Metabolic responses to combined water deficit and salt stress in maize primary roots
2021-12-14LlPengchengYANGXiaoyiWANGHoumiaoPANTingYANGJiyuanWANGYunyunXUYangYANGZefengXUChenwu
Ll Peng-cheng ,YANG Xiao-yi,WANG Hou-miao ,PAN TingYANG Ji-yuanWANG Yun-yunXU Yang ,YANG Ze-feng , ,XU Chen-wu ,
1 Jiangsu Key Laboratory of Crop Genetics and Physiology/Key Laboratory of Plant Functional Genomics,Ministry of Education/Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding/Agricultural College,Yangzhou University,Yangzhou 225009,P.R.China
2 Jiangsu Co-Innovation Center for Modern Production Technology of Grain Crops,Yangzhou University,Yangzhou 225009,P.R.China
3 Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education/Yangzhou University,Yangzhou 225009,P.R.China
Abstract Soil water deficit and salt stress are major limiting factors of plant growth and agricultural productivity. The primary root is the first organ to perceive the stress signals for drought and salt stress. In this study,maize plant subjected to drought,salt and combined stresses displayed a significantly reduced primary root length relative to the control plants. GC-MS was used to determine changes in the metabolites of the primary root of maize in response to salt,drought and combined stresses. A total of 86 metabolites were measured,including 29 amino acids and amines,21 organic acids,four fatty acids,six phosphoric acids,10 sugars,10 polyols,and six others. Among these,53 metabolites with a significant change under different stresses were identified in the primary root,and the content of most metabolites showed down-accumulation. A total of four and 18 metabolites showed significant up-and down-accumulation to all three treatments,respectively. The levels of several compatible solutes,including sugars and polyols,were increased to help maintain the osmotic balance.The levels of metabolites involved in the TCA cycle,including citric acid,ketoglutaric acid,fumaric acid,and malic acid,were reduced in the primary root. The contents of metabolites in the shikimate pathway,such as quinic acid and shikimic acid,were significantly decreased. This study reveals the complex metabolic responses of the primary root to combined drought and salt stresses and extends our understanding of the mechanisms involved in root responses to abiotic tolerance in maize.
Keywords:maize,primary root,combination stress,drought,high salt stress,metabolomics
1.lntroduction
Abiotic stresses,including drought and high salt stress,are some of the main factors limiting crop productivity and yield in agriculture,which represent a grave threat to global food demand. It was estimated that drought alone affects 45% of the world’s agricultural land,whilst a further 20%of irrigated land is salt-affected (Abdelraheemet al.2019).A combination of two or more abiotic stresses results in more yield loss than a single stress. More than 50% of the arable land lost worldwide was caused by the combination of drought and salinization (Abdelraheemet al.2019).Hence,understanding the tolerant mechanisms to these combined stresses is imperative for the improvement of salt and drought tolerance in crops.
The adverse changes caused by drought and salt stresses in plant growth are orchestrated at the morphological,physiological,gene expression,metabolites,and protein levels (Huanget al.2012). Drought and salt stresses reduce the water uptake ability of plants,causing osmotic and oxidative stresses.Additionally,salt stress causes ionic stress and Na+toxicity (Ruan and Silva 2011). In response to these stress conditions,plants deploy a number of adaptive strategies,including the accumulation of compatible solutes or osmoprotectants and alterations in the direction of growth to escape from salt and drought (Ruan and Silva 2011; Pierik and Testerink 2014). Roots are the major organ that anchor the plant in the soil and are critical for the uptake of nutrients and water. Root development is highly plastic and flexible,acting as a sensory system to a changing environment to adjust the root morphology to reduce exposure to stress (Pierik and Testerink 2014).Most studies and breeding selection mainly focus on enhancing traits in the aboveground tissues to withstand these stresses,whereas the roots (the ‘hidden half’ of a plant’s architecture) are still an under-utilized source of crop improvement (Koevoetset al.2016). In maize,the root system comprises primary,seminal,crown,brace,and lateral roots. The primary root (PR) is the first organ to appear when a seed germinates,and therefore,the first organ to perceive the stress signals for drought and salt stress. It plays a significant role in stress sensing and signal transduction to the shoot tissue (Julkowskaet al.2017).Primary root elongation can be reduced by salt,drought and combined stresses inArabidopsis,maize and barely(Julkowskaet al.2014; Zhanget al.2015; Osthoffet al.2019). Responses to abiotic stress are controlled by multigene pathways,which are shared across multiple abiotic stresses (Peleget al.2011). Understanding the adaptive responses of the primary root is of fundamental importance to develop more stress-tolerant crops.
Plant responses to salt and drought stresses may involve several metabolic pathways,such as the accumulation of osmoprotectants,ion transport,hormone synthesis,and energy metabolism (Hareet al.1998; Crameret al.2007;Huanget al.2012). Currently,metabolomics has become a powerful tool for analyzing a large number of compounds that could provide a comprehensive understanding of systematic adjustment in metabolic processes (Jorgeet al.2016). Several metabolomic analyses have been conducted to investigate the mechanisms of abiotic stress tolerance and nutrition deficiency. The results suggest that both primary and secondary metabolites play an important role in plant responses to drought and salt stresses.The metabolomics profile of tobacco and soybean roots and leaves facing dehydration stress revealed the high accumulation of 4-hydroxy-2-oxoglutaric acid in tobacco roots and coumestrol in soybean roots (Rabaraet al.2017).Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species revealed that 21 metabolites,including sugars,amino acids,organic acids,and low molecular weight compounds,increased in both the leaves and roots (Ullahet al.2017). Under salt stress,the maintenance of cell division and root elongation in a salttolerant barley cultivar was associated with the synthesis and accumulation of amino acids,sugars and organic acids(Sheldenet al.2016). In rice,metabolite changes,such as allantoin and glutamine,were found to be positively correlated with the growth potential and salt tolerance(Namet al.2015). WhenArabidopsiswas exposed to drought,heat or combined stresses,the accumulation of sucrose,maltose and glucose was significant (Rizhskyet al.2004). Analyses of metabolomic data showed that the differential accumulation of several key metabolites(carbohydrates,amino acids,lipids,cofactors,nucleotides,peptides,and secondary metabolites) occurred in soybean leaves in response to drought and heat stresses (Daset al.2017). These results revealed that metabolic responses are important indicators for evaluating the drought and salt stress tolerance in plant. Even with these findings,studies tend to focus on plant responses to individual stresses,and limited information is available for metabolite profiling to combined salt and drought stresses in the primary root of maize.
In this study,we analyzed the metabolic responses of the primary root to individual and combinatorial stresses,with the aim to explore the early metabolic changes of the primary root exposed to individual and combinatorial stresses. This study will provide new insights into metabolic adaptations to salt and drought stresses in maize.
2.Materials and methods
2.1.Plant materials,growth conditions,treatment,and measurements of the primary root length
For phenotyping and metabolomics experiments,seeds of the maize inbred line B73 were disinfected with 10% H2O2solution for 20 min and rinsed with distilled water. The sterilized seeds were soaked in saturated CaSO4for 6 h to synchronize germination,then germinated in the dark on moist filter paper at 28°C and a relative humidity of 80%.After 2 days,eight germinated seeds were transferred to brown germination paper (Anchor Paper Company,St Paul,MN,USA). On day 3,the distilled water was replaced with Hoagland solution and complemented with either NaCl solution (100 mmol L-1) to induce salt stress,PEG8000 solution (-0.8 MPa) to simulate water deficit,or a combination of both (Opitzet al.2016; Zhanget al.2019).The nutrient solution was renewed every 2 days. Hydroponic experiments were conducted in a growth chamber at a temperature of 28/22°C with a 14/10 h light/dark cycle,relative humidity of 60% and light intensity of 400 μmol m-2s-1. The five primary roots per biological replicate were sampled to measure the root length. For each treatment,two independent biological replicates were analyzed.
2.2.Metabolite extraction
At day 5,the primary root length (PRL) showed significant reduce under all stress conditions,because the metabolites changed earlier than the phenotype (Fiehn 2002),we selected day 4 to sample for metabolic profiling. Thirty primary roots per treatment were sampled per biological replicate after 24 h of drought,salt stress and combined stress on day 4 and four independent biological replicates were analyzed. The sampled plant material was immediately frozen in liquid nitrogen and stored at -80°C. Fresh plant samples (100 mg) were transferred into 5 mL centrifuge tubes,five steel balls were added,then samples were placed into liquid nitrogen for 5 min. Tubes were put through the high flux organization grinding apparatus (70 Hz min-1). After grinding,1 400 μL of pre-cooled methanol was added and vortexed for 30 s,then 60 μL of Ribitol (0.2 mg mL-1) was added as an internal quantitative standard and vortexed for 30 s. Then,the tubes were placed into an ultrasound machine at room temperature (25°C) for 30 min,after which 750 μL of pre-cooled chloroform and 1 400 μL of deionized water (dH2O) were added. The tubes were vortexed for 60 s and centrifuged for 10 min at 14 000 r min-1. Next,1 mL of the supernatant was transferred into a new centrifuge tube,and samples were blow-dried in a vacuum concentrator.Then,60 μL of 15 mg mL-1methoxyamine pyridine solution was added,vortexed for 30 s,and left to react for 120 min at 37°C. Finally,60 μL of BSTFA reagent was added into the mixture,left to react for 90 min at 37°C,and then centrifuged at 1 2000 r min-1(4°C) for 10 min. The supernatant was then transferred into a bottle for GC-MS detection.
2.3.Metabolite analysis by GC-MS
Metabolites were analyzed by GC-MS using a modified method of Lisecet al.(2006). All samples were tested for their metabolite contents using a 7890A GC/5975C MS System (Agilent,USA). The injection,chromatography and mass spectrometer parameters were set accordingly. The peak identification,data baseline filtering and integration were processed using XCMS Software. Metabolite annotations were based on the retention time,massto-charge ratio (m/z) and mass spectral signature of all detectable ions. The samples were identified using the golm metabolome database (GMD) and NIST MS Search Program database. Moreover,the qualitative identification of 43 chemicals were confirmed using standard chemicals.
2.4.Data analysis
The contents of all the metabolites were normalized using SIMCA-P (v13.0). The identified metabolites were compared by principal component analysis (PCA),using the “factoextra” and “FactoMineR” packages in R-3.4.3,and partial least squares-discriminant analysis (PLS-DA)by SIMCA-P. Differentially expressed metabolites were detected by the first principal component of VIP (variable importance in the projection) values (VIP>1) and Student’st-test (P<0.05). The fold-change of metabolites between the treatment and control was transformed by the logarithmic base of 2. Differentially expressed metabolites were displayedviaa heatmap,using the “Pheatmap” packages in R.
3.Results
3.1.Primary root performance of maize under abiotic stress treatments
The PRL per treatment was evaluated every day (Fig.1),commencing on day 4. Only the PRL subjected to the combined stress treatment was significantly shorter than that of the control plants (P<0.05). At day 5,both the PRLs of plants subjected to the salt and combined stress treatments displayed extremely significantly reduced PRLs relative to the control plants (P<0.01),whilst a significant decrease in the plant was observed in drought conditions. By day 6,PRL subjected to water deficit,salt and combined stress treatments displayed significantly reduced PRL relative to the control plants. At day 9,PRLs deceased by 54.3,53.8 and 62.3% in the plants experiencing water deficit,salt stress and a combination of the both,respectively.
3.2.Metabolic changes in the responses to abiotic stress treatments
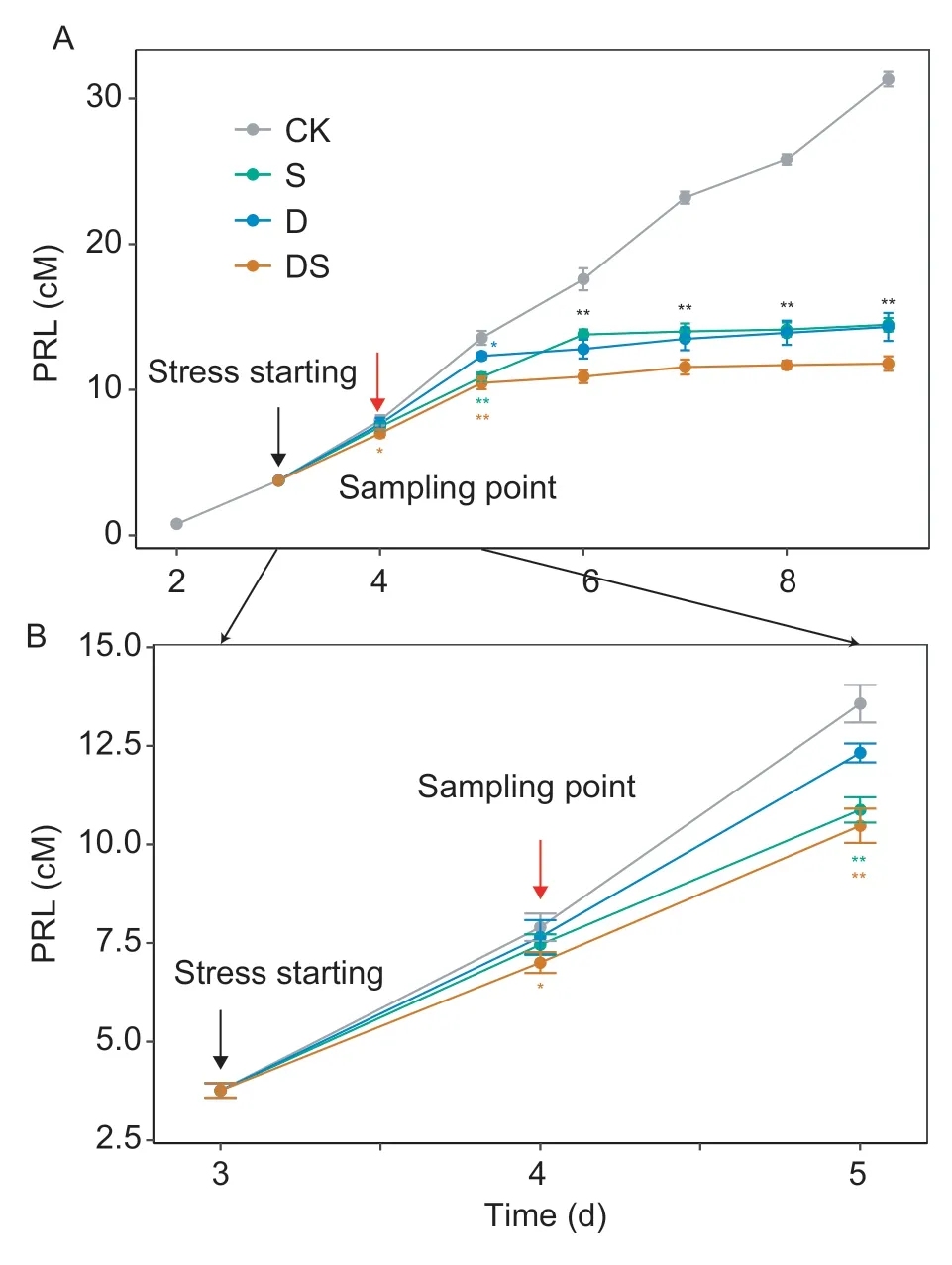
Fig.1 Comparison of the primary root lengths (PRL) between the control,salt,drought,and combined stress treatments.A,the PRL from 2 to 9 days after germination. B,detailed figure between days 3 and 5. At each time point,the significant differences in the means were calculated using the t-test. * and**,significant differences at P<0.05 and P<0.01,respectively.
To survey the metabolic changes in the different treatments of primary roots,seedling primary roots subjected to drought,salt stress and a combination of the both for 24 h were collected for metabolomics analysis by GC-MS. In total,86 metabolites were measured,including 29 amino acids and amines,21 organic acids,four fatty acids,six phosphoric acids,10 sugars,10 polyols,and six others. PCA was conducted on the detected metabolites,and the first two major principal components (PC1 and PC2) could explain 45.7 and 15.2% of the variance,respectively (Fig.2-A).The main metabolites contributing to PC1 were organic acids/polyols,such as benzoic acid,fumaric acid,glucaric acid,1-monooctadecanoylglycerol,and glycerol. PC2 was dominated by amino acids/sugars,mainly including glutamate,lysine,glucose,fructose,and sucrose (Fig.2-B).The PCA analysis showed clear separations between the samples in different stress treatments.
3.3.Metabolic profiles in response to different stresses in the primary root
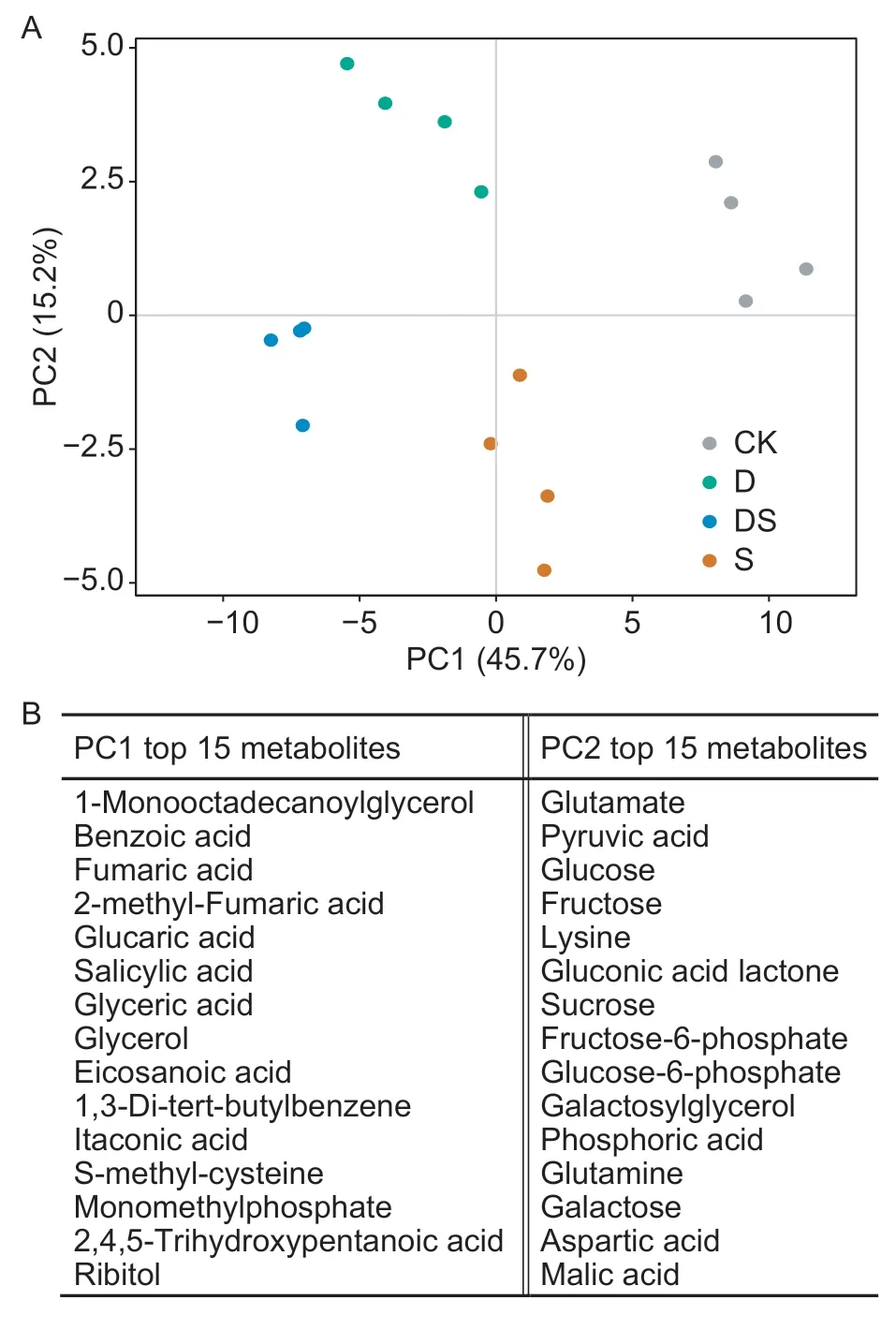
Fig.2 Principal component (PC) analysis of the root metabolome. A,score scatter plots among different samples.CK,the control; D,drought; DS,combined stress; S,salt.B,the top 15 metabolites contributing to PC1 and PC2.
There were 53 metabolites with a significant change under different stresses in the primary root,and the content of most metabolites showed down-accumulation (Figs.3 and 4). The salt stress resulted in the smallest number of differentially expressed metabolites (5 and 20 significant up-and downaccumulation metabolites,respectively). Under water deficit stress,10 and 31 metabolites showed significant up-and down-accumulation,respectively. The most severe impact on the content of metabolite was observed in the combined water deficit and salt treatment,with six and 39 metabolites showing significant up-and down-accumulation,respectively(Fig.3-A). A cross-comparison of the differentially expressed metabolites between the different stresses found that a large proportion of the metabolites were responsive to all three treatments,with four and 18 metabolites showing significant up-and down-accumulation to all three treatments,respectively (Fig.3-B). A set of 10 metabolites were unique to combination treatments,while the salt (one metabolite)and drought (six metabolites) treatments resulted in less uniquely metabolites than combined stress. A set of 12 metabolites were shared between drought and combination treatments. Similar expression patterns were observed between the drought and combination treatments (Fig.4).
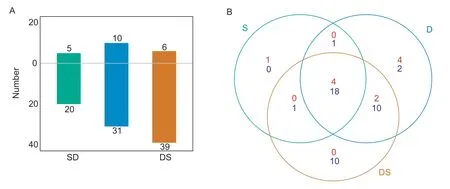
Fig.3 Overview of differentially expressed metabolites (DEMs) between the control and stress-treated samples. A,Pairwise comparisons of DEMs among different conditions. B,Venn diagram showing the overlap between DEMs responsive to salt (S),drought (D) and combined stress (DS). The number of DEMs is indicated by red (up-accumulation) and blue (down-accumulation)numbers.
Under stress conditions,the accumulation of some kinds of sugar was enhanced in the roots (Fig.5),a dramatic increase in sucrose was observed in all three treatments,the accumulation of fructose was only enhanced under drought,while the level of glucose was unaltered. Some metabolites involved in glycolysis were altered by different stresses. The level of glucose-6-phosphate was increased by drought,while glyceric acid decreased in all three treatments. The contents of metabolites in the shikimate pathway (quinic and shikimic acids) were significantly decreased under drought and combined stress conditions. The analysis of amino acids from intermediates of glycolysis revealed an increase in the levels of glycine (drought and combined stress) and alanine(all three treatments) during stress conditions. The levels of metabolites involved in the TCA cycle,including citric acid(combined stress),ketoglutaric acid (drought and combined stress),fumaric acid (all three treatments),and malic acid(salt and combined stress),were reduced in the primary root.The contents of the oxaloacetate/aspartate family of amino acids were altered by different stresses. The content of asparagine decreased under drought and combined stress conditons,homoserine was decreased by combined stress conditons,and the decrease of methionine was caused by salt and drought stresses. While,the levels of lysine were increased by drought stress. Salt stress caused an increase in glutamate,but the proline,glutamine and ornithine levels were unaltered by any stress treatment. Moreover,the GABA content was significantly increased under all three stress treatments,while urea significantly decreased under the combined stresses (Fig.5). Stress caused a dramatic decrease in several fatty acids,including eicosanoic acid(1.45,2.33 and 2.99 folds decrease for salt,water deficit and combined stress,respectively),exadecenoic acid (1.83,3.25 and 4.59 folds decrease for salt,water deficit and combined stress,respectively),heptadecanoic acid (1.83,3.25 and 4.59 decrease for salt,water deficit and combined stress),and octadecanoic acid (1.63,2.99 and 4.50 fold decrease for salt,drought and combined stress,respectively; Fig.6 and Table 1). Moreover,the levels of several organic acids,phosphoric acids and polyols were significantly decreased(Fig.6; Table 1).
4.Discussion
Over 400 million years ago,plants successfully colonized on land by developing root-like organs. To cope with highly heterogeneous soil,the ancestral root-like organ was replaced by a complex root system during plant evolution,which was suited for effective water and nutrient acquisition and plant anchorage (Motte and Beeckman 2019). Under heterogeneous environments,abiotic stresses,such as nutrient deficiency,drought and salt stress,are major reasons for yield losses. Recently,the importance of root adaptations has received increasing attention. The manipulation of the root system could deliver gains in resource use efficiency and yield,so plant scientists and breeders have begun to focus belowground (Bishopp and Lynch 2015). In natural habitats,plants are usually subjected to a combination of abiotic conditions,such as drought and salt (Suzukiet al.2014). Drought and salt stresses have been extensively studied,while little is known about how their combination impacts the primary root in maize. In the present study,we surveyed the single and simultaneous effects of drought and high salt stress on root development and the global metabolite profiles of maize primary roots. Growth inhibition of the shoots and roots is a common response to salt and drought stresses (Suzukiet al.2014). Here,the length of the primary root was reduced by either a single stress or the combined stresses,and the growth rate decreased with an increase in the treatment time (Fig.1). The effect of the combined stresses was greater than that of a single stress.A similar result was observed in the seminal roots of barley to individual and simultaneous effects of water deficit and high salt stress (Osthoffet al.2019). Combined stresses also caused severe outcomes on the development of the aboveground tissue and photosynthetic rate in maize (Sunet al.2015). Drought and combined stresses resulted in more DEMs than salt stress in this study. Several studies have revealed that water deficiency could lead to a more severe effect than salt stress on the plant growth,gene expression and metabolites (Crameret al.2007; Osthoffet al.2019; Sunet al.2015). The results support the notion that the number of responsive genes or metabolites may be associated with the complexity and intensity of the imposed stress.
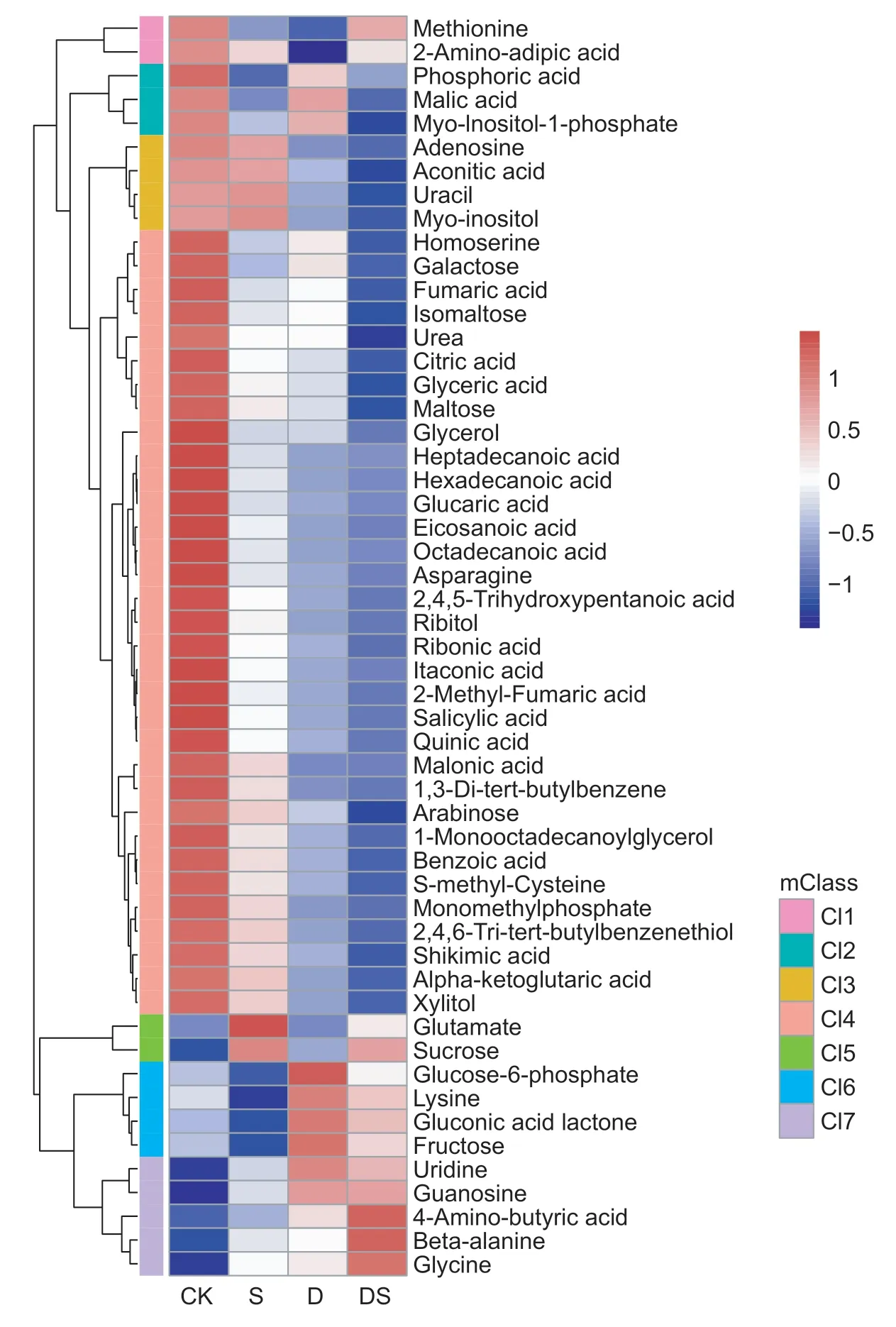
Fig.4 Clustered heatmap of the differentially expressed metabolites (DEMs) under the control (CK),salt (S),drought (D),and combined stress (DS) conditions. mClass,the clustered group of metabolites.
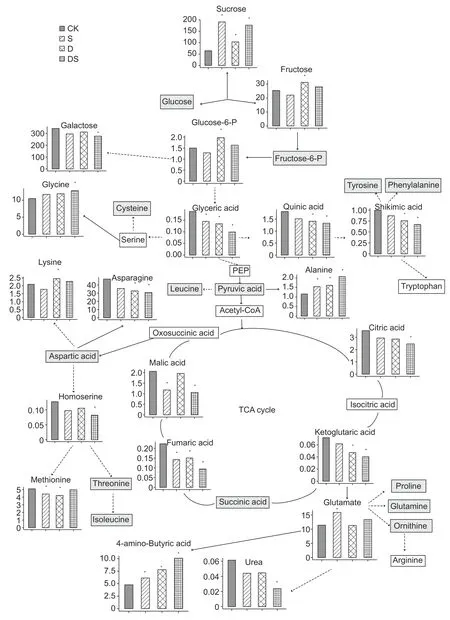
Fig.5 Changes in the metabolism pathways of the primary root of plants under control (CK),salt (S),drought (D),and combined stress (DS) conditions. x-axis are treatments; y-axis are relative metabolite contents. Metabolites without a significant difference between treatments are indicated by a grey square,a white square indicates that the metabolites were not measured; continuous arrows indicate a one-step reaction,and broken arrows indicate a series of biochemical reactions. *,significant difference (P<0.05).
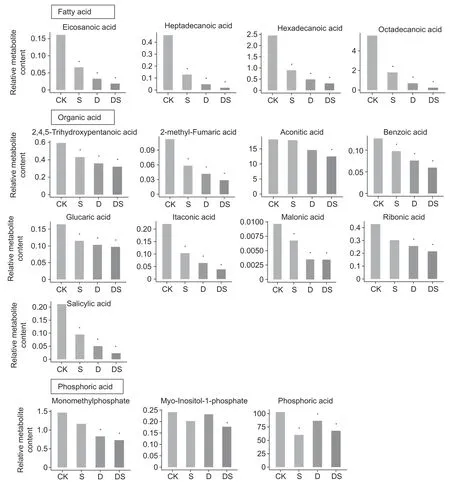
Fig.6 Changes in the fatty acids,organic acids and phosphoric acids in the primary root of plants under control (CK),salt (S),drought (D),and combined stress (DS) conditions. Asterisks indicate differentially expressed metabolites at P<0.05.
A comparison of the differentially expressed metabolites found that 4 and 18 metabolites were significantly up-and down-accumulated under all of the stress conditions,respectively (Fig.2-B). This high proportion of overlap among the three stresses showed the similar adaptive responses of the primary root. The osmotic stress imposed by drought and salt stress is thought to act during the early stages of the response,as the accumulation of compatible solutes/osmolytes is an adaptive response of plants to help maintain the osmotic balance (Hareet al.1998; Chen and Murata 2002; Munns 2002). Several compatible solutes have been identified in plant cells,such as sugars(sucrose and trehalose),N-containing compounds (proline and glycine betaine) and polyols. Our results showed that the level of sucrose dramatically increased under all stress conditions. Another study has shown that sucrose increased in a salt tolerant barley genotype in response to high salt stress (Widodoet al.2009; Wuet al.2013). Sucrose is also accumulated in many plant tissues in response to environmental stresses,including water deficit,salt stress and cold stress (Samiet al.2016). Moreover,the expression of sucrose transporters (AtSUC9) was induced by abiotic stresses. The disruption ofAtSUC9could lead to sensitive responses to abiotic stress (Jiaet al.2015). GABA plays a crucial role in several processes,including maintainingthe carbon/nitrogen balance,pH regulation and energy production (Rameshet al.2015),and accumulates rapidly when plants are exposed to stress that has been shown to be a stress marker. The level of GABA was dramatically increased under both individual and combined stresses.Moreover,inArabidopsis,the accumulation of high levels of GABA results in the inhibition of cell elongation and represses cell wall synthesis genes (Renaultet al.2011). GABA also accumulates in the cell division zone in the primary root of barley (Sheldenet al.2016). Inhibition of primary root growth may be associated with GABA accumulation under stress conditions. Recently,a study found that endogenous GABA accumulation not only inhibited adventitious root growth,but also suppressed or delayed adventitious root formation in poplar trees (Xieet al.2019). Proline is one of the most common compatible osmolytes when plants are subjected to osmotic stress,caused by drought or salt stress. Several previous studies have highlighted the role of proline (Delauney and Verma 1993; Hayatet al.2012).Proline was highly accumulated in the leaves and roots under salt and drought stresses conditions in barley,rice and wheat (Wuet al.2013; Zhaoet al.2014; Ullahet al.2017; Kanget al.2019). However,in this study,the proline content did not significantly increase under individual or combined stresses.The reason may be that the primary roots have been exposed to stresses for too short a time.Fatty acids,as the main component of membrane lipids,are considered to be important in stress resistance of plants(Upchurch 2008). Here,the levels of four fatty acids were decreased under all three stress conditions (Fig.6),which was a new finding in the present study.
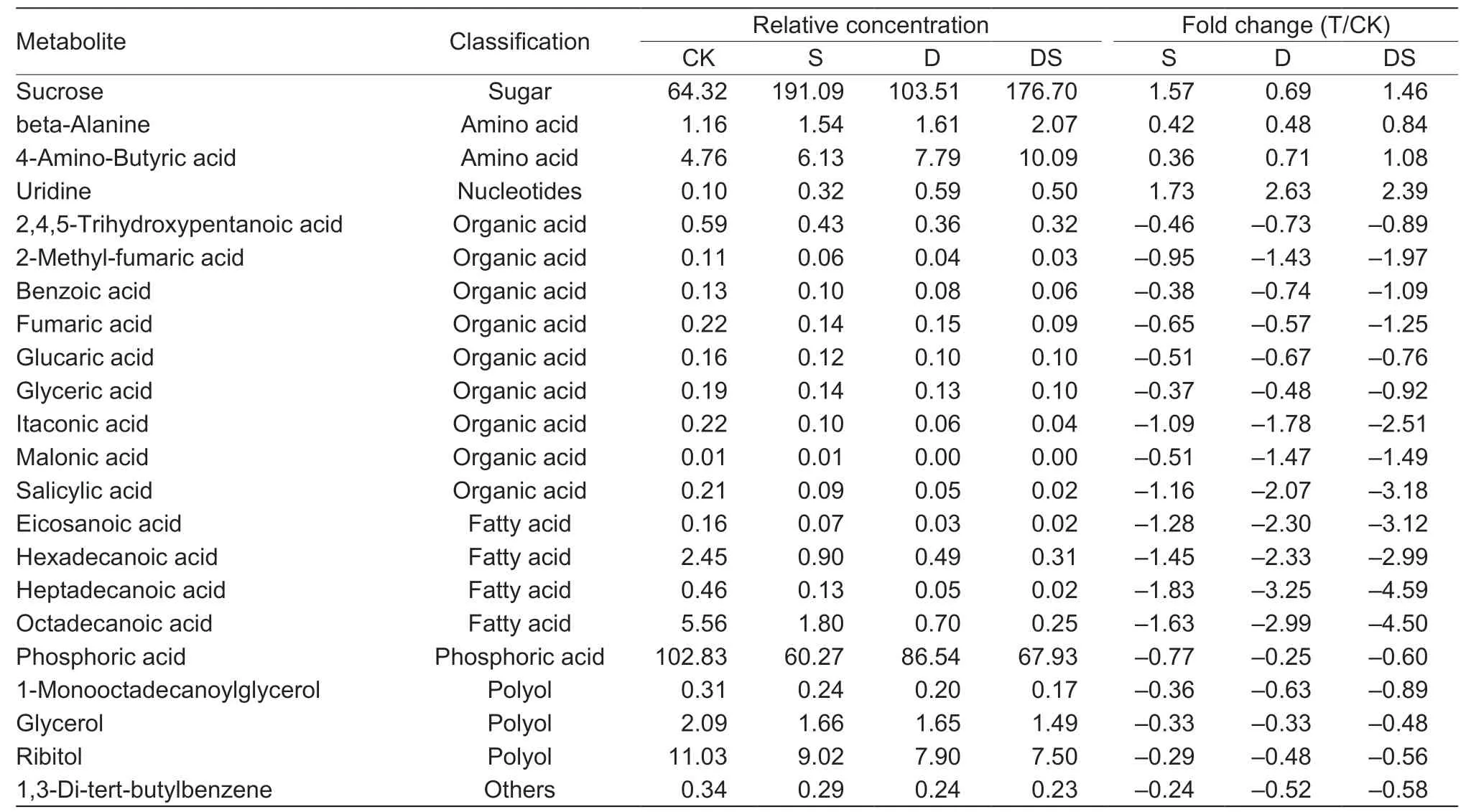
Table 1 Relative concentration and fold changes of major metabolites in primary root under control (CK),salt (S),drought (D),and combined stress (DS) conditions1)
The biosynthesis and transport of auxin and its signaling play a crucial role in root elongation and adventitious root formation (Sainiet al.2013). IAA (indole-3-acetic acid)is synthesized through the tryptophan (Trp)-dependent pathway or Trp-independent pathway. In both pathways,the Trp biosynthesis is considered to be one of the critical steps (Zazimalova and Napier 2003). Tryptophan is the precursor for auxin synthesis,which is synthesized mainly through the shikimic acid pathway (Maeda and Dudareva 2012). A previous study found that an increase in auxinviathe shikimic acid pathway could enhance shoot and root growth under mixed nitrogen conditions (Wanget al.2019). The observed differences in the down-accumulation of quinic acid and shikimic acid in stress may be a reason for the inhibition of the primary root. The TCA cycle is an important source of energy and carbon skeleton for the synthesis of some amino acids (Sweetloveet al.2010). Root growth depends upon the availability of carbon and energy required for cell division (Mulleret al.1998; Sheldenet al.2016). The levels of TCA cycle intermediates,including citric acid,ketoglutaric acid,fumaric acid,and malic acid,were decreased in roots exposed to stress. Similar response to stresses were observed in root of barley,rice and wheat(Wuet al.2013; Zhaoet al.2014; Ullahet al.2017; Kanget al.2019). This may be the other reason for the inhibition of the primary root.
5.Conclusion
In maize,plants subjected to drought,salt and combined stresses displayed significantly reduced primary root lengths relative to the control plants. GC-MS metabolomics was used to identify the differences between the stress conditions of the root. There were 53 metabolites with a significant change under different stresses in the primary root,and the content of most metabolites showed down-accumulation. A total of four and 18 metabolites showed significant up-and down-accumulation to all three treatments,respectively.The levels of several compatible solutes,including sugars and polyols,were increased to help maintain the osmotic balance. The decreased shikimic acid pathway and TCA cycle may be the reasons for the inhibition of the primary root. These data provide new insights into metabolomics changes in the primary root of maize in response to stress.
Acknowledgements
This work was supported by grants from the National Key Technology Research and Development Program of Ministry of Science and Technology of China (2016YFD0100303),the National Natural Science Foundation of China(31972487,31902101 and 31801028),the Key Technology Research and Development Program of Jiangsu,China(BE2018325),the Natural Science Foundation of Jiangsu Province,China (BK20180920),and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions,China (PAPD).
杂志排行
Journal of Integrative Agriculture的其它文章
- lnfectious disease-resistant pigs:Will they fly?
- Crop photosynthetic response to light quality and light intensity
- Alginate oligosaccharides preparation,biological activities and their application in livestock and poultry
- Genome-wide pedigree analysis of elite rice Shuhui 527 reveals key regions for breeding
- TaSnRK2.4 is a vital regulator in control of thousand-kernel weight and response to abiotic stress in wheat
- Development and characterization of new allohexaploid resistant to web blotch in peanut
