不同基质对闭鞘姜生长发育和光合作用的影响
2021-12-23刘晓荣吴志徐扬韩庆斌王代容
刘晓荣 吴志 徐扬 韩庆斌 王代容



摘 要:采用隨机区组设计,研究不同配比的红壤、泥炭、椰糠和珍珠岩6种基质配方对闭鞘姜生长的影响。测定6种基质的物理和化学性质,观测萌芽率、叶片数、茎粗、株高、株幅、光合日变化、根茎鲜重和根茎干重。结果显示,基质S4(泥炭+椰糠+珍珠岩=1∶2∶2)的植株净光合速率(Pn)显著高于其他基质处理。在6种基质生长的植株叶片净光合速率曲线呈单峰或双峰变化,而蒸腾速率曲线呈单峰变化。最大株高、最大根茎鲜重和根茎干重也出现在基质S4种植的植株。从以上结果可知,基质S4比较适合闭鞘姜的生长和根状茎干物质积累。
关键词:闭鞘姜;盆栽植物;无土栽培基质;泥炭;椰糠;光合特性
中图分类号:S682.19 文献标识码:A
1 Introduction
Costus speciosus is a rhizomatous perennial herb with pinkish white flowers on reddish bracts. It has increased popularity in recent years due to its medicinal and ornamental properties. Its traditional potting substrate is soil, which is heavy and ine¬fficient for transport.
Substrate is a key factor that affects plant growth in soilless cultivation. In addition to supporting and fixing, substrate is important for transferring adequate oxygen, water, and nutrients from the nutrient solution to plant roots. Peat has been widely used in soilless cultivation over the last century due to its excellent physical and chemical properties, especially at the seedling stage[1-3]. Ho¬wever, as a non-renewable resource and increasing price, peat has raised concerns among environmental, scientific, and governmental agencies[4-7], which has resulted in policy changes and governmental regulations of its use in several European countries.
Coir is now widely used in the soilless cultivation across the world as an environmentally friendly substrate which has abundant resources. It is lightweight, good aeration, and a high water-holding capacity that is more than eight times of its own weight[8]. Previous studies found that coconut coir is a good alternative to peat[9-11]. It is also cost efficient for raising plant growth, which has been widely used for growing various fruits, vegetables, and flowers since the beginning of the century[12-14].
Although coir has a high water-holding capa¬city, it has poor aeration. Mixed and combined with other coarser material could make up this shortcoming. Pan et al[15] demonstrated that Oncidium grew best in a substrate combination of crushed stone, bark, coconut shell and charcoal in a 2∶2∶1∶1 ratio. A hanging ornamental plant was proved that soil mixture (1 part cocopeat:1 part topsoil:1 part sand) was significantly better than cocopeat only[16]. Bhardwaj[17] reported that the medium (coil + vermicompost + sand + pond soil) gave maximum seed germination and seedling growth.
Although, the effects of different substrate mixtures on flower growth and development have been previously investigated, there were few reports available on C. speciosus growth. The objective of this study was to assess red soil, peat, coir, and perlite in different combinations on C. speciosus growth and development, to develop a labor-effi¬cient and cost-saving substrate.
2 Materials and Methods
2.1 Plant and growth conditions
Rhizomes annually of C. speciosus were wild germplasm obtained from native. One or two buds were divided and individually grown in plastic pots with a diameter of 10 cm and height of 8.0 cm. The experiment was conducted in the greenhouse in En-vironmental Horticulture Institute, Guangdong Aca-demy of Agricultural Sciences, China (113°15 E, 23°08 N) from April, 2018 to October, 2018. The temperature and relative humidity were recorded by ZDR-20 data loggers (Hangzhou Zeda Instruments Co. Ltd., Hangzhou, China). The minimum and ma¬ximum average temperature was 24.3 ℃ and 33.9 ℃, respectively. Relative humidity was maintained at the range of 70% to 80%.
2.2 Substrate treatment
Six substrates consisting of red soil, peat, coir, and perlite in different proportions were used for the experiment. The red soil was the native field soil. Peat, coir and perlite were purchased from a hor¬ticultural supplier’s corporation (DGSTAR, Guangzhou, China). The mixtures by volume were as follows: S1 (red soil + perlite; 3∶1); S2 (peat + perlite; 3∶1); S3 (coir + perlite; 3∶1); S4 (peat + coir + perlite; 1∶2∶2); S5 (peat + coir + perlite; 2∶1∶2) and S6 (peat + coir + perlite; 2∶2∶1).
Coir was supplied in the form of compressed bricks (30 cm × 30 cm × 12 cm), and peat was sup¬plied as compressed bails (300 L). Both substrates were hydrated according to the manufacturer’s instructions. Initial substrate samples of each treatment were collected. The potential of hydrogen (pH) and electrical conductivity (EC) of extracted substrate solutions were analyzed using the pour thr¬ough method[18]. The bulk density (BD), total po¬rosity, and aeration porosity of the media were measured and analyzed[19].
2.3 Experimental design
Plants were arranged in a randomized complete block design, and each treatment replicated three times, and in each replicate consisted of 10 plants. Plants were fertilized using a 20 N-20 P-20 K com¬mercial water-soluble fertilizer (COMPO Expert GmbH, Munster, Germany) and irrigated two or three days with tap water. The EC and the pH value of water are 0.23 mS·cm–1 and 7.4 respectively.
2.4 Data collection
Data regarding all growth indices were collected in late June before flowering time, including the plant height, plant width, number of leaves, leaf length, and leaf width of the third mature leaf from the top of the plant. Rhizome fresh weight (RFW) and rhizome dry weight (RDW) were measured in October. Leaf gas exchange was measured using a portable photosynthesis measuring system (LI-6400; LICOR, Lincoln, NE, USA). Stomatal conductance, intercellular carbon dioxide (CO2), net photosynthetic rate (Pn), and transpiration rate (Tr) were recorded. Water use efficiency (WUE) was calculated using the following equation: WUE = Pn/Tr.
Diurnal photosynthetic variations were deter-mined from 8∶30 to 16∶30 in three sunny days using five plants per treatment, and from the top the third leaf per plant was selected. Leaf length, leaf width, chlorophyll content was determined using the same leaves as those used for other growth parameters above. 3 SPAD readings (Minolta Camera Co., Osaka, Japan) were taken on each leaf (inter area).
2.5 Statistical analysis
The data were analyzed using statistical soft¬ware (SAS version 8.1; SAS Institute, Cary, NC). It was used one-way PROC ANOVA to evaluate variance of substrate pH, EC, density, total porosity, aeration porosity, hold-water porosity and gas-water porosity ratio, number of leaves, stem base diameter, plant height, leaf length and width, RFW and RDW, stomatal conductance, intercellular CO2, Pn, Tr and WUE and leaf SPAD. Mean separation used least significant difference (LSD) at P = 0.01 or 0.05.
3 Results
3.1 Substrate physical and chemical proper-ties
The physical characteristics of the six sub-strates were provided in Tab. 1. S1 and S2 had the lower pH values (5.58 and 4.84, respectively) sig-nificantly different from each other. No significant differences were detected among S3, S4, S5, and S6 with regard to pH values. S1 had the lowest EC value, although there was no significant difference between S1 and S3. There was the highest bulk density (0.972 g·L–1) And lowest water holding capacity (54.82%) in S1. S4 had the greatest total porosity (84.67%) and water holding porosity (76.88%), but had a lower bulk density. No significant differences were detected in the aeration porosity or gas- water porosity ratios among the six media treatments.
3.2 Effects of different substrates on vegeta-tive parameters
The six substrates did not significantly affect the sprouting rate or leaf length (Tab. 2). The greatest number of leaves was observed in S4 (25.3). Although the greatest stem base diameter was observed in S6, no significant differences were detected among S3, S4, and S6. Plant height was greater in S3 and S4 than in S5. The smallest leaf width was observed in S1 (4.47 cm), but no significant differences were detected among the other five substrates. TheRFW (231.85 g) and RDW (44.80 g) of S4 were greater than those of S1, S2. The lowest RFW (146.03 g) and RDW (20.81 g) were observed in S1.
3.3 Effects of different substrates on photo-synthetic physiological characteristics
No significant differences were detected in sto-matal conductance and Tr among the six substrates (Tab. 3), but intercellular CO2 concentration, Pn, and WUE were significant. The intercellular CO2 concentration of S1 was greater than S2, and S6. The Pn of S4 was significantly greater than S1, S2, S5, and S6. The WUE of S3 was greater than S1, S5 and S6.
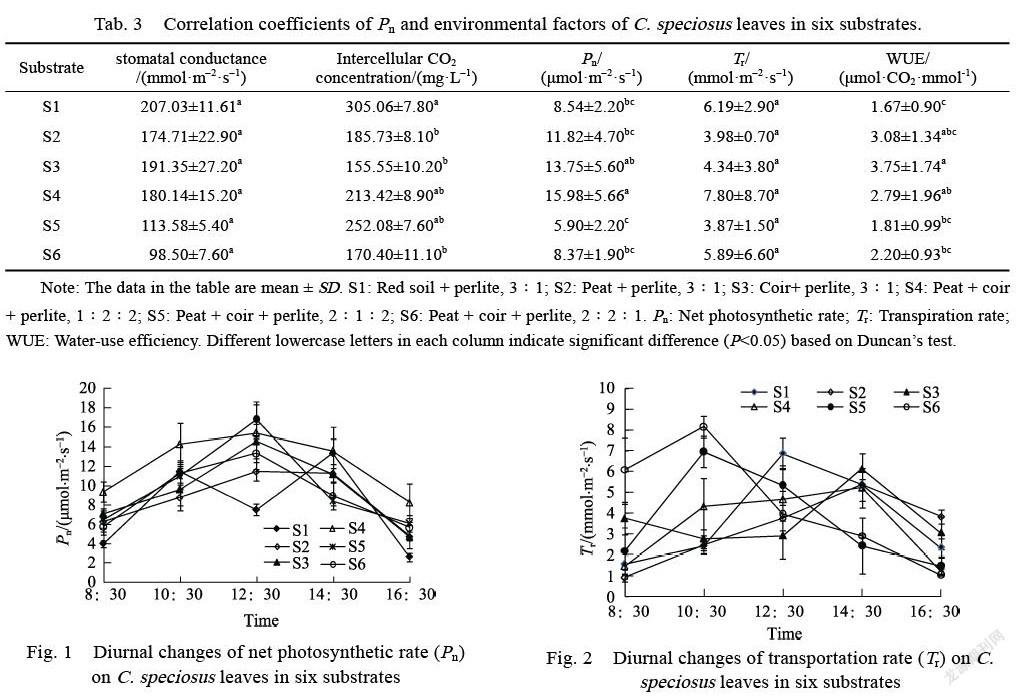
3.4 Diurnal changes of leaf photosynthetic parameters
The diurnal variation curve of leaf Pn in S1 dis-played two single peaks (Fig. 1). The first peak was appeared at 10:30 (11.48 μmol·m–2s–1), and the second peak was at 14:30 (13.35 μmol·m–2s–1). The diurnal variation curves of leaf Pn in the other five substrates were similar and displayed one peak at 12:30. The average diurnal Pn of the six substrates were 7.77, 8.50, 9.43, 12.16, 9.71 and 9.00 μmol·m–2s–1, respectively.
The diurnal variation of Tr of all six substrates displayed one peak (Fig. 2), but the times were dif-ferent. The peak in S1 appeared at 12:30, while the peaks in S2, S3, and S4 reached their maximum at 14:30. In S5 and S6, the peak appeared at 10:30. The maximum leaf Tr was observed in S6 (8.17 mmolm–2s–1), while the minimum was observed in S4 (5.22 mmolm-2s-1). The average diurnal Tr of the six substrates were 3.53, 3.28, 3.72, 2.99, 3.50, and 3.70 mmol·m–2s–1, respectively.
The diurnal variation of WUE in S1, S3, and S6 exhibited a linear rise-fall pattern (Fig. 3). The peaks of S1 and S3 appeared at 10:30 (4.70 μmol CO2mmol–1 H2O) and 12:30 (5.0 μmol CO2mmol–1 H2O) respectively. From 8:30 to 12:30, S6 rose in a straight line, slowly decreased at 14:30, and subsequently rose to 5.22 μmol CO2mmol–1 H2O. In contrast to S1, S2, and S6, the diurnal variation of WUE in S2, S4, and S5 exhibited a linear fall-rise pattern. The S2 exhibited a linear downward trend from 8:30 to 16:30. From 8:30 to 14:30, S4 exhibited a downward trend and rose after 14:30 to 6.52 μmol CO2·mmol–1 H2O. The S5 reached its minimum level (1.57 μmol CO2·mmol–1 H2O) at 10:30, and subsequently increased.
3.5 Effects of different substrates on foliar SPAD readings
The SPAD readings of S1 were the highest (44.1), while S3 was the lowest (34.7) (Fig. 4). The order of SPAD readings among the six substrates was as follows: S1>S2>S4>S6>S5>S3. No significant differences were detected among S1, S2, and S4. The SPAD readings of S1 was significantly higher than S3 (P<0.05), which was about 1.27 times.
4 Discussion
Soil and peat were the most commonly used growing substrates in the container production of annual and perennial ornamental plants[20]. However the density of soil was heavy, difficult to move, and contains many potentially harmful micro-organisms. Peat was uneconomical or unrecyclable, making growers look for alternatives.
In this study, the greatest of S4 over the other combinations probably related to its characteristics including higher total porosity and hold-water porosity. The number of leaves, RFW, and RDW of S4 significantly increased compared with other five substrates. Although sprouting rate and leaf lengths were not significantly different.
The total porosity and maximum water holding capacity are important factors for plant growth. However, porosity and bulk density are interacted each other. Bulk porosity is low and the air content is reduced. The air porosity of the substrate is large; therefore it is more suitable for plant growth. Middle density was more suitable at the seedling stage; similar findings were also reported by Chen[21].
The results revealed that the stem base diameter, RFW and RDW were lower in S1 the soil-based substrate potentially due to its large bulk density (0.972 g·L–1), matching the findings that the density range of substrate was 0.19~0.70 g·L–1 for most potting commercial horticultural crops[22].
Different substrates affected Pn, Tr and pore conductance of two gerbera[23]. This study revealed that the Pn differed among the six substrates and the greatest value observed in S4. Intercellular CO2 and WUE also differed among the six substrates, in the following orders S1>S5>S4>S2>S6>S3 and S3> S2>S4>S6>S5>S1, respectively. However, like Pn, the diurnal variation curves of leaf photosynthesis were similar and exhibited one peak, except S1. Interestingly, the Tr of the six substrates displayed single peak, but the times were different.
The maximum value of Pn was in S4, which promoted plant leaf growth and increased rhizome accumulation. The results confirmed previously reported findings, in which Pn directly reflected plant light energy and the ability to accumulate photosynthetic products[24].
SPAD-502 meter has been provided a rapid and nondestructive measurement of leaf chlorophyll content. Several studies demonstrated that SPAD readings were significantly related to extracted chlorophyll[25-28]. In the study, the greenest leaves were observed in S1. Although no significant differences were detected between S1 and S4, the degree of leaf greenness reflected plant growth and physiological health. In future studies if combined with fertilizer management, the leaf chlorophyll content would be improved. Therefore, S4 would be an excellent substrate for C. speciosus growth and development.
References
[1] Kaveriappa K M, Phillips L M, Trigiano R N. Micropropa-gation of flowering dogwood (Cornus florida) from seedl-ings[J]. Plant Cell Reports, 1997, 16(7): 485-489.
[2] Worrall R J. Comparison of composted hardwood and peat-based media for the production of seedlings, foliage and flowering plants[J]. Scientia Horticulturae, 1981, 15(4): 311-319.
[3] 欒亚宁, 孙向阳, 刘克林, 等. 几种泥炭基质物理性质比较研究[J]. 中国农学通报, 2008, 24(9): 137-140.
Luan Y N, Sun X Y, Liu K L, et al. Comparisons of physical properties of several peats as growing mediums[J]. Chinese Agricultural Science Bulletin, 2008, 24(9): 137-140.
[4] Barkham J P. For peat’s sake: Conservation or exploita-tion?[J]. Biodiversity and Conservation, 1993, 2(5): 556-566.
[5] Carlile W R. Growing media and the environmental lobby in the UK. 1997-2001[J]. Acta Horticulturae, 2004, 644: 107-113.
[6] Defra SP08019: Availability and supply of alternative mate-rials for use in growing media to meet the UKBAP target on reduced peat use in horticulture[Z]. 2009.
[7] Gruda N. Current and future perspective of growing media in Europe[J]. Acta Horticulturae, 2012, 960: 37-43.
[8] 蔡东宏, 韦开蕾. 我国椰子业现状发展前景和对策[J]. 世界热带农业信息, 1999(4): 8-10.
Cai D H, Wei K L. Prospects and countermeasures of coco-nut industry in China[J]. World Tropical Agriculture Infor-mation, 1999(4): 8-10.
[9] Alexander P D, Bragg N C, Meade R, et al. Peat in horticul-ture and conservation: the UK response to a changing world[J]. Mires and Peat, 2008, 3(8): 1-10.
[10] Evans M R, Stamps R H. Growth of bedding plants in sphagnum peat and coir dust-based substrates[J]. Journal of Environmental Horticulture, 1996, 14(4): 187-190.
[11] Meerow A W. Coir dust, a viable alternative to peat moss[J]. Greenhouse Product News, 1997, 1: 17-21.
[12] Xiong J, Tian Y Q, Wang J G, et al. Comparison of coconut coir, rockwool, and peat cultivations for tomato production: nutrient balance, plant growth and fruit quality[J]. Frontiers in Plant Science, 2017, 8(2): 1327.
[13] Khayyat M, Nazari F, Salehi H. Effects of different pot mixtures on pothos (Epipremnum aureum Lindl. and Andre ‘Golden Pothos’) growth and development[J]. Ameri-can-Eurasian Journal of Agricultural and Environmental Science, 2007, 57(4): 492-493.
[14] Usman M, Shah M, Badar A, et al. Media steaming and coco-coir enhance growth of rough lemon (Citrus Jambhiri L.) stock[J]. Pakistan Journal of Agricultural Sciences, 2014, 51(3): 617-625.
[15] 潘英文, 林明光, 陈施明. 文心兰切花产业化栽培基质的筛选研究[J]. 热带农业科学, 2009, 29(7): 32-35.
Pan Y W, Lin M G, Chen S M. Screening of substrates for commercial culture of cut-flower Oncidium orchid[J]. Chi-nese Journal of Tropical Agriculture, 2009, 29(7): 32-35
[16] Khelikuzzaman M H. Effect of different potting media on growth of a hanging ornamental plant (Tradescantiasp)[J]. Journal of Tropical Agriculture and Food Science, 2007, 35(1): 41–48
[17] Bhardwaj R L. Effect of growing media on seed germination and seedling growth of papaya cv. ‘Red lady’[J]. African Journal of Plant Science, 2014, 8(4): 178-184.
[18] Wright R D. The pour-through nutrient extraction proce-dure[J]. Hortscience, 1986, 21: 227-229.
[19] Niedziela C E, Nelson P V. A rapid method for determining physical properties of undisturbed substrate[J]. Hortscience, 1992, 27(12): 1279-1280.
[20] Baiyeri K P, Mbah B N. Effects of soilless and soil-based nursery media on seedling emergence, growth and response to water stress of African breadfruit (Treculia africana Decne)[J]. African Journal of Biotechnology, 2006, 5(15): 1405-1410.
[21] 陈凤真. 不同基质对小青菜穴盘苗生长和光合特性的影响[J]. 中国土壤与肥料, 2014(1): 75-78, 100.
Chen F Z. Effects on growth and photosynthetic characteristics of greengrocery (Brassica chinensis L.) in plugs under different substrate[J]. Journal of Soil and fertilizer sciences in china, 2014(1): 75-78, 100.
[22] Bilderback T E, Warren S L, Owen Jr J S, et al. Healthy substrates need physicals too[J]. Hort Technology, 2005, 15: 747-751.
[23] Issa M, Ouzounidou G, Maloupa H, et al. Seasonal and diurnal photosynthetic responses of two gerbera cultivars to different substrates and heating systems[J]. Scientia Horticulturae, 2001, 88(3): 215-234.
[24] Lincoln Taiz, Eduardo Zeiger. Plant physiology-fifth edi-tion[M]. Sinauer Associates, Inc., Publishers, 2015: 92-212.
[25] Azia F, Stewart K A. Relationships between extractable chlorophyll and spad values in muskmelon leave[J]. Journal of Plant Nutrition, 2001, 24(6): 961-966.
[26] Ruiz-Espinoza F, Fenech-Larios L, Beltran-Morales A, et al. Field evaluation of the relationship between chlorophyll content in basil leaves and a portable chlorophyll meter (spad-502) readings[J]. Journal of Plant Nutrition, 2010, 33(3): 423-438.
[27] Wang Q B, Chen J J, Stamps R H, et al. Correlation of visual quality grading and SPAD reading of green-leaved foliage plants[J]. Journal of Plant Nutrition, 2005, 28(7): 1215-1225.
[28] Yamamoto A, Nakamura T, Adu-Gyamfi J J, et al. Rela-tionship between chlorophyll content in leaves of sorghum and pigeonpea determined by extraction method and by chlorophyll meter (SPAD-502)[J]. Journal of Plant Nutri-tion, 2002, 25(10): 2295-2301.
責任编辑:谢龙莲
