Liver involvement in inflammatory bowel disease:What should the clinician know?
2021-12-06GiuseppeLosurdoIreneVitaBresciaChiaraLilloMartinoMezzapesaMicheleBaroneMariabeatricePrincipiEnzoIerardiAlfredoDiLeoMariaRendina
Giuseppe Losurdo,Irene Vita Brescia,Chiara Lillo,Martino Mezzapesa,Michele Barone,Mariabeatrice Principi,Enzo Ierardi,Alfredo Di Leo,Maria Rendina
Giuseppe Losurdo,Irene Vita Brescia,Chiara Lillo,Martino Mezzapesa,Michele Barone,Mariabeatrice Principi,Enzo Ierardi,Alfredo Di Leo,Maria Rendina,Section of Gastroenterology,Department of Emergency and Organ Transplantation,University of Bari,Bari 70124,Italy
Abstract Inflammatory bowel disease(IBD)may show a wide range of extraintestinal manifestations.In this context,liver involvement is a focal point for both an adequate management of the disease and its prognosis,due to possible serious comorbidity.The association between IBD and primary sclerosing cholangitis is the most known example.This association is relevant because it implies an increased risk of both colorectal cancer and cholangiocarcinoma.Additionally,drugs such as thiopurines or biologic agents can cause drug-induced liver damage;therefore,this event should be considered when planning IBD treatment.Additionally,particular consideration should be given to the evidence that IBD patients may have concomitant chronic viral hepatitis,such as hepatitis B and hepatitis C.Chronic immunosuppressive regimens may cause a hepatitis flare or reactivation of a healthy carrier state,therefore careful monitoring of these patients is necessary.Finally,the spread of obesity has involved even IBD patients,thus increasing the risk of non-alcoholic fatty liver disease,which has already proven to be more common in IBD patients than in the non-IBD population.This phenomenon is considered an emerging issue,as it will become the leading cause of liver cirrhosis.
Key Words:Inflammatory bowel disease;Liver;Primary sclerosing cholangitis;Viral hepatitis;Immunosuppression;Non-alcoholic fatty liver disease
INTRODUCTION
Inflammatory bowel disease(IBD)consists of two separate disease entities,ulcerative colitis(UC)and Crohn’s disease(CD),affecting the gastrointestinal tract[1].However,IBD does not exclusively affect the gut.The gut-liver axis refers to the bidirectional relationship between the gut and its microbiota,and the liver,resulting from the integration of signals generated by dietary,genetic and environmental factors[2].Therefore,a perturbation of this axis may mirror pathologic conditions both in the gut and the liver.Based on this consideration,the relationships between IBD and liver disorders are noteworthy and should always be considered by the clinician.The association between IBD and primary sclerosing cholangitis(PSC)is the most known and studied model,as it has several implications,the most important ones are the increased risk of both colorectal cancer and cholangiocarcinoma.Additionally,hepatotoxicity due to drugs such as thiopurines or biologic drugs is a relevant issue that should also be taken into account when planning IBD treatment[3].It should not be forgotten that IBD patients may have concomitant chronic viral hepatitis,such as hepatitis B(HBV)and hepatitis C(HCV)[3].Chronic immunosuppressive regimens may cause a hepatitis flare or reactivation of a healthy carrier state;therefore,careful monitoring of these patients is necessary.Finally,the obesity epidemic has involved even IBD patients,thus increasing the risk of non-alcoholic fatty liver disease(NAFLD),which has already proven to be higher than the control population in IBD patients[3].This phenomenon is considered an emerging issue,as it will become the leading cause of liver cirrhosis.
Therefore,we aimed to perform a narrative review describing the main interactions between IBD and corresponding liver involvement,with a particular focus on PSC and other autoimmune liver disorders,drug-induced hepatitis,HBV,HCV and NAFLD(Table 1).
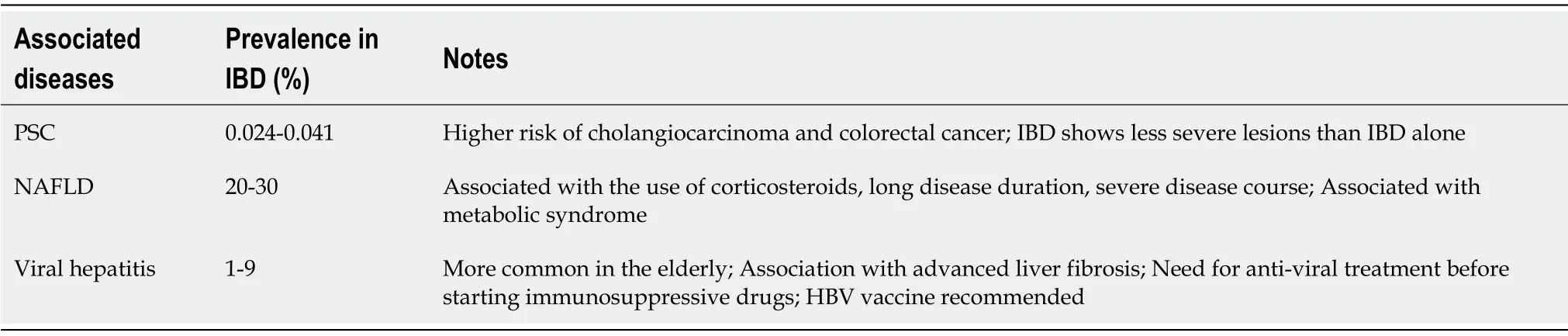
Table 1 Main liver comorbidities associated with inflammatory bowel disease
IBD AND PRIMARY SCLEROSING CHOLANGITIS
IBD and PSC are two pathologic entities that can occur alone or in combination.In this case they create a phenotypically different disease known as PSC-IBD.PSC-IBD prevalence is uncertain and differs in several studies,but it is agreed that it is very low(0.024%-0.041%)[4-6].PSC and IBD may occur simultaneously or sequentially.Indeed,PSC patients develop IBD in 20%-70% of cases,with a stronger association with UC(80%)than with CD(10%)and indeterminate colitis(IC)(10%)[7].Conversely only 5% of patients with UC show concomitant PSC.
Primary Sclerosing Cholangitis and Ulcerative Colitis
UC represents the underlying IBD in most cases of PSC-IBD.In patients with PSC and UC(PSC-UC),UC characteristically tends to be mild,quiescent and may even appear endoscopically normal(in this case,the diagnosis is based simply on histological analysis)[8].Therefore,random biopsies during the first colonoscopy should always be performed to reveal an underlying UC in patients with PSC.Similarly,PSC may be underdiagnosed in patients with UC,as it can be asymptomatic.Thus,liver function tests,including cholestatic and hepatocellular damage markers,should always be recommended in the follow-up of UC.If a patient with UC is found to have hepatocellular injury or a cholestatic pattern,magnetic resonance cholangiopancreatography(MRCP)should be performed to confirm the diagnosis[9].The onset of the two disorders may vary.Typically,UC occurs first,with a median time interval of 10 years[10].Nevertheless,in a minority of cases,UC may appear some years after the diagnosis of PSC,even after orthotopic liver transplantation[11].The degree and the extension of colorectal inflammation in PSC-UC differ from UC alone.Indeed,the incidence of pancolitis appears increased in PSC-UC patients when compared with UC-only patients,as shown by Boonstraet al[12]In their series,PSC-UC patients were affected by pancolitis in 94% of cases,while pancolitis was demonstrated only in 62% of patients affected by UC alone.Patients with PSC-UC usually have a greater prevalence of backwash ileitis and rectal sparing(51% and 52%,respectively)than controls with UC alone(7% and 6%,respectively)[13].However,the mild degree of colitis and the low rate of endoscopically visible inflammation may overestimate rectal sparing,when random biopsies are not performed[12,14].Even though,the extension of colitis tends to be more diffuse,and in PSC-UC the severity of the mucosal inflammation seems less pronounced.Patients with PSC-UC have less significant bowel symptoms,a lower need for steroids and undergo fewer hospitalizations than patients with UC alone[15].
Primary Sclerosing Cholangitis and Crohn’s Disease
Similar to patients affected by PSC-UC,patients with PSC and CD(PSC-CD)have a phenotypical and clinical pattern that sharply differs from patients with CD alone.Indeed,isolated ileal involvement,which occurs in about 30% of patients affected with CD,is rare in patients with PSC-CD(2%-5%)[12,16].As shown with PSC-UC,the degree of endoscopically visible inflammation is milder in patient with PSC-CD than in those affected by UC.Likewise,the incidence of CD complications seems low in PSC-CD[12,16,17].
Main characteristics of PSC in IBD
While IBD in PSC-IBD has specific phenotypical patterns as listed above,PSC does not show significant differences in terms of histologic findings such as periductal fibrosis,inflammation and portal edema or fibrosis[18].From a clinical point of view,according to Yanaiet al[19]PSC outcomes,including cirrhosis incidence and transplant-free survival,did not differ in PSC-IBD compared with PSC alone patients.Conversely,Feveryet al[20]reported higher rates of liver-related death and malignancies in patients with PSC-UC when compared to patients with PSC-CD.Interestingly,Nordenvallet al[21]found that patients with PSC-UC who underwent colectomy,seemed to have a lower risk of mortality,morbidity and the need for liver transplantation.
Risk of colorectal cancer(CRC)and hepatobiliary carcinomas in PSC-IBD
Although both PSC and IBD patients do not have a general higher risk of malignancies than the general population,patients with PSC-IBD show a significantly more marked risk of developing colorectal carcinoma(CRC)and cholangiocarcinoma(CCA),and hepatocellular carcinoma(HCC).In a meta-analysis,Zenghet al[22]found that patients with PSC-IBD have a strikingly higher risk for the development of CRC than patients with IBD alone.In detail,the stratification by IBD type showed a three-fold increased risk for the development of CRC and colorectal dysplasia in patients with PSC-UC compared to those with UC alone.A non-significant increase in the risk of neoplasia was shown in patients with PSC-CD,in contrast to that found in patients with CD alone.For these reasons,patients with PSC-IBD(especially those with PSCUC)require close colorectal neoplasia endoscopic surveillance.Major American and European Societies recommend that annual CRC screening should be started at the time of PSC-IBD diagnosis.In PSC-IBD patients an increased risk of hepatobiliary malignancies such as CCA,gallbladder carcinoma(GBC),and HCC has been demonstrated.Gulamhuseinet al[23]demonstrated that prolonged duration of IBD is associated with an increased risk of CCA in patients with PSC-IBD.They also observed that the risk of CCA was not modified after colectomy,thus suggesting that colonic resection itself does not reduce the risk of CCA.European and American Societies recommend that CA 19-9 and biliary imaging should be completed every year for these patients[24,25].IBD could be an additional risk factor that further increases the hazard of CCA in PSC.In particular,a long duration of IBD is associated with CCA with a hazard ratio of 1.37[23].
There are no studies demonstrating an increased risk of GBC in PSC-IBD patients,even if that risk is demonstrated in PSC-alone patients[26].Saidet al[27]found in their cohort of patients affected with PSC,that 6% had gallbladder masses,of which 56% were malignant.The American Association for the Study of Liver Disease(AASLD)guidelines support cholecystectomy for polyps of any size in these patients,given the high likelihood of malignancy[28].HCC seems to be a rare malignancy in PSC-IBD.Zanouziet al[29]analyzed a cohort of PSC-cirrhosis patients and found no cases of HCC.However,in the same cohort of patients,IBD was found in 65%.
As both CCA and CRC are likely to occur in PSC-IBD patients,a chemopreventive strategy could be proposed.A meta-analysis[30]showed that low dose ursodeoxycholic acid may have a protective effect on both CRC and colonic dysplastic lesions,with an odds ratio of 0.19.However,the studies were performed on small populations in tertiary centers,and were often retrospective,therefore the strength of evidence is not high[31].Even mesalazine has demonstrated,in vitroand in animal models,an anti-proliferative effect as well as the ability to inhibit the Wnt/β-Catenin pathway and epithelial growth factor receptor activation;therefore,it may be a promising agent for CRC prevention,despite the chemopreventive effect of mesalazine only being documented for patients with UC alone so far[32].Unfortunately,no effective approach for CCA chemoprevention has emerged,therefore surveillance remains the mainstay for early CCA detection in PSC patients.
Therapeutic perspectives
The pathogenetic mechanisms underlying PSC-IBD remain unknown,even though many hypotheses have been proposed.Understanding the basis of the disease could lead to the identification of a new targeted therapy.One of the most interesting assumptions suggests that intestinal mucosal lymphocytes may migrate to the liver following activation in the bowel of IBD patients,thus promoting liver inflammation[33].It has been shown that adhesion molecules and chemokine receptors normally expressed only in the gut can be aberrantly expressed within the liver to promote the homing of gut-associated lymphocytes.One of these adhesion molecules is α4β7 integrin.A monoclonal antibody directed against α4β7,vedolizumab,has been approved for the treatment of IBD.It was hypothesized that vedolizumab could provide hepatic anti-inflammatory benefits.Nevertheless,Christensenet alfound that,after treatment with vedolizumab,symptoms and intestinal clinical activity were significantly decreased,but the Mayo PSC Risk Score and liver damage biomarkers were only slightly improved[34].
Aberrant microbiota epitope recognition and gut dysbiosis seem to have a role in the pathogenesis of PSC-IBD,while genetics,gut mucosal permeability and autoimmune mechanisms have a controversial role[35].Further studies are needed to improve our knowledge on the pathogenesis of PSC-IBD in order to provide new and efficient therapeutic strategies.
When PSC causes end-stage liver disease,liver transplantation is the only curative treatment.Regarding this point,some studies found that IBD does not worsen survival in patients who undergo liver transplantation for PSC.Only exposure to azathioprine seems to increase post-transplant mortality,while IBD per se increases the risk of cytomegalovirus infection[36].
PRIMARY BILIARY CHOLANGITIS AND AUTOIMMUNE HEPATITIS IN IBD
PBC is an autoimmune liver disease characterized by inflammatory cell infiltration of intralobular biliary ducts,with consequent biliary duct damage,which can progress towards fibrosis.Currently,there is no solid link between IBD and PBC,as only a few case reports have been published.The most consistent case series involving six PBC patients in a cohort of IBD subjects during the period 2006-2016(3 CD and 3 UC),who were diagnosed with PBC by liver biopsy responded to ursodeoxycholic acid therapy[37].In a genetic association study,it was found that TNFSF15 and ICOSLG-CXCR5 might be a shared pathogenic pathway in the development of PBC and CD[38].
Similarly,only some case reports on the association between IBD and autoimmune hepatitis(AIH)have been published.A systematic review found approximately 109 cases,which were mostly overlap syndrome with PBC.The authors reported that jaundice was the most common onset sign and that response to steroids was good,with a low mortality rate[39].Interestingly,a case report of AIH onset after starting adalimumab has been described,which underlines the possibility that an immunogenic drug may alter an equilibrium in the immune system[40].
HEPATIC STEATOSIS IN IBD
Hepatic steatosis is defined as intrahepatic fat accumulation of at least 5% of liver weight.Prolonged hepatic lipid storage may lead to liver metabolic dysfunction,inflammation,and advanced forms of NAFLD.Non-alcoholic hepatic steatosis is associated with obesity,type 2 diabetes and dyslipidemia.Several mechanisms are involved in the accumulation of intrahepatic fat,including increased flux of fatty acids to the liver,increasedde novolipogenesis,and/or reduced clearance through βoxidation or very-low-density lipoprotein secretion[41,42]in the absence of secondary causes of lipid overload such as significant alcohol intake.
A link between hepatic steatosis and IBD has been studied since 1873,when Thomas[43]described for the first time the association between “ulceration of the colon” and a “much enlarged fatty liver”.In recent years,due to the spread of obesity in the context of IBD[44],fatty liver disease has been increasingly recognized in IBD.The intestinal inflammatory state and gut barrier perturbation secondary to IBD might increase toxin and bacterial constituents translocation from the gut to the portal vein;this event has been recognized as a possible pathophysiologic mechanism underlying NAFLD[45].Moreover,diets poor in high fiber foods,such as fruits and vegetables,frequently consumed by IBD subjects to avoid intestinal symptoms,could lead to a great prevalence of NAFLD[46].Moreover,food components and alimentary habits with high proteins and fats,excessive sugar intake and less vegetables and fiber can influence the composition of the intestinal microbiome,and play a role in driving IBD pathogenesis and fat metabolism leading tog NAFLD onset[47].
A recent meta-analysis showed that the overall pooled prevalence of NAFLD in IBD patients was 27.5%[48].NAFLD,in particular,was more common among patients with features of severe IBD,such as longer disease duration or a history of abdominal surgery.
Another study by Bessisowet al[49]showed a frequency of NAFLD in IBD of 33.6% and demonstrated that disease activity,duration of IBD and prior surgery were predictors of NAFLD development.
Conversely,in a Japanese study[50],the ultrasonographic prevalence of NAFLD in CD was 21.8% and this was the only study in which NAFLD was identified as an independent predictor of a negative C-reactive protein level and higher rate of remission,so NAFLD might offer a protective effect in patients with CD.
Nevertheless,most studies did not include non-IBD patients as a control group.
Glassneret al[51]examined 3 groups of patients:IBD + NAFLD,IBD alone,and NAFLD alone.A total of 168 patients were evaluated,56 patients in each group.They found an overall NAFLD prevalence of 13.3% in IBD patients.IBD patients with NAFLD had longer IBD disease duration and developed NAFLD even in the absence of metabolic risk factors when compared to patients with NAFLD alone.
A study performed in 2018 by Principiet al[52]included 465 IBD patients and 223 non-IBD patients.The prevalence of NAFLD was higher in IBD than in non-IBD patients(28.0%vs20.1% respectively,P =0.04);furthermore,younger age was observed in NAFLD-IBD than in non-IBD individuals,whereas no other differences were found between these two subgroups.Regarding risk factors,diabetes and fasting blood glucose were associated with development of NAFLD in IBD,without any difference in the populations without IBD,with only a higher waist circumference in IBD compared to non-IBD patients.No IBD-related variable was associated with NAFLD.
There are no studies on the progression of NASH in IBD.However,since IBD may induce gut barrier perturbation and an increase in toxin and bacterial translocation,it is possible that in patients with NAFLD,the coexistence of IBD can trigger the progression from simple steatosis to NASH.A single study,on the other hand,has shown that progression of fibrosis,estimated by the NAFLD fibrosis score,is quite rare in IBD[53].
In conclusion,NAFLD is common in patients with IBD.Screening,prevention,and early treatment of NAFLD might be recommended in IBD patients.However,a better understanding of the underlying mechanism of the coexistence of IBD and NAFLD is necessary to improve management.The treatment of NAFLD in IBD does not differ from other cases.In particular,so far only diet and physical exercise have been proved to be effective[54].
CHRONIC VIRAL HEPATITIS IN IBD
Chronic viral hepatitis,in particular HBV and HCV-related,is a very common infection and a worldwide health issue.It is estimated that over 350 million people in the world have chronic HBV infection and over 250 million people have chronic HCV infection,with a mean prevalence of 5% and 2% for HBV and HCV,respectively[55,56].
With regard to the prevalence of chronic hepatitis B(CHB)and chronic hepatitis C(CHC)in IBD,recent evidence[57-61]shows that it was comparable to a control population,ranging from 1% to 9%.A recent Italian study by Losurdoet al[62]on 807 IBD patients and 189 controls,found a prevalence of 3.4% for CHC and 0.9% for CHB,a result which agrees with recent literature reports[57,58,61].This analysis demonstrated that advanced age was independently associated with increased risk of CHB/CHC.It is possible that surgery performed before the diffusion of presurgical hepatitis screening could explain this result,also taking into account that CHC was more common in patients operated before 1990.Indeed,the introduction of the HBV vaccine and HCV routine detection led to an improvement in the prevention measures against viral hepatitis transmission during surgery or blood donation,thus reducing the risk of infection in young generations[62].
As the treatment of IBD is based in selected cases on immunosuppressive agents(thiopurines and biologic drugs such as monoclonal antibodies),an accurate clinical and laboratory assessment is preliminarily required to look for chronic infections that may have a severe flare under biologic drugs[57,63].Among these,chronic viral hepatitis and in particular CHB and CHC,are advised to be investigated by the guidelines before starting immunosuppressive treatment[64].
According to the guidelines,all IBD patients should be tested for HBV(HBsAg,anti-HBs,anti-HBc)at diagnosis of IBD to determine HBV status.In patients with positive HBsAg,viremia(HBV-DNA)should also be quantified.Moreover,HBV vaccination is recommended in all HBV anti-HBc seronegative patients with IBD.All HBsAg positive subjects should start anti-viral agents before undergoing biologic treatment to prevent potentially serious hepatitis B flares[64,65].A number of case series and study cohorts suggest that nucleotide/nucleoside analogues are safe and effective in IBD patients on immunomodulator treatment[66].Entecavir and tenofovir are preferred for IBD patients due to their rapid onset of action,high anti-viral potency and low incidence of resistance.On the other hand,patients with HBsAg positive(chronic HBV infection)should receive anti-viral agents before,during and for at least 12 mo after immunomodulator treatment has ceased[64].Additionally,HBV vaccination is strongly advised by the guidelines,possibly before starting any immunosuppressive treatment and preferably at the moment of diagnosis,if anti-HBs level is not protective.This approach should be followed in any region,irrespective of HBV prevalence.
With regard to CHC,present knowledge shows in some cases mild liver dysfunction and an amplified detrimental effect by the simultaneous presence of other viruses(HBV/HIV)in relation to immunomodulator assumption[67,68];therefore,HCV antibody testing and HCV-RNA should be investigated.Immunomodulators are not contraindicated but should be used with caution.The decision depends on the severity of IBD and the stage of liver disease.In the past years,an interferon-based treatment for HCV infection in CD has generally not been recommended,as it could worsened the intestinal disorder;however,this aspect remains controversial[69].Conversely,in UC,interferon therapy did not appear to have an adverse effect[70].In addition,the administration of ribavirin plus interferon or triple anti-viral therapy(interferon,ribavirin and protease inhibitors)could have increased the toxicity of drugs used for IBD maintenance(for example azathioprine,methotrexate)[64].Therefore,the risk that anti-viral therapy or drug interactions with IBD therapy might exacerbate IBD should assessed cautiously when considering the need for HCV treatment[64].However,over the last years,concomitant IBD and HCV infection management has completely changed due to the recent introduction of direct-acting anti-virals(DAAs).Recently published data on DAAs are very encouraging also in IBD patients[71].There are three possible timing strategies for administration in patients requiring biological therapies:(1)Sequential strategy,meaning the choice of treating firstly the active IBD with biologics and then,once the acute phase has been controlled,treating the HCV infection;(2)Concomitant strategy,that is the contemporaneous initiation of DAAs and biologic drug administration;and(3)Inverted sequential strategy,i.e.,the administration of anti-viral therapy before biologics.The timing strategy could depend on several factors,including IBD activity and patient comorbidity.This means that a case-by-case decision could be the best choice[72].The opportunity to eradicate HCV should always be taken into account,as it has demonstrated that a sustained viral response may reduce liver stiffness in these patients[73].
IBD AND DRUG-INDUCED LIVER INJURY
In the last decade,treatment options for IBD have included new molecules acting at different target levels.Usually,as new drugs are introduced,their side effects should also be considered,and liver toxicity is one of the most meaningful among these.
Drug-induced liver injury(DILI)caused by these drugs can be classified into three forms:hepatocellular,cholestatic or a mixed pattern.Moreover,some forms of druginduced AIH should also be considered.This issue leads to a schedule of specific screening before starting therapy for IBD,and a follow-up to monitor liver enzymes is necessary[74,75].
In Table 2,we summarize the main knowledge on DILI in IBD patients.
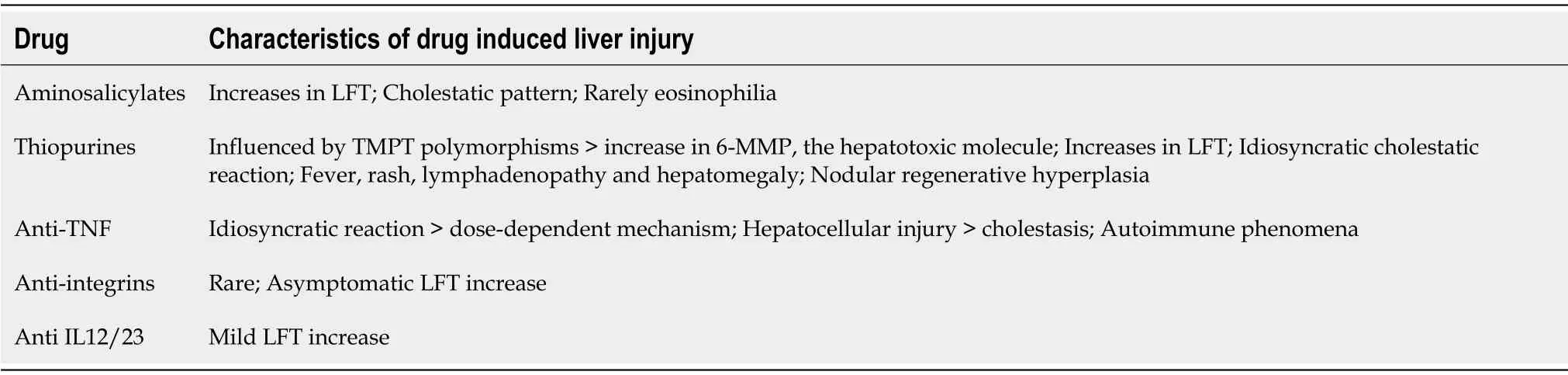
Table 2 Main features of drug-induced liver injury in inflammatory bowel disease
Thiopurines
Thiopurines,in particular azathioprine(AZA)and 6-mercaptopurine(6-MP)are used for induction and maintenance of remission in IBD.Studies have shown that AZA/6-MP as add-on to infliximab can reduce the development of antibodies against infliximab.Thiopurines act as DNA synthesis inhibitors by incorporating purine analogues into DNA with cytotoxic and immunosuppressive effects.AZA is metabolized in the liver to 6-MP,which is metabolized by three enzymes,including thiopurine S-methyltransferase(TMPT)to 6-methylmercaptopurine(6-MMP).AZA and 6-MP are prodrugs of 6-thioguanine(6-TGN),the real effective metabolite.Some studies have suggested that some TMPT polymorphisms could cause a rise in 6-MMP level,thereby amplifying hepatotoxicity.In a cohort study of 270 patients treated with 6-MP,47 patients showed evidence of altered liver function tests(LFT)in the first 20 weeks of treatment and > 80% of these patients had elevated levels of 6-MMP in the first week[76].Another study proved that patients with high concentrations of 6-MMP had not only a strong risk of side effects but also a reduction in therapeutic response[77].Conversely,Donget al[78]found that the presence of TMPT polymorphisms increased bone marrow toxicity but not hepatotoxicity.A recent meta-analysis of 10 studies(recruiting 1875 patients)proved that TMPT polymorphisms were not linked with liver injury.The physiopathology of liver injury due to thiopurine is still unclear.
The prevalence of thiopurine-induced liver toxicity can vary between 0% and 17%.In a systematic review of 34 studies with 3485 patients,the prevalence of hepatotoxicity induced by AZA/6-MP was 3.4% with no differences between the two drugs[79].Additionally,Chaparroet al[80]in a study of 3931 patients with IBD treated with thiopurine reported that hepatotoxicity was one of the most common side effects,with a prevalence of 4%.CD,smoking and preexisting NAFLD seemed to be risk factors,while the prevalence was lower in females.In a study by Shroder,who analyzed 259 patients undergoing immunosuppressive treatment with AZA,6MP and MTX,liver steatosis was found in 28.2% of them,and patients with steatosis also had a higher risk of having elevated alanine transaminase(ALT)blood levels[81].
On the other hand,dose independent,idiosyncratic liver reactions have been described for thiopurines.Acute dose-independent toxicity is caused by an idiosyncratic cholestatic reaction accompanied by fever,rash,lymphadenopathy and hepatomegaly with increased alkaline phosphatase level.The median onset time of hepatotoxicity is 110 days,and in most cases is self-limiting with a good prognosis.
Another atypical,long-term liver injury caused by thiopurines is characterized by vascular endothelial lesions.Nodular regenerative hyperplasia(NRH),is the mostfrequent of these lesions,while peliosis hepatis and sinusoidal obstruction syndrome(SOS)are less common.NRH is frequently asymptomatic.The mechanism underlying NRH is still unknown,it is possible that hepatocyte atrophy and portal venules destruction could be involved;risk factors seem to be male sex,CD with stricturing behavior and previous small bowel resection.In a large French study,NRH was found in 37 cases,with a cumulative risk of 0.5% at five years and a median onset time of 48 mo[82].A recent study observed a similar prevalence of NRH between patients treated with thiopurines and patients thiopurine-naïve[83].On the other hand,it was found that thiopurines are associated with NRH when the dose is high(tioguanine > 40 mg/day)or in male patients with small bowel resection > 50 cm[84,85].The evolution of NRH after stopping thiopurine therapy is still unclear.
There is no agreement on thiopurine toxicity management.In a large study with a long-term follow-up only 3.6% of patients needed to discontinue therapy[86].In another study,90% of patients had normalization of LFT by reducing thiopurine doses[87].It is unclear whether the frequency of hepatotoxicity is the same for AZA and 6-MP treatment:a study of 135 patients reported that 6-MP was well tolerated in 71% patients who had shown liver toxicity with AZA[88].Coadministration of allopurinol(a xanthine-oxidase inhibitor)seems to reduce 6-MMP levels as it leads to a higher concentration of 6-MP converted to 6-TGN.However,since allopurinol is a xanthineoxidase inhibitor,the AZA dose should be reduced.A retrospective cohort study of 105 patients reported that coadministration of allopurinol allowed long-lasting therapy and transaminase normalization[89].Also,in another study by Krejineof,among 211 patients with liver toxicity,86% experienced an improvement by lowering the dose of thiopurines in association with allopurinol[90].A larger study by Vasuvedan analyzed 767 patients on thiopurine therapy and demonstrated that allopurinol should be started to reduce side effects,as 94% of patients who had hepatotoxicity achieved resolution by changing to co-therapy[91].As TMPT polymorphisms are likely to be involved in hepatotoxicity,some authors have proposed that these polymorphisms should be identified before starting therapy,but a review by the American Gastroenterological Association Institute stated that the benefits of these tests were low[92].On the contrary,a consensus guideline by the British Society of Gastroenterology focused on TMPT activity and recommended the administration of a half-dose of thiopurines to patients with low TMPT activity[93].
LFT should be monitored routinely,but there is no agreement on their timing.Mottetet al[93]recommended LTF every wk for the first mo,then twice a mo during the second mo and then once every 3 mo.
Sulfasalazine and mesalamine
Sulfasalazine is used for mild UC.It has been associated with acute hepatitis,cholestatic hepatitis,granulomatous hepatitis and rarely with acute liver failure[94].The incidence of hepatotoxicity is low:A review by Ransfordet alwho analyzed 4.7 million prescriptions in the period from 1991 and 1998,reported only 9 cases of hepatitis caused by sulfasalazine[95].
Mesalamine(oral and rectal)is approved for mild UC.Authors in the last three years have demonstrated that the prevalence of liver toxicity caused by mesalamine is low,between 0% and 4%.The use of mesalamine may be associated with asymptomatic elevations in LFT,hepatitis and cholestatic hepatitis[96].A recent review reported that LTF should be monitored every year and therapy should be stopped in the case of abnormal increases,while treatment with corticosteroids should be considered if fever,rash,or eosinophilia are observed.The same review demonstrated that most cases of hepatotoxicity quickly reversed with drug withdrawal[97].
Methotrexate
Low doses of methotrexate(MTX)are used for mild CD,and it is widely used for rheumatologic disease;therefore,in this field its hepatotoxicity has been more extensively studied.The underlying mechanism is still not clear;several polymorphisms of enzymes involved in folic acid metabolism are thought to be involved.Two systematic reviews on this topic reported opposite results:the first review found an association between MTX hepatotoxicity and C677T polymorphism of methylenetetrahydrofolate reductase(MTHFR)gene,while the second review did not confirm this result[98,99].MTX can cause different histological liver findings according to the Roenigk’s classification including:(1)Normal;(2)Mild fatty infiltration,nuclear alterations or portal inflammation;(3)Moderate to severe fatty infiltration,nuclear alterations,or portal infiltration and mild fibrosis;(4)Moderate to severe fibrosis;and(5)cirrhosis[100].
Some studies reported that the prevalence of abnormal LTF in these patients ranged from 15 to 50%,while most recent evidence demonstrated a lower prevalence.A metaanalysis of patients with IBD treated with MTX reported a rate of abnormal LTF(defined as ALT higher than normal values but less than x2 upper normal limit(ULN))of 1.4 per 100 person-month and a rate of hepatotoxicity(defined as ALT higher than two times normal values)of 0.9 per 100 person-month[101].It should be noted that,in CD,methotrexate is giveni.m.,with a dose of 25 mg/wk at induction and 15 mg/wk for maintaining remission.Considering that this dose is higher than in rheumatologic patients,this could explain the more frequent liver adverse events.
Before starting MTX treatment,patients should be screened for preexisting medical conditions,such as alcohol intake,viral hepatitis,steatosis and family history of liver disease.Rheumatological consensus guidelines recommend monitoring LFT every two wk for the first 2 mo,then every 2 or 3 mo[102].Liver biopsy should be considered in some cases,such as when liver laboratory tests remain abnormal despite dose reduction or when there are high blood levels of drug in patients with known risk factors for hepatotoxicity.Treatment should be stopped in the case of severe fibrosis or cirrhosis and daily doses should be reduced in the case of LFT elevation.Co-administration with folic acid or folinic acid seems to reduce the frequency of serum transaminase elevation[103].Elastography(Fibroscan)and laboratory tests are emerging tools to diagnose fibrosis as reported byLabadie et al[104].Furthermore,in a case control study of 518 patients treated with MTX,8.5% showed Fibroscan and FibroTest abnormalities,i.e.,severe fibrosis[105].A multivariate analysis reported that elastography should be used mainly in patients with an alcohol habit or obesity,or affected by NAFLD.Similar results were reported in a study by Herfathet al[106].
Tumor necrosis factor alpha inhibiting agents
Currently several molecules belonging to this class have been approved to treat IBD:infliximab(IFX),adalimumab(ADA),golimumab and certolizumab pegol.Few data are available on the hepatotoxicity of golimumab and certolizumab,while most of the literature reports DILI by IFX and ADA.
The Food and Drug Administration(FDA)in 2004 after 130 cases of liver injury in patients treated with IFX and etanercept(which has no indication in IBD),issued an alarm statement of severe hepatic adverse reactions,including acute liver failure,autoimmune hepatitis(AIH)and cholestatic hepatitis during IFX therapy[107].In an Icelandic study by Bjornsson that included patients with IBD,rheumatological and dermatological disorders,the occurrence of DILI in patients treated with IFX or ADA was 1:120 and 1:270,respectively[108].Sheltonet al[109]in a retrospective study analyzed 1753 patients under anti-TNF therapy(1170 IFX,575 ADA,8 certolizumab),and found that 102 patients had high blood levels of ALT,but in 54 of these patients,additional risk factors for liver injury were found and,of the remaining 48 patients(45 IFX,3 ADA),only 4 were considered to be affected by anti-TNF induced liver injury.Kolleret al[110]in a recent observational study of 251 patients with IBD,monitored liver injury in 163 receiving IFX.Twenty-six patients(16%)showed a grade 1 liver injury(ALT < x3 ULN),4 patients(2.5%)a grade 2(ALT > x3 ULN);grade 1 alkaline phosphatase elevation was seen in 11 patients(6.7%)and grade 2 alkaline phosphatase elevation(> x2.5 ULN)in none.Liver injury in these patients was associated with high BMI,hepatic steatosis and longer duration of IBD[110].In an Australian retrospective cohort study of adult patients with IBD treated with IFX(IDLE STUDY),out of 175 patients(149 with CD and 26 with UC),57 showed abnormal liver laboratory tests.In this study,the authors used the Roussel Uclaf Causality Assessment Method (RUCAM)score to predict the risk of hepatic injury caused by drugs.A score of 0 rules out DILI,1-2 means unlikely DILI,3-5 possible DILI,6-8 probable DILI,and > 8 highly probable DILI.Eleven patients had a RUCAM score > 3,but just one patient had a score > 8.Usually,liver injury due to IFX occurs after multiple infusions and a mean latency of 14-18 wk from induction.In this context,the RUCAM score is not a diagnostic test,but it is useful to predict DILI relying on LFT,timing of drug initiation and cessation,and on liver biopsy,when performed[111].
Although IFX,ADA and etanercept are anti-TNF drugs,they are structurally different.This explains the different responses to these agents and the different capacity to induce liver injury.Some authors have described how patients tolerate successful treatment with another molecule after a prior DILI episode induced by an anti-TNF agent.This suggests a lack of cross-toxicity within this class of drugs.
The pathogenetic mechanism underlying anti-TNF hepatotoxicity is still unknown.As liver injury can occur after a singular infusion it seems more an idiosyncratic injury rather than a dose-dependent one[107].A genetic predisposition may be considered.Another hypothesis is that anti-TNF agents may trigger a pre-existing autoimmune disorder or generate autoantibodies:the binding of IFX to the transmembrane TNFalpha can lead to apoptosis of monocytes and T-lymphocytes with exposure of nucleosomal autoantigens and the production of autoantibodies[112,114].Another possibility is that anti-TNF drugs inhibit T-lymphocytes activity,thus suppressing auto-reactive B cells;this may lead to increased humoral autoimmunity[114].However,there are several cases without evidence of autoimmunity,in which direct liver injury is involved.
DILI caused by anti-TNF agents can show different patterns:Hepatocellular injury in 75% cases,but also a mixed pattern,most rarely with cholestasis,while few cases of acute liver failure have been described.Colinaet al[115]reported histological necroinflammation caused by IFX,with bridging and massive necrosis in the most severe cases and some features of autoimmune injury with piecemeal necrosis in the periportal interface and prominent plasma cells infiltration.Liver injury caused by anti-TNF drugs is associated with the presence of autoimmunity markers in some patients:anti-nucleus,anti-DsDNA and anti-smooth muscle actin positivity and/or histologic features of AIH are described for IFX,ADA and etanercept.In a study analyzing 34 patients undergoing anti-TNF treatment with DILI,22 were positive for such antibodies and showed higher levels of ALT than seronegative patients.Fifteen out of 22 subjects underwent liver biopsy that revealed clear features of autoimmunity[116].Indeed,it is difficult to distinguish between AIH and drug-induced AIH,since these conditions may have similar clinical,biochemical,serological and histological features.Actually,IFX-induced AIH is rare in IBD patients and is described more often in rheumatology patients.In several studies,autoimmunity features were treated with corticosteroids,achieving in some cases a reduction or disappearance of autoantibodies titer;this suggests an immune-mediated DILI rather than an anti-TNF induced AIH.Ierardiet al[117]reported a case of acute liver injury after a single IFX administration.Analogously,Adaret al[118]described the first case of AIH caused by ADA that resolved after treatment cessation and corticosteroid therapy.
There is still a lack of consensus on the management of DILI induced by anti-TNF agents.The prognosis is usually favorable with normalization of LFT without cessation of anti-TNF therapy.Liver enzymes should be monitored before starting treatment and then monitored periodically,especially during the first 3 mo.If ALT remains < x3 ULN,anti-TNF can be continued until resolution;if ALT is persistently elevated > x3 ULN or in the case of jaundice,corticosteroids and liver biopsy should be considered.If a DILI is documented,anti-TNF withdrawal is still controversial.Also,the necessity to obtain an autoimmune panel before starting anti-TNF treatment is debated:several studies demonstrated that this practice does not predict the risk of developing drug-induced AIH and that anti-TNF therapy could be continued in the presence of asymptomatic anti-nucleus positivity[102].
Anti-Integrins
Natalizumab and vedolizumab were approved some years ago for the treatment of IBD.Both drugs have shown a good safety profile,but in the post-marketing phase,6 cases of significant DILI associated with natalizumab were reported to the FDA[119].Liver injury caused by natalizumab is rare with a 5% rate of asymptomatic liver enzymes elevation and it can manifest with both the hepatocellular and cholestatic pattern and can be associated with jaundice.Some cases with autoimmune features(autoantibodies positive)have also been described[120].The guidelines recommend monitoring LFT before starting the treatment and then every 3 or 6 mo[121].Nevertheless,the use of natalizumab is quite rare in IBD due to possible severe neurologic complications such as progressive multifocal leukoencephalopathy[122].
Similar to natalizumab,liver injury associated with vedolizumab is rare,less than 2% in clinical trials,with both the hepatocellular or cholestatic pattern[123].Similar to natalizumab,the guidelines recommend monitoring liver enzymes every 3-6 mo.
Anti IL12/23
Ustekimumab was approved for CD treatment in 2016 and UC treatment in 2019.Most of the data regarding hepatotoxicity induced by ustekimumab comes from dermatologic studies.In PHOENIX 1 and 2,both studies evaluated the efficacy and safety of ustekimumab in patients with psoriasis,and the rate of liver enzymes abnormalities was low(between 0.5% and 2%)and similar between the case and control group[124,125].A small retrospective study including 44 patients with psoriasis treated with ustekimumab described cases of mild elevation of liver enzymes and no cases of severe DILI[126].Some case reports described spontaneous regression of liver injury after ustekinumab withdrawal[127].
Small molecules
Tofacitinib was approved for UC treatment in 2018.Liver enzymes elevation with a hepatocellular pattern has been rarely described[128].One case of possible AIH was reported,but liver injury due to other drugs could not be excluded[129].Monitoring liver enzymes periodically during tofacitinib treatment is recommended.
Ozanimod is a new molecule introduced for IBD treatment.Aspartate transaminase increases 32 wk after drug exposure were described in 2% and 1% of patients treated with 0.5 mg and 1 mg of ozanimod,respectively.Preliminary data suggest a low rate of hepatotoxicity associated with these new therapeutic approaches[102].
PORTAL VEIN THROMBOSIS
Portal vein thrombosis(PVT)is a common event in IBD.Indeed,IBD patients have a high risk of thromboembolism due to systemic inflammation and alterations in the concentrations of some coagulation factors,such as high factor V and VIII or low antithrombin III[130].
In a retrospective study,the incidence of thromboembolic events in patients with IBD rose from 5.65% in 2000 to 7.17% by 2009[131].In particular,the prevalence of PVT in IBD has been estimated to be about 0.17%[132].There are several causes of PVT,including inflammation,immobilization,major extent of colon disease,disease severity,surgery,use of corticosteroids and smoking.For that reason,the guidelines recommend starting heparin when facing an acute flare of UC,for PVT prophylaxis[133].
After the onset of PVT,complications such as portal hypertension,bleeding or even death are not common,but early anticoagulation is safe and associated with a better outcome,and the use of novel direct oral anticoagulants was associated with particularly favorable outcomes in this setting[134].
CONCLUSIONS
In conclusion,the scenario of liver involvement of IBD patients is quite extensive.The relationship between IBD and PSC is the most studied.PSC is a disease that currently has no effective medical therapy;therefore,research on drugs that may be effective for both hepatic and intestinal disorders is required.Moreover,the strategies for early neoplasia screening(both CCA and CCR)in these patients are not sufficiently efficient at present,and this is a pitfall that needs to be resolved.
NAFLD in IBD is another focal issue,as this novel comorbidity may complicate the management of IBD patients due to its multifaceted aspects.
As viral hepatitis may soon become a thing of the past,due to the advent of drugs with very high success rates,some patients will still require careful monitoring,especially when immunosuppression for IBD is required.
Among the drugs currently in use to treat IBD,thiopurines,mesalazine derivatives and methotrexate are the most studied,and periodic assessment of LFT is still required.However,the field of DILI is expected to expand quickly,as several novel molecules for the treatment of IBD(tyrosine kinase inhibitors,small molecules and others)have been developed,and their possible hepatotoxicity will be a matter of debate.
杂志排行
World Journal of Hepatology的其它文章
- Incidence of umbilical vein catheter-associated thrombosis of the portal system:A systematic review and meta-analysis
- Role of endoscopic ultrasound in the field of hepatology:Recent advances and future trends
- Porta-caval fibrous connections—the lesser-known structure of intrahepatic connective-tissue framework:A unified view of liver extracellular matrix
- Promising diagnostic biomarkers of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:From clinical proteomics to microbiome
- Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis
- Chelation therapy in liver diseases of childhood:Current status and response
