Evaluation of Antibiotic Pollution in Soil of Vegetable Base in Yangling District, Shaanxi Province
2021-12-31GuoxiuLILihuiCUIYingshaLIU
Guoxiu LI, Lihui CUI, Yingsha LIU
Department of Biological Engineering, Yangling Vocational and Technical College, Yangling 712100, China
Abstract [Objectives] To evaluate the pollution status of antibiotics in the soil of vegetable bases in Yangling District. [Methods] The contents of 19 antibiotics in 4 categories of quinolones, tetracyclines, sulfonamides and macrolides in soil samples from 20 bases were detected and analyzed using HPLC-MS/MS. [Results] The quinolones, sulfonamides and tetracyclines were all detected in 100% of soil samples, and the detection rate of macrolides was 62%. The average contents of the four antibiotics from high to low were quinolones (51.76 μg/kg) >tetracyclines (12.77 μg/kg)> sulfonamides (1.14 μg/kg) >macrolides (0.28 μg/kg), and norfloxacin, oxytetracycline, sulfamethazine and erythromycin were the main contents, respectively. According to the trigger value of ecological toxic effect of antibiotics in soil (100 μg/kg) proposed by the Steering Committee of the International Coordinating Committee for Veterinary Medicine (VICH), the contents of the four classes of antibiotics in the soil of the study area were all lower than the trigger value, indicating a small ecological risk. [Conclusions] Compared with other domestic research reports, the vegetable bases in this study area are also at a relatively low level of antibiotic pollution.
Key words Vegetables, Soil, Antibiotics, Pollution evaluation
1 Introduction
With the rapid development of social economy, the demand for high-quality food, especially organic food, is increasing day by day. At present, there are hundreds of countries in the world to carry out organic agricultural production. Since this century, China has also made great efforts to develop organic agriculture, with an area of 3 million ha, making it the third largest producer and consumer of organic food in the world. Different from the traditional agricultural model, organic agriculture forbids pesticides, chemical fertilizers and other synthetic products, advocates the use of organic fertilizers and has strict requirements on the environmental quality of the producing areas. Therefore, most organic farms directly or indirectly use livestock manure as fertilizer, resulting in a large number of pollutants into the soil.
Antibiotics are one of the main pollutants in livestock feces. The application of livestock manure or irrigation with breeding wastewater can cause a large number of antibiotics to enter the farmland soil, then be absorbed and accumulated by crops, threatening human health. Vegetable is not only an indispensable food in people’s daily life, but also a very important economic crop. Its safety is not only an issue of great concern to the government and ordinary people, but also an important issue for the improvement of the quality and efficiency of agricultural products and sustainable development. A large number of studies have shown that vegetables can absorb and accumulate antibiotics from the soil. This study made a comprehensive detection and analysis of the pollution status of antibiotics in the soil of Yangling District and its surrounding vegetable base, which can provide a scientific basis for the safe production of vegetables and ensuring the quality and safety of vegetables in this area.
2 Materials and methods
2.1 Instruments and reagents
AB4500 Triple Quadrupole LC/MS (AB Sciex Company, USA); OSJ-UP-V ultra-pure water system (Shandong Boke Scientific Instrument Co., Ltd.); RE-52C rotary evaporator (Zhengzhou Yarong Instrument Co., Ltd.); water-bathing constant temperature vibrator (Shanghai Qiqian Electronic Technology Co., Ltd.); MD-200 nitrogen blower (Hangzhou Aosheng Instrument Co., Ltd.); Mixplus vortex mixer (Hefei Ebenson Scientific Instrument Co., Ltd.); KQ-AS1000GVDE ultrasonic cleaning machine (Kunshan Ultrasonic Instrument Co., Ltd.); DZF-6090 vacuum drying oven (Jinan Olabo Scientific Instrument Co., Ltd.); TG1650-WS desktop high-speed centrifuge (Shanghai Luxiangyi Centrifuge Instrument Co., Ltd.); AR2140 electronic balance (Ohaus, USA); SPE-12 solid phase extraction device (Tianjin Evio Science and Technology Development Co., Ltd.).The standard solution of 19 antibiotics (Table 1) (100 μg/mL, purchased from Beijing Tan-Mo Technology Co., Ltd.); methanol and acetonitrile are of chromatographic purity; 85% phosphoric acid, 36% glacial acetic acid, citric acid and n-hexane, hydrochloric acid, anhydrous sodium sulfate, Disodium hydrogen phosphate dodecahydrate, ammonium acetate, Ethylenediaminetetraacetic acid disodium salt are all analytically pure, and the water used in the experiment is ultra-pure water.
2.2 Sample collection and pretreatment
Yangling is located in the middle of Guanzhong Plain, which belongs to Xianyang City, Shaanxi Province, Shaanxi Province, It is a national agricultural high-tech industry demonstration zone. From October 2018 to September 2019, 20 vegetable production bases were selected from Yangling Demonstration Zone and the surrounding Jiangzhang Town in Fufeng County, Yabai Town in Zhouzhi County, Wugong Town in Wugong County, and soil samples were collected according to theTechnical
Specification
for
Soil
Environment
Monitoring
(HJ/T 166-2004). The 5-point sampling method was used in each sampling base, and the surface soil samples of 0-20 cm were mixed. The soil samples to be tested were laid flat on an air-drying plate, the impurities in the soil were removed and dried naturally in a ventilated and cool place. After grinding, they were sifted through a 60-mesh sieve and stored in a refrigerator to avoid light.2.3 Pretreatment of soil samples
According to the method reported in the reference, different kinds of antibiotics were pretreated as follows.2.3.1
Pretreatment of quinolones antibiotics. 1.00 g of soil samples were weighed accurately, placed in a 10 mL centrifuge tube, 5 mL of 50% magnesium nitrate-10% ammonia water (96/4,v/v
) was added, shaken for 5 min, treated with ultrasonic extraction for 15 min, centrifuged at 4 500 r/min for 10 min, and the supernatant was collected. The residue was extracted twice by the above method, combined with the supernatant, and then extracted and enriched by HLB solid phase extraction cartridge (passing through 6 mL of methanol and 6 mL of water respectively). The cartridge was cleaned with 6 mL of high purity water, vacuum-dried for 10 min, and then eluted with 3 mL of 1% acetic acid-acetonitrile. The eluent was blown to near dry with nitrogen at 40 ℃. The volume of the eluent was fixed to 1 mL with acetonitrile-water (20/80,v/v
). The solution passed through the 0.22 μm filter membrane and collected in the sample bottle to be tested.2.3.2
Pretreatment of tetracycline antibiotics. About 1.00 g of soil samples were accurately weighed and placed in a 10 mL centrifuge tube, 5 mL of NaEDTA-Mcllvaine buffer/methanol (1/1,v/v
) was added, shaken for 5 min, treated with ultrasonic extraction for 15 min, centrifuged at 4 500 r/min for 10 min, and the supernatant was collected. The residue was extracted twice according to the above method, and the supernatant was combined. It was evaporated to constant volume, and then extracted and enriched by HLB solid phase extraction cartridge (passing through 6 mL of methanol and 6 mL of NaEDTA-Mcllvaine buffer solution respectively). The cartridge was cleaned with 6 mL high purity water, dried in vacuum for 10 min, and then eluted with 3 mL of methanol. The eluent was blown to near dry with nitrogen at 40 ℃. The volume of the eluent was fixed to 1 mL with methanol-water (3/2,v/v
). The solution passed through the 0.22 μm filter membrane and was collected in the sample bottle to be tested.2.3.3
Pretreatment of sulfonamides and macrolides. About 1.00 g of soil samples were weighed accurately and placed in a 10 mL centrifuge tube, then 5 mL of citric acid-MgClbuffer solution/ethylonitrile (1/l,v/v
) was added, shaken for 5 min, treated with ultrasonic extraction for 15 min, centrifuged at 4 500 r/min for 10 min, and the supernatant was collected. The residue was extracted twice according to the above method, and the supernatant was combined. It was evaporated to constant volume, and then extracted and enriched by HLB solid phase extraction cartridge (passing through 6 mL of methanol and 6 mL of water respectively). The cartridge was cleaned with 6 mL high purity water, dried in vacuum for 10 min, and then eluted with 3 mL of methanol. The eluent was blown to near dry with nitrogen at 40 ℃. The volume of the eluent was fixed to 1 mL with methanol-water (3/2,v/v
). The solution passed through the 0.22 μm filter membrane and was collected in the sample bottle to be tested.2.4 Detection methods of antibiotics
High performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) was used to detect 19 kinds of antibiotics in soil samples (Table 1).(i) Chromatographic condition parameters. Chromatographic column: Kinetex Bipheny C(50×30 mm, 2.6 μm); the mobile phase A was 0.1% formic acid aqueous solution, and the mobile phase B was 0.1% formic acid acetonitrile solution. Gradient elution procedure (B%): 0-5 min, 20%-90%, 5-7 min, 90%. Flow rate: 0.4 mL/min. Column temperature: 35 ℃. Injection volume: 5 μL.
(ii) Mass spectrometry condition parameters. The relevant mass spectrometry parameters are shown in Table 1.
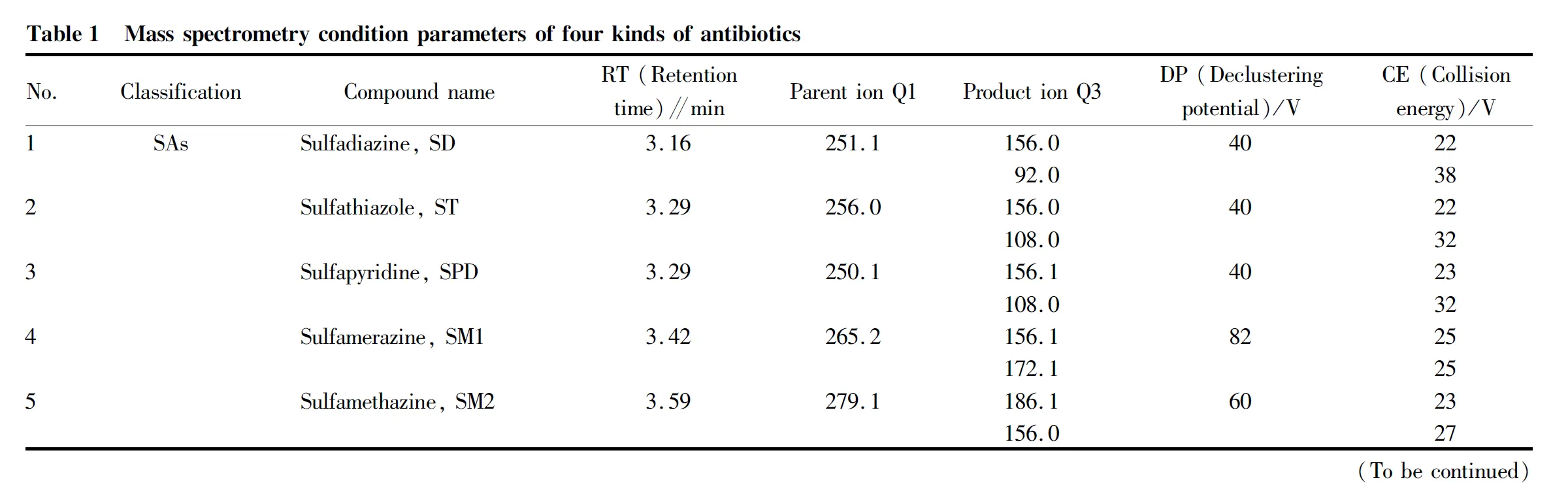
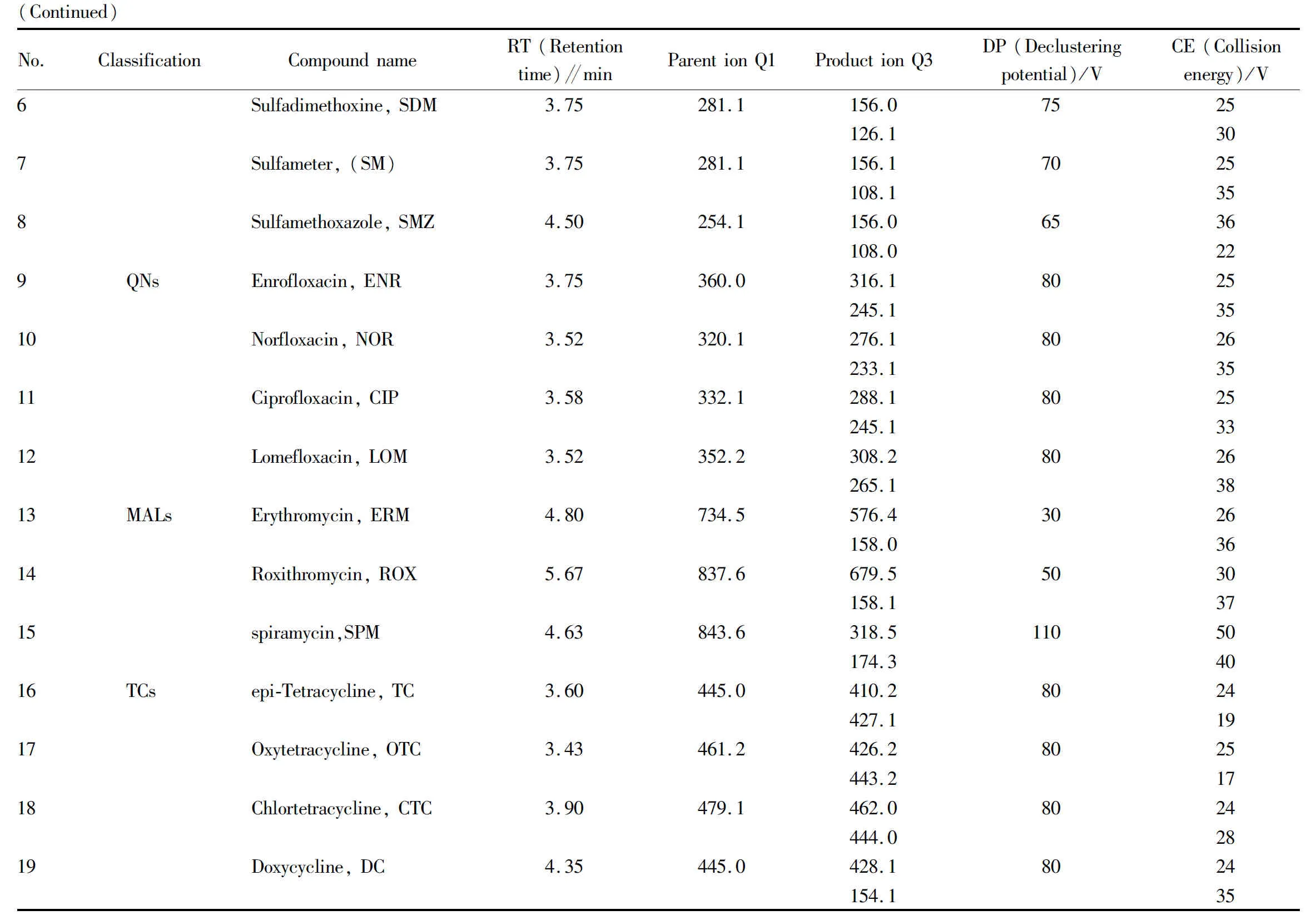
(iii) Ion current diagram. The ion chromatogram analyzed by mass spectrometry is shown in Fig.1.
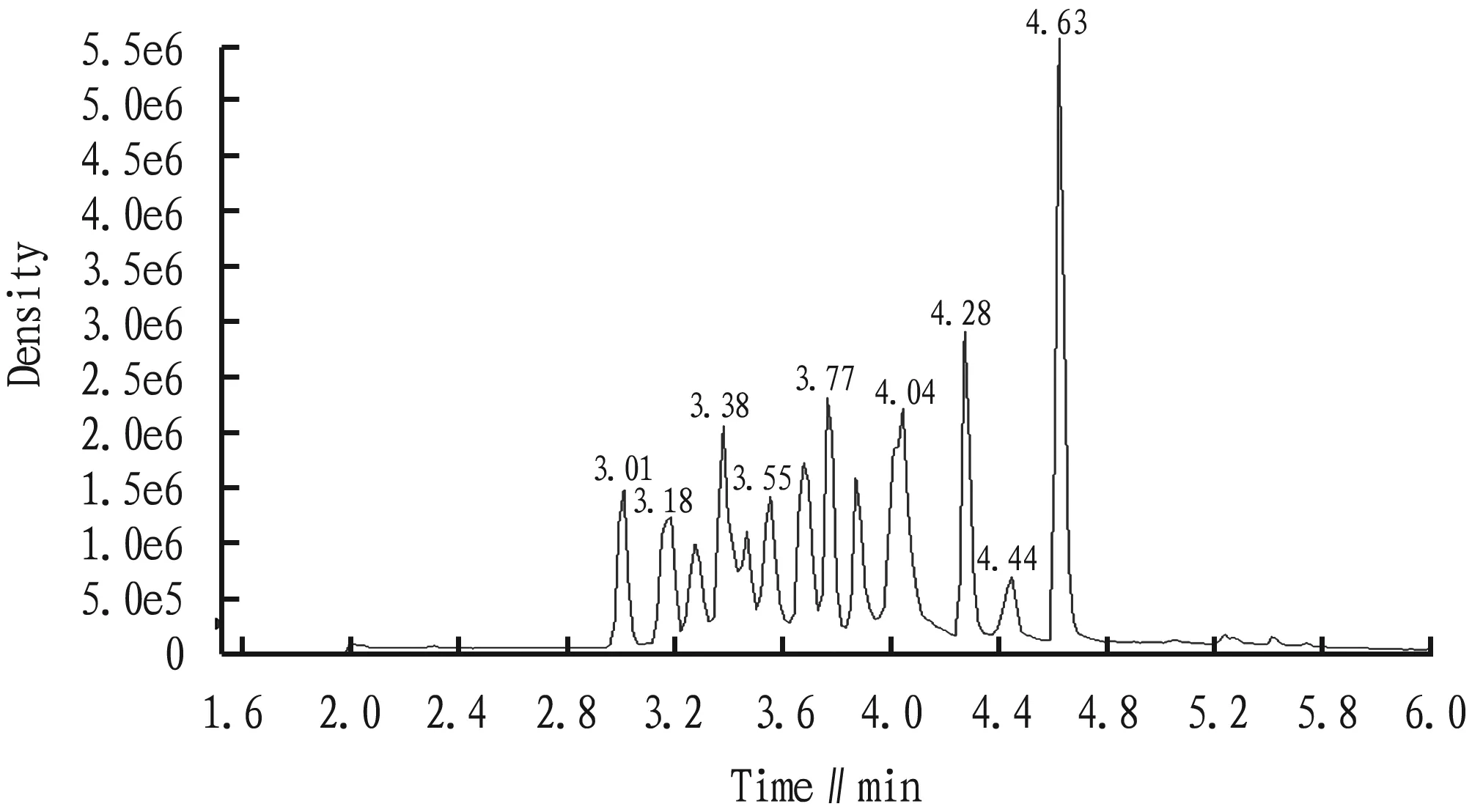
Fig.1 Ion chromatogram analyzed by mass spectrometry analysis
2.5 Data processing
Excel 2013 and SPSS 17.0 were used for data processing and analysis.3 Results and analysis
3.1 Content characteristics of quinolone antibiotics in soil
The content characteristics of quinolone antibiotics in soil samples are shown in Table 2. In 20 soil samples, the total content of four quinolone antibiotics (ΣQNs) ranged from 14.64 to 103.37 μg/kg, with an average of 51.76 μg/kg. The distribution of total content is shown in Fig.2, with 20% below 30 μg/kg, 45% between 30 and 60 μg/kg, 25% between 60 and 90 μg/kg, and 10% above 90 μg/kg. The detection rates of norfloxacin, ciprofloxacin and enrofloxacin were all 100%, and the detection rate of lomefloxacin was 85%, and a small quantity of them (15.4%) were lower than the limit of quantitation. The average content of the four compounds from high to low is norfloxacin > ciprofloxacin > enrofloxacin > lomefloxacin. The composition of quinolones in soil was mainly dominated by norfloxacin, followed by ciprofloxacin and enrofloxacin, while the content of lomefloxacin was very low. Four kinds of quinolones could be detected in most soil samples at the same time, but the content of these compounds was mainly less than 50 μg/kg. The content of quinolone antibiotics in soil of vegetable base in this study area was lower than that reported by Zou Huiyun(average of 161.07 μg/kg), and similar to that of Tai Yiping, Zhao Huinanand Duan Xiazhen.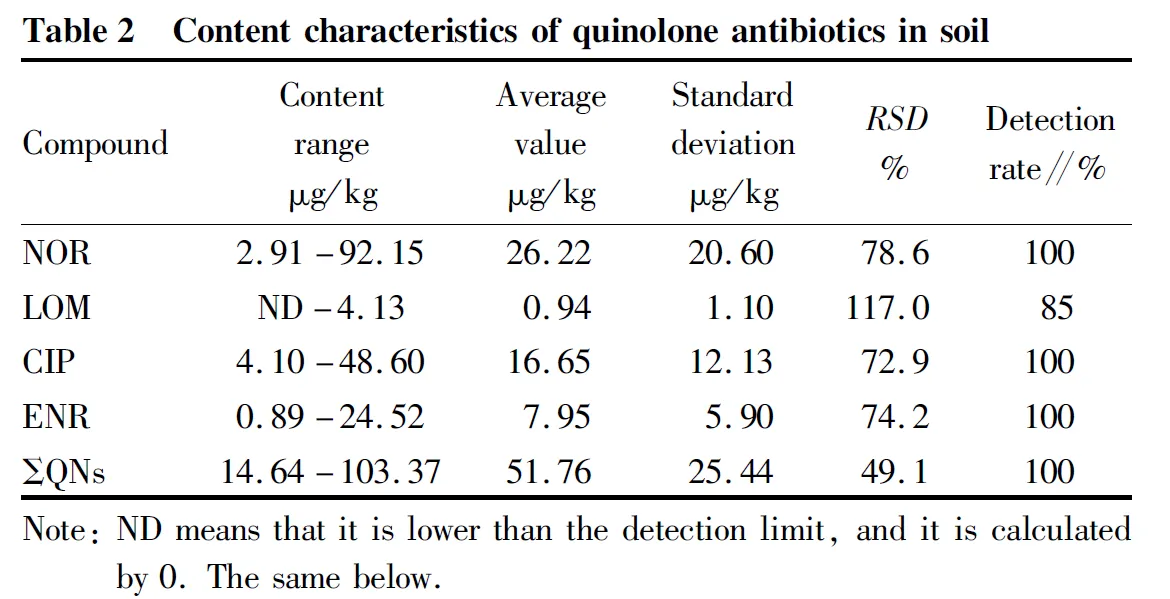
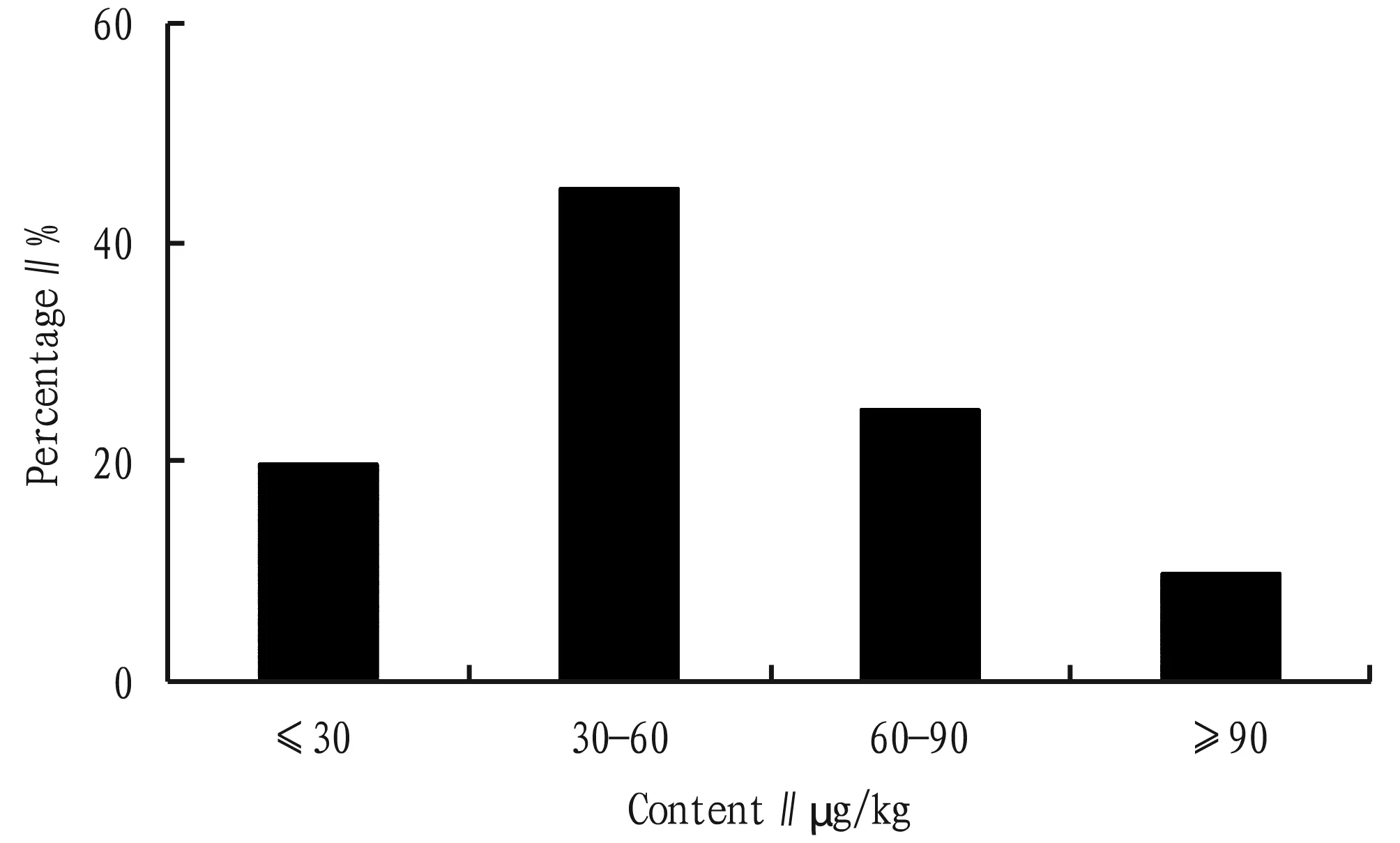
Fig.2 Range distribution of total content of quinolone antibiotics in soil
3.2 Content characteristics of tetracycline antibiotics in soil
Table 3 showed that the total content of tetracycline antibiotics (ΣTCs) ranged from 2.41 to 36.96 μg/kg, with an average of 12.77 μg/kg, all less than 50 μg/kg. The total content distribution is shown in Fig.3, with 40% below 10 μg/kg, 55% between 10 and 30 μg/kg and 5% between 30 and 50 μg/kg. The detection rates of the four tetracyclines were all higher than 80%, and the average content was 6.62, 1.15, 3.91 and 1.09 μg/kg, respectively. The tetracycline compounds in soil were mainly composed of oxytetracycline, followed by chlortetracycline, while the content of tetracycline and doxycycline was low. Four tetracycline compounds could be detected in most soil samples at the same time, but the content of these compounds was mainly less than 10 μg/kg. The content of tetracycline compounds in the soil of vegetable base in this study area was lower than that reported by Zhang Lanhe, Tai Yiping, Li Yanwen, Lang Langet
al.
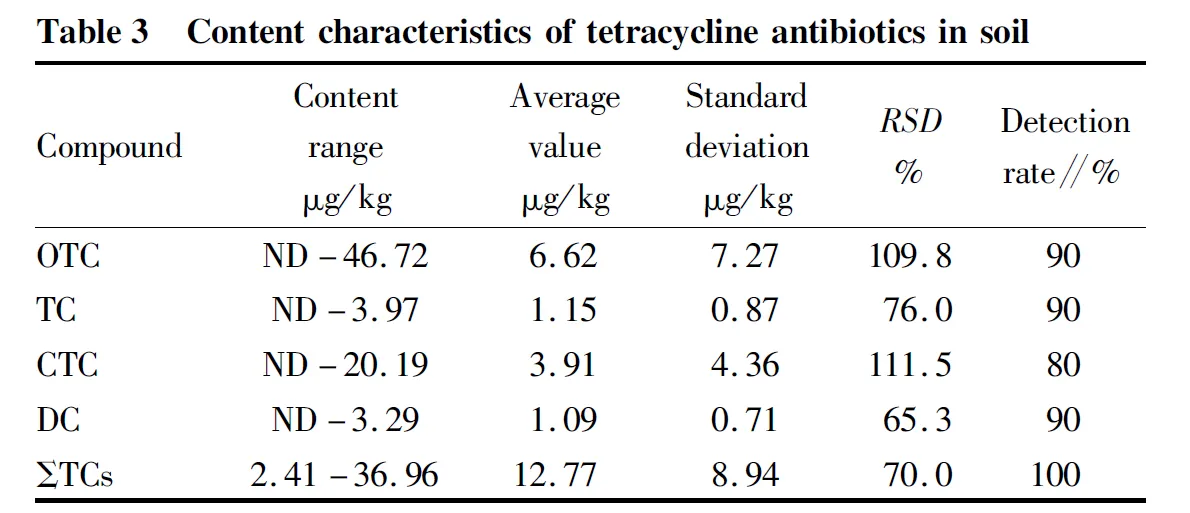

Fig.3 Range distribution of total content of tetracycline antibiotics in soil
3.3 Content characteristics of sulfonamides in soil
The content characteristics of sulfonamides in soil samples is shown in Table 4. The total content of 8 sulfonamides (ΣSAs) ranged from 0.10 to 2.94 μg/kg, with an average of 1.14 μg/kg. Only sulfamethazine (SM2) was detected, and the highest content was 2.61 μg/kg. The content of other seven sulfonamides was all less than 1 μg/kg. The average content of single compound is in the order of sulfamethazine (SM2) > sulfamethoxazole (SMZ) > sulfameter (SM) > sulfadiazine (SD) > sulfapyridinee (SPD) > sulfadimethoxine (SDM) > sulfamethyldiazine (SMl) > sulphathiazole (ST). Therefore, SM2 (average of 0.69 μg/kg) is the main sulfa antibiotic in soil. Cheng Yutinget
al.
reported that the total content of sulfonamides in the soil of typical organic vegetable bases in Guangzhou ranged from 0.73 to 973 μg/kg, with an average of 116 μg/kg. Zhang Lanheet
al.
reported that the content of sulfonamides in greenhouse soil of vegetable field ranged from 4.13 to 34.70 μg/kg, with an average content of 13.41 μg/kg, which was higher than that in the soil samples of vegetable base in this study area, indicating that the pollution level in this study area was low.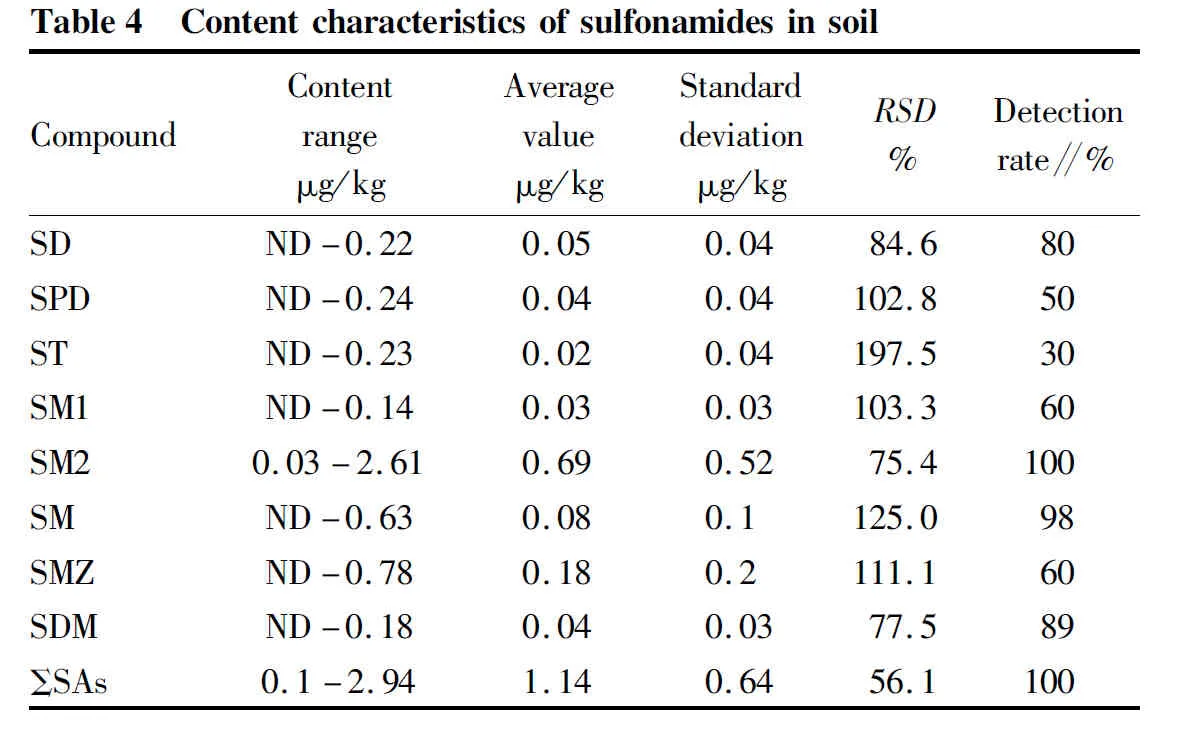
3.4 Content characteristics of macrolide antibiotics in soil
The content characteristics of macrolide antibiotics in soil samples are shown in Table 5.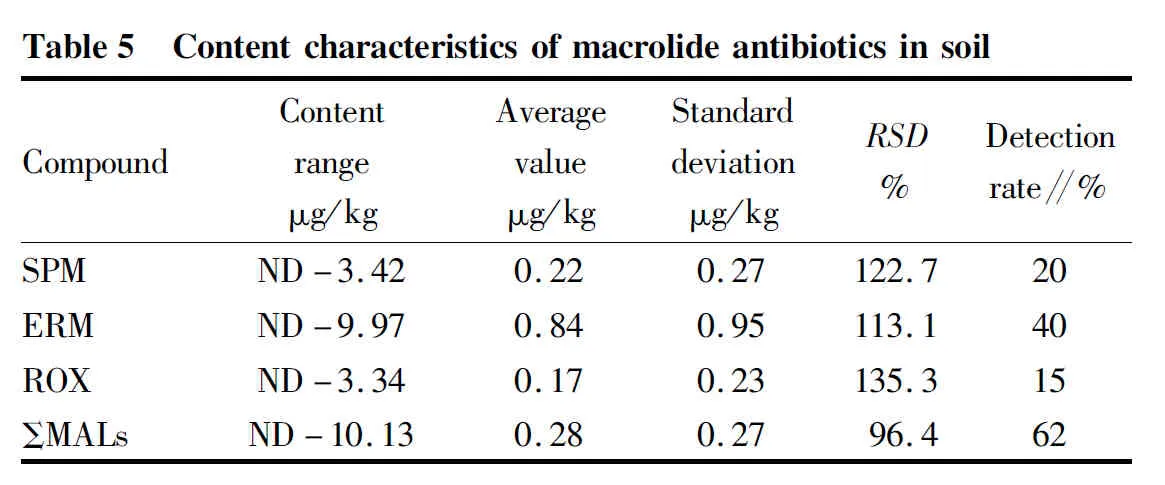
The total content ranged from 0 (below the detection limit) to 10.13 μg/kg, and the average content was 1.27 μg/kg. The detection rates of spiramycin, erythromycin and roxithromycin were 20%, 40% and 15%, respectively. The average content of the three compounds from high to low was erythromycin > spiramycin > roxithromycin.
4 Conclusion
The content of quinolones, tetracyclines, sulfonamides and macrolides in soil samples from 20 vegetable production bases in Yangling District was detected and analyzed. The results showed that quinolones, sulfonamides and tetracyclines were 100% detected in the soil of the vegetable base in the study area, and the detection rate of macrolide antibiotics was 62%. The average content of four kinds of antibiotics from high to low was quinolones (51.76 μg/kg) > tetracyclines (12.77 μg/kg) > sulfonamides (1.14 μg/kg) > macrolides (0.28 μg/kg). Norfloxacin, oxytetracycline, SM2 and erythromycin were the main antibiotics, respectively. At present, there is no limit requirement for antibiotics in soil in China. According to the trigger value of ecotoxic effect of antibiotics in soil (100 μg/kg) put forward by the Veterinary International Conference on Harmonization (VICH), the content of four kinds of antibiotics in soil is less than this trigger value, and the ecological risk is small. Compared with the research reports in other parts of China, the pollution degree of antibiotics in the vegetable base in this study area is also at a relatively low level.
Four kinds of typical antibiotics were detected in the soil of the vegetable base in the study area, indicating that the soil was polluted by antibiotics to a certain extent. As the accumulation of antibiotics in soil will affect the growth and reproduction of normal microflora in soil, and will further migrate to planted crops, it will endanger the safety of ecological environment and food safety. Antibiotics in soil mainly come from sewage irrigation and direct application of livestock and poultry manure. Vegetable production workers should establish awareness of the quality and safety of agricultural products, irrigate without sewage and apply organic fertilizer reasonably to avoid the accumulation of antibiotics in the soil.
杂志排行
Asian Agricultural Research的其它文章
- Niche Characteristics of Herbaceous Plants under Pinus tabuliformis Plantation Forest in Feldspathic Sandstone Area
- Design of Trinity Framework for Cultivated Land Protection
- Current Situations and Recommendations for Development of Agricultural Sci-Tech Park District of Luzhou City
- Study on Virus-free Breeding Technology of Potato Microtuber
- Labor Flow Characteristics of Taian City in the Context of Rural Revitalization
- Villagers’ Satisfaction Evaluation of Rural "Toilet Revolution" in Qinghai Province: A Case Study of Huzhu Tu Autonomous County
