Fungal infections following liver transplantation
2021-12-06MadihaKhalidRiteshNeupaneHumayunAnjumSalimSurani
Madiha Khalid,Ritesh Neupane,Humayun Anjum,Salim Surani
Madiha Khalid,Department of Medicine,Orlando Health Medical Center,Orlando,FL 32806,United States
Ritesh Neupane,Department of Medicine,Penn State Health Milton S Hershey Medical Center,Hershey,PA 17033,United States
Humayun Anjum,Department of Medicine,University of North Texas,Denton,TX 76203,United States
Salim Surani,Department of Pulmonary Critical Care and Sleep Medicine,Texas A&M Health Science Center,Corpus Christi,TX 78405,United States
Abstract With increasing morbidity and mortality from chronic liver disease and acute liver failure,the need for liver transplantation is on the rise.Most of these patients are extremely vulnerable to infections as they are immune-compromised and have other chronic co-morbid conditions.Despite the recent advances in practice and improvement in diagnostic surveillance and treatment modalities,a major portion of these patients continue to be affected by post-transplant infections.Of these,fungal infections are particularly notorious given their vague and insidious onset and are very challenging to diagnose.This mini-review aims to discuss the incidence of fungal infections following liver transplantation,the different fungi involved,the risk factors,which predispose these patients to such infections,associated diagnostic challenges,and the role of prophylaxis.The population at risk is increasingly old and frail,suffering from various other co-morbid conditions,and needs special attention.To improve care and to decrease the burden of such infections,we need to identify the at-risk population with more robust clinical and diagnostic parameters.A more robust global consensus and stringent guidelines are needed to fight against resistant microbes and maintain the longevity of current antimicrobial therapies.
Key Words:Invasive fungal infections;Liver transplantation;Candidiasis;Antifungal prophylaxis;Aspergillosis;Cryptococcus
INTRODUCTION
Liver transplantation is one of the principal treatment modalities for the treatment of many hepatic diseases,mainly but not limited to chronic and end-stage liver disease.Despite advances in the field of transplantation,invasive fungal infections remain a major source of morbidity and mortality.This is attributed to delay in diagnosis,nonspecific clinical features[1],fastidious nature of these organisms,lack of consensus on prophylactic regimens,and rise of antifungal resistant species.
Moreover,with an increase in the number of grafts being offered,there is a trend towards recipients being older,debilitated,and having more non hepatic comorbidities which contributes to the burden and subsequently leads to a higher rate of fungal infections[2].
In this article,we aim to discuss the incidence and trend of invasive fungal infections(IFI)in liver transplant(LT)patients,associated risk factors,diagnostic challenges,and data on prophylaxis.
IFI DEFINITION
IFIs,according to the Invasive Fungal Infections Cooperative Group in Europe and the Mycoses Study Group in the United States,are divided into 3 categories:proven,probable and possible.
Proven IFI is defined as a positive fungal culture or histological proof of fungal or hyphal elements in a sterile site biopsy.This also includes positive cryptococcal antigen in cerebrospinal fluid.
Probable and possible IFIs have a wider definition and inclusion criteria.This is based on several host factors along with various clinical and mycological criteria[3].
Some studies evaluating prophylactic regimens,in this regard have been a focus of criticism as their IFI’s were considered colonization rather than infection[4].
INCIDENCE AND RESPONSIBLE FUNGI
The incidence of IFI after LT has decreased in recent years and this is attributable to advancement and improvement in surgical techniques along with more aggressive post-operative care.Previously,in one study by Funget al[5],the incidence of IFI after LT was reported to be 6.6% with a mortality of 54.5%.The ninety-day cumulative mortality after invasive candidiasis has been reported to be 26% and 1-year survival after invasive aspergillosis is about 59% according to TRANSNET in 2010[6].
More recently,according to some cohort studies,the overall incidence of IFI after solid organ transplant is about 1%-4%[7-9].1-year cumulative probability of IFI in LT was 1.8%[7].This shows a promising trend and is related to improvise surgical techniques and timely recognition of risk factors that make certain patients more susceptible to IFIs.
However,in underdeveloped nations,it remains higher at 14.7% with an in-hospital mortality rate of 77%[10].A future streamlined approach to the problem with specific guidelines might be one of the ways to improve these numbers.
The three major fungi involved areCandidaspp.,Cryptococcus,andAspergillusspp.Candidapredominates with 81% followed byAspergillus(16%)andCryptococcus(3%).Non-Albicans Candidaaccounted for 68% of allCandidainfections[11].The rise of resistant non-Albicans Candidaespecially C.parapsilosis was felt to coincide with the increased use of fluconazole[11].C.parapsilosis is associated with increased mortality in these patients.This increase in resistant fungal species indicates a dire need for a patient-specific prophylactic regimen based on risk factorsvsa universal approach.
The distribution of the fungal species remains similar in the East withCandidarepresenting 64.1% andAspergillus35.8% of the IFIs in LT patients.
Despite the highly variable clinical presentation,these pathogens most commonly affect the respiratory system followed by renal and gastrointestinal tract[10].According to a retrospective study in 2015 by Eschenauer and colleagues,intraabdominal candidiasis(73%)was the most common IFI[12].The common clinical manifestations of various fungal organisms are shown in Table 1.
TIMING FROM TRANSPLANT TO INFECTION
There has been a shift in the time duration between the developments of IFIs after LT.It was initially thought to occur in the early post-operative phase most commonly within the first couple of months.
Grauhanet al[13]in 1994 reported a median time from LT to IFI of 2 mo.
According to Husainet al[14]in 2003,the median time to infection for invasive candidiasis was 13.5 d with 72% of the IFIs happening within the first month after LT.
Aspergillustends to present later as compared toCandida.Results from one study by Singh and colleagues in 2003 reported 55% of theirAspergillusIFI occurring after 90 d[15]and Gravaldaet al[16]also described 43% of their IFIs as late onsetAspergillus.
In transplant centers with a higher risk ofAspergillusbased on epidemiology,this delayed time to presentation is important to consider while deciding on the length of prophylactic regimen in high-risk patients.Moreover,clinicians need to be mindful of this time frame while diagnosing an already difficult-to-diagnose disease.
RISK FACTORS
Multiple factors have been observed over time to be associated with the development of fungal infections in LTs.Identifying patients that are at high risk for developing IFI can be of immense help as that can aide in decreasing the diagnostic delay and assure appropriate prophylaxis.By adopting this targeted method of prophylaxisvsuniversal approach,we can also potentially reduce the incidence of drug-resistant fungi,lower the morbidity due to side effects and interactions of these medications particularly with immunosuppressants,and mitigate the overall cost.
Many scientists over the past few decades have worked on identifying these attributes.These can be categorized into pre-operative,operative,and post-operative factors as shown in Table 2.Risk factors forAspergillusspecifically seem to depend more on post-operative factors as highlighted in Figure 1.

Figure 1 Risk factors for Candida and Aspergillus.FQ:Fluoroquinolone;HLA:Human leukocyte antigen;CMV:Cytomegalovirus;HD:Hemodialysis;LT:Liver transplant.
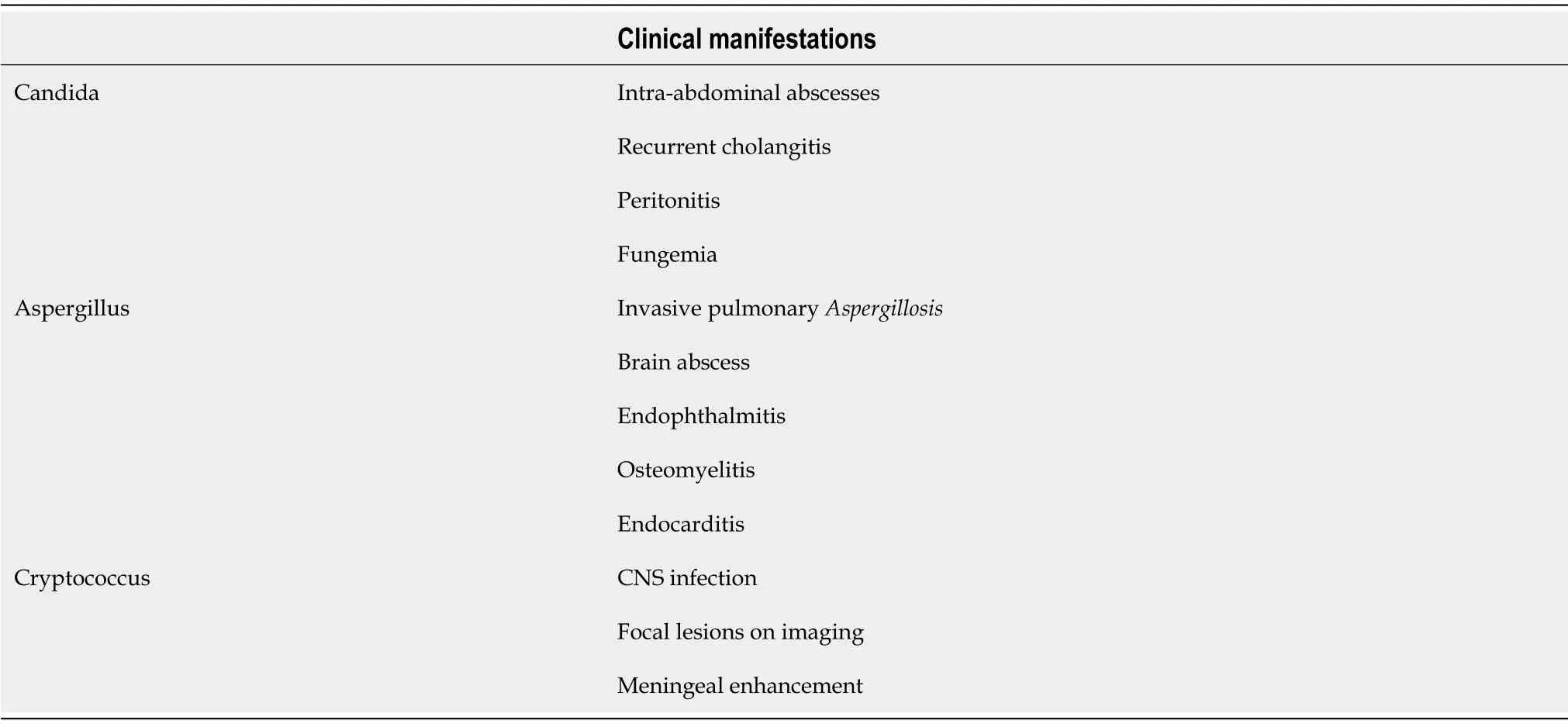
Table 1 Common clinical manifestations of invasive fungal infection
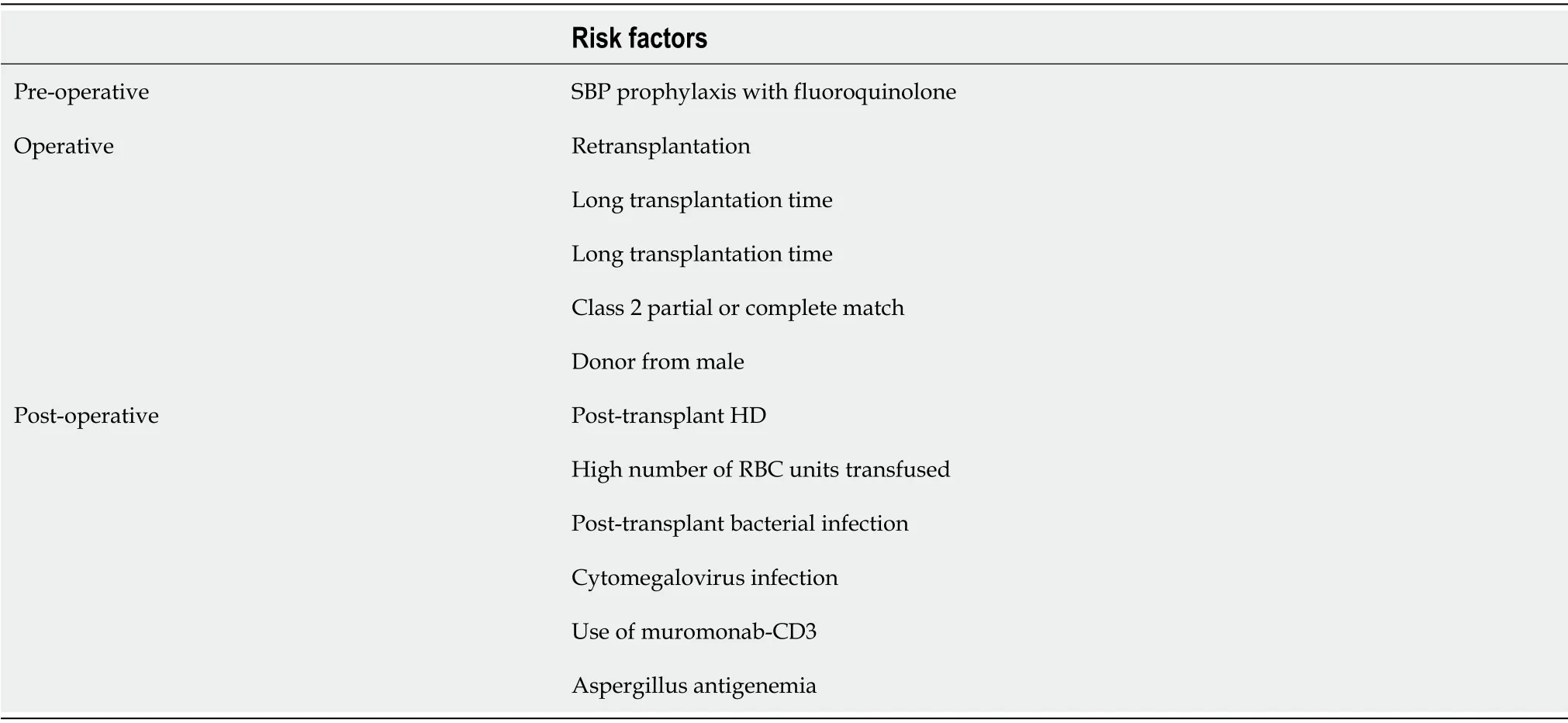
Table 2 Risk factors for invasive fungal infections
Collinset al[17]in 1994 identified the following as potential risk factors:renal insufficiency,length of transplant operation,rate of re-transplantation,abdominal or intra-thoracic reoperation,and cytomegalovirus infection.
Other studies showed that model for end-stage liver disease(MELD)scores > 25,post-transplant acute kidney injury(Cr > 2 or risk,injury,failure,loss of kidney function,and end-stage n criteria I- or F-)and pre-transplant fungal colonization seem to be the culprits identified with IFIs[11,18].
One of these was an important and common risk factor of daily prophylactic fluconazole dose of < 200 mg,which was thought to cause a rise in drug-resistant non-Albicans Candidaspp[11].
Although very rare,a French study also identified contamination during organ procurement as a risk factor with a 1.33% prevalence ofCandidaspp.in preservation fluid.This was associated with a higher rate of IFI and impaired survival[19].
Alongside predictable risk factors like diabetes and hemodialysis dependence,Vermaet al[10]pointed out prior antibiotic use,cerebral and respiratory organ failures,chronic liver failure(CLIF)organ failure/CLIF-consortium acute-on-chronic liverfailure as predictors of IFIs.Non-survivors in their study also had higher levels of 1.3-beta D glucan(BDG)levels.BDG levels have been studied as a diagnostic marker and look promising.
There has been a general shift in the trend of risk factors over the last 2 decades,which is attributable to better surgical techniques.Singhet al[20]studied 190 liver transplants during 1990 and 2000 and demonstrated improvement in length of operation,intraoperative transfusion requirements,use of roux-en-Y biliary anastomosis,re-transplantation,rate of rejection over time,and cold ischemic time.This led to a decrease in the incidence of invasive candidiasis in this study population from 9%-1.7% without any use of antifungal prophylaxis.
In 2015,Eschenauer and colleagues identified bile leaks within the first 30 d posttransplant and living donor liver transplants as new independent risk factors for IFIs.This is becauseCandidahas an affinity for growth in the biliary tract.Moreover,living donor liver transplants are highly technical procedures that are not commonly performed in the United States.The increased length and complexity of these procedures along with higher disruption of the biliary tract is responsible for these findings.The authors recommended instituting antifungal prophylaxis in all living donor liver transplants[12].
A small study recently in 2020 by Jorgensonet al[21]studied the effects of pretransplant roux-en Y gastric bypass on liver transplant outcomes.There were increased rates of fungal infection in patients with bariatric surgery before transplant and might be associated with loss of defense provided by gastric acid.This study is limited by its retrospective nature and its size.
DIAGNOSTIC CHALLENGES
In general,fungal infections do not present themselves vividly and are increasingly difficult to grow in culture media.It makes it even more challenging in patients who have chronic liver disease,are immunosuppressed,or have other underlying comorbidities.They are difficult to detect clinically and also objectively in laboratories.Hence,prevention becomes essential,and it has significantly improved in the last decade with the advancement in surgical techniques,intense pre-operative evaluation,and appropriate use of antifungal prophylactic agents in high-risk patients.
Distinguishing between colonization and true infection can be challenging for the clinician.Apart from 'proven IFI' as discussed above,the other two categories are vague and have plenty of variable factors.In these clinical scenarios,the use of newer diagnostic tools like BDG and galactomannan(GM)can be helpful.Polymerase chain reaction fungal assays are promising but not yet approved by the Food and Drug Administration.
BDG has been studied and looks promising as a diagnostic marker in serum.In a study from 2017,with 271 transplant patients,weekly BDG was tested and monitored for IFIs.95% of the patients with IFI had positive BDG and a very promising negative predictive value of 96% was seen.The sensitivity of BDG was 75% and specificity was 65%,making it a very good tool to rule out IFIs[22].
The GM test is an enzyme-linked immunosorbent assay that detects the GM antigen released byAspergillushyphae when they invade host cells.
The patient’s epidemiological risk factors should be considered strongly which would help guide better towards increasing clinical suspicion and ordering appropriate tests and guided treatments.Objective risk factors such as the MELD score,the overall duration of need for total parenteral nutrition,length of the operative procedure,and removal of abdominal drains and other catheters or lines should be evaluated[23].
PROPHYLAXIS
Fungal infections in liver transplant recipients are mostly attributed toAspergillusandCandida.Three agents are mainly used in prophylaxis-fluconazole,liposomal amphotericin B,and itraconazole.The studies involving these agents have been confounded by the difficulty of differentiating colonization and a true infection,the variability between patient selection,therapeutic agent(s)used in comparison with placebo or each other,and variable duration of treatment.
Data on the effectiveness of antifungal prophylaxis in LT over the past 10 years have been summarized in the Table 3 below.
There have been three meta-analyses as summarized in Table 3.Playfordet al[24]and Crucianiet al[25]published two in 2006,with 10 and 6 studies respectively.These summarized that universal fungal prophylaxis leads to a reduction in proven IFIs without any mortality benefit.This universal approach leads to a significantly higher proportion of episodes of non-Albicans Candidainfection.
In 2014,Evanset al[26]published a meta-analysis of studies on prophylaxis to prevent IFIs after LT and concluded that the odds of proven IFI and IFI related mortality were lower in patients receiving antifungal prophylaxis,even if the overall mortality did not change.It was also demonstrated that the efficacy of fluconazole compared to liposomal amphotericin was similar with the latter having the benefit of not altering the cytochrome P450 system and therefore not affecting the calci-neurin inhibitor levels.However,fluconazole is favored because of its cost-effectiveness and safety profile.This meta-analysis did not reveal any information on echinocandins,however,it was different from their counterparts in that they did a mixed treatment comparison and was more recent of the few meta-analyses already on the subject matter.
Studies since 2014(after the last meta-analysis)on prophylaxis are summarized in Table 4.

Table 3 Effectiveness of antifungal prophylaxis in liver transplant

Table 4 Studies since 2014(after the last meta-analysis)on prophylaxis for liver transplant
In 2015,Eschenauer and colleagues performed a retrospective study involving liver transplant patients that were divided into three main groups.Group 1 included 145 patients who received targeted prophylaxis with either voriconazole in 54%,fluconazole in 5% or no antifungal which was the case of 38% of these patients.This was compared to a group of 237 patients,who received universal prophylaxis with voriconazole.These regimens were continued for a median time of 11 d in the targeted group and for 6 d in the universal group,with a significantPvalue.There was no statistical difference between incidence of IFI between both groups(6.8% in targeted and 4.2% in universal).Similarly,thePvalue was not statistically significant for the mortality rates over 100 d from IFIs in both groups(10% for targeted and 7% for universal group).They,therefore concluded that targeted approach to antifungal use in liver transplant patients was a safe,cost effective strategy and prevented unnecessary side effects[12].
With regards to echinocandins,Salibaet al[27]in 2015 compared micafunginvsstandard treatment and found them equally effective.Standard therapy was centerspecific and included IV fluconazole,liposomal amphotericin,or IV caspofungin.
Similarly,in a study from Spain in 2016,caspofungin was compared to fluconazole in high-risk patients and similar efficacy was reported to prevent global IFIs.In this study caspofungin was related to decrease in breakthrough IFIs and also led to a lower rate of invasive aspergillosis[28].
Echinocandins should be considered as prophylactic agents,where appropriate,especially in areas of increased prevalence of drug-resistant non-Albicans Candida.Unfortunately,these too come with a higher price tag compared to fluconazole which can affect their use,especially in non-affluent countries.
According to the Infectious Disease Society of America guidelines,patients who meet 2 or more of the following risk factors to be considered for prophylaxis:creatinine more than 2 mg/dL,need for re-transplantation,choledochojejunostomy,more than 11 h of operative time,need to transfuse with ≥ 40 units of blood products,evidence of fungal colonization in immediate pre and post-operative days.Suggested duration of antifungal use is 14-21 d.
However,since the current data suggest that the incidence and risk of fungal infection overall in the general liver transplantation population is low,these agents should be utilized for higher-risk patients as unguided use is associated with drugresistant non-Albicans Candidainfection and higher mortality in these patients[23].
CONCLUSION
Fungal infections following liver transplantation remain an influential cause of morbidity and mortality in these patients,despite the low incidence.Identification of high-risk patients based on risk factors discussed above and starting an appropriate prophylactic antifungal regimen based on epidemiology,calcineurin inhibitor use,and renal function is the first step in avoiding dealing with this evasive disease.
Prophylactic antifungals are generally well tolerated but can lead to drug-resistantCandidaspp.,hence the importance of selecting the appropriate patient and agent.Using BDG as a negative predictive tool and having a high degree of suspicion,even if the time from transplant exceeds 2 mo,can prevent diagnostic delays.
Further randomized controlled trials comparing azoles,amphotericin,and echinocandins are needed to develop an updated standard of care.
杂志排行
World Journal of Hepatology的其它文章
- Incidence of umbilical vein catheter-associated thrombosis of the portal system:A systematic review and meta-analysis
- Role of endoscopic ultrasound in the field of hepatology:Recent advances and future trends
- Porta-caval fibrous connections—the lesser-known structure of intrahepatic connective-tissue framework:A unified view of liver extracellular matrix
- Promising diagnostic biomarkers of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:From clinical proteomics to microbiome
- Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis
- Liver involvement in inflammatory bowel disease:What should the clinician know?
