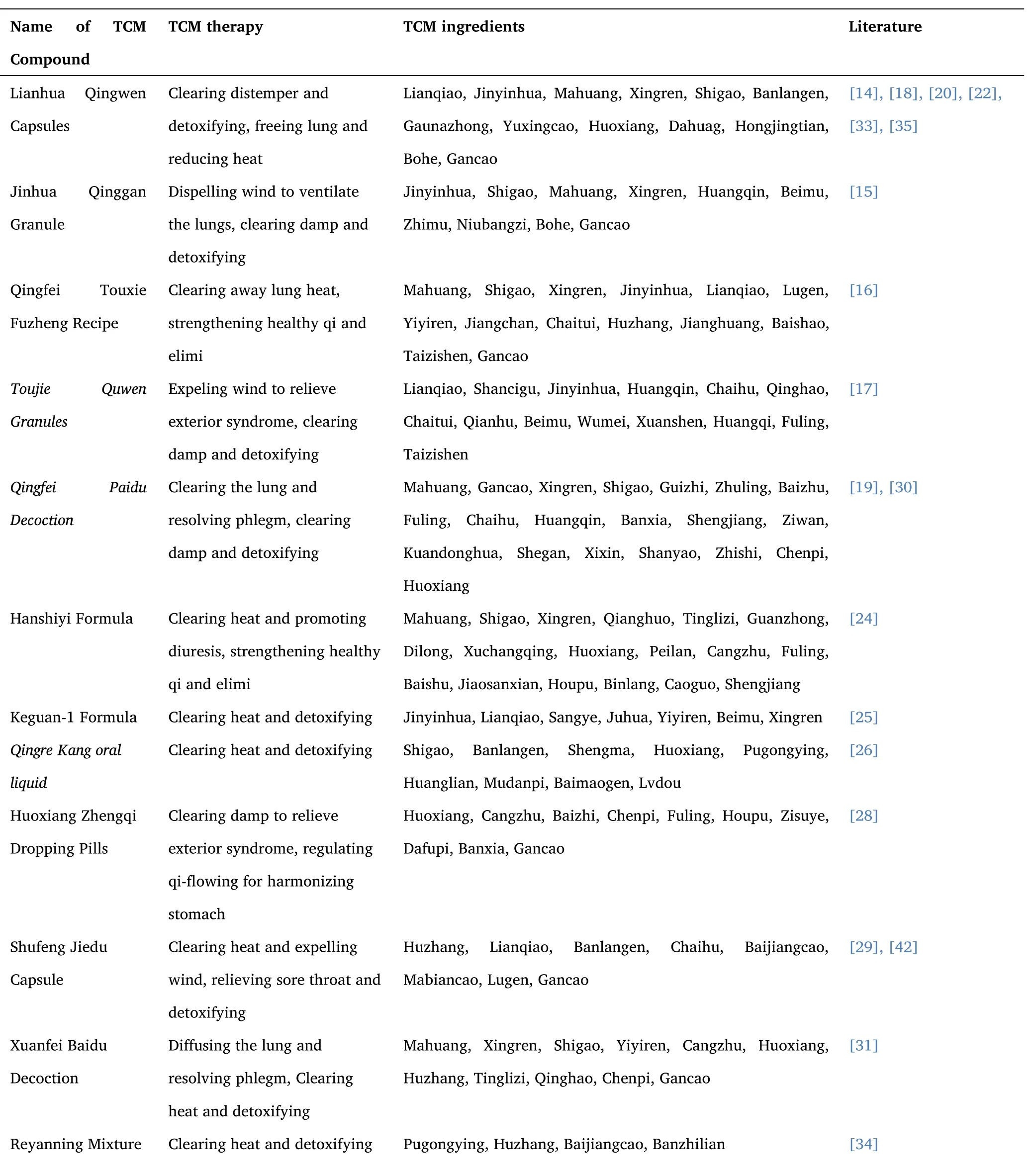
Table 2 The composition of Chinese traditional medicine compound

Discussion
TCM believes that COVID-19 belongs to the category of“epidemic disease”,which is caused by the pestilential pathogen.As early as the"Huang Di Nei Jing" there are records that "when the plague comes,people are easy to get sick,and the disease is similar".Zhang Zhongjing'sTreatise on Febrile Diseasesis a clinical summary of the treatment of exogenous diseases represented by epidemics."The theory of the plague","identification of warm disease" and "compendium on epidemic febrile disease" in the later generations were all the fruits of fighting against epidemic diseases in different periods,and all recorded a large number of principles and prescriptions for preventing and treating epidemic diseases [43].Pathogenic microorganisms is changing,and the human body after infection pathogenic microorganisms symptoms are similar,different from WM treated by a single target,draw lessons from the ancient people for thousands of years of traditional Chinese medicine disease exploration and thinking,by distinguishing between good and evil viscera of ups and downs and the blood and viscera,such as a multi-angle and pathways and targets of the overall treatment and comprehensive control,effectively prevent the damage of pathogenic microorganisms on the body and improve the body's ability to fight pathogenic microorganisms,but also to the prognosis of the disease[44].
Previous studies showed that the herbs of clearing distemper and detoxifying contains flavonoids,alkaloids,polysaccharides,organic acids,lignans,volatile oils,phenylethanol glycosides,triterpenes,phenolic acids,iridoid glycosides,quinazolines and other effective chemical components,which have the pharmacological effects of antiviral,anti-inflammatory,analgesic,antipyretic,anti-oxidation,anti-fibrosis,and improving immune function [45].Scutellaria baicalensis is mainly composed of flavonoids,which has the therapeutic effects of anti-virus,anti-lung injury and hepatoprotective.Baicalein,wogonin,wogonoside and baicalin are the main components of broad-spectrum antiviral activity against H1N1,Dengue virus,HSV-1 and HBV [46].Lui et al.found that the crude extract of Scutellaria baicalensis could inhibit the replication of SARS-CoV-2 in Vero E6 cells.Docking model showed that 6-OH in baicalein plays an important role in inhibiting RNA replication of SARS-CoV-2 virus [47].Angiotensin-converting enzyme 2 (ACE2) is a vital component of the angiotensin-regulating system and is the host cell entry receptor for SARS-CoV-2.Berbamine has high binding energy for ACE2,TMPRSS2 and GRP78 receptors,which exhibits efficacy for the treatment of COVID-19 [48-49].Phillyrin is extracted from the traditional Chinese medicine Forsythia suspensa,which has good pharmacological effects against lung inflammation,traumatic brain injury and acute kidney injury.Studies have shown that phillyrin could inhibit the replication of SARS-CoV-2 by suppressing the nuclear factor kappa B (NF-κB) signaling pathway [50-51].Lianhuaqingwen can inhibit the replication of SARS-COV-2,reduce the production of pro-inflammatory cytokines (TNF-α,CXCL-10/IP-10,CCL-2/MCP-1),and lead to the abnormal particle morphology of virion in cells,thus affecting virus morphology and exert anti-inflammatory activity in vitro [52].
This systematic review included the current published clinical research literature on the treatment of COVID-19 withICWM,including 17 RCTs and 13 CRSs.As an auxiliary therapy for Covid-19,theICWMimproved the total effective rate,shortened the hospital stay,Promoted inflammatory absorption in the lungs and the transformation of nucleic acid of novel coronavirus into negative,reduced the conversion rate from mild or moderate to severe and mortality.Additionally,patients who treated withICWMshowed reduced the symptoms (fever,cough,fatigue) disappearance time,increased white blood cell counts and percentage of lymphocytes,reduced c-reactive protein levels,and reduced adverse reactions.
The study has some limitations.Firstly,the included research literature included some CRSs,with only 17 RCTs,which may lead to publication bias and selectivity bias.Secondly,the quality of the current evidence is still limited and unsatisfactory due to the small sample size,absence of placebo,absence of blinding,inappropriate outcome endpoints,retrospective design,and other methodological drawbacks.Finally,the particularity of TCM interventions prevented prevented the study from being published in a high-impact journal.Since the difficulty of preparing TCM placebo,especially decoction,and the urgency of the epidemic,all studies used open label design instead of placebo-controlled and blind methods.In addition,it is difficult for TCM prescriptions to be accepted abroad because of their complex composition and lack of basic data of chemical composition and pharmacological research.
Conclusion
TheICWMin the treatment of COVID-19 can indeed improve the symptoms of patients,shorten the length of stay,and improve clinical efficacy and safety.However,the specific mechanism of TCM treatment of COVID-19 is still unclear.High-quality,large-sample,multi-center RCTs are required to confirm the efficacy of traditional Chinese medicine intervention COVID-19.
杂志排行
Clinical Research Communications的其它文章
- Clinical analysis of laparoscopic lateral peritoneal suspension in the treatment of severe pelvic organ prolapse
- Dexmedetomidine:chaperone of anesthesia
- Study on the pharmacological mechanism of chaihu shugan powder in the treatment of hepatocellular carcinoma
- The application research progress of intelligent robot in orthopedics
