Current strategies to induce liver remnant hypertrophy before major liver resection
2021-12-06CelesteDelBassoMartinGaillardPanagiotisLainasStellaZervakiGabrielPerlemuterPierreChaguLaurenceRocherCosminSebastianVoicanIbrahimDagherHadrienTranchart
Celeste Del Basso,Martin Gaillard,Panagiotis Lainas,Stella Zervaki,Gabriel Perlemuter,Pierre Chagué,Laurence Rocher,Cosmin Sebastian Voican,Ibrahim Dagher,Hadrien Tranchart
Celeste Del Basso,Martin Gaillard,Panagiotis Lainas,Stella Zervaki,Ibrahim Dagher,Hadrien Tranchart,Department of Minimally Invasive Digestive Surgery,Antoine Béclère Hospital,Clamart 92140,France
Gabriel Perlemuter,Cosmin Sebastian Voican,Department of Hepato-Gastroenterology and Nutrition,Antoine Béclère Hospital,Clamart 92140,France
Pierre Chagué,Laurence Rocher,Department of Radiology,Antoine Béclère Hospital,Clamart 92140,France
Abstract Hepatic resection is the gold standard for patients affected by primary or metastatic liver tumors but is hampered by the risk of post-hepatectomy liver failure.Despite recent impro-vements,liver surgery still requires excellent clinical judgement in selecting patients for surgery and,above all,efficient pre-operative strategies to provide adequate future liver remnant.The aim of this article is to review the literature on the rational,the preliminary assessment,the advantages as well as the limits of each existing technique for preparing the liver for major hepatectomy.
Key Words:Liver regeneration;Major hepatectomy;Liver insufficiency;Future liver remnant;Portal vein embolization
INTRODUCTION
Hepatic resection is the gold standard for patients affected by primary or metastatic liver tumors but is hampered by the risk of post hepatectomy liver failure(PHLF).Indeed,PHLF is considered the most frightening complication of liver surgery,representing a major source of severe morbidity and mortality[1].Despite recent improvements,liver surgery still requires excellent clinical judgement in selecting patients for surgery and,above all,efficient pre-operative tools to provide an adequate future liver remnant(FLR).
The liver has a unique capacity of preserving its volume due to regeneration.The atrophy-hypertrophy phenomenon is a prime example of the liver’s pathophysiologic(atrophy)and restorative(hypertrophy)response to injury[2].It occurs whenever there is impairment of bile or blood flow:the liver reacts with atrophy of the region concerned and with compensatory hypertrophy of the less or not impaired regions,resulting in characteristic gross deformity of the organ and,in some instances,in rotation of the liver around a virtual hilar axis[3].The mechanisms that induce cellular division are complex and based on different inflammatory cytokines.The Hepatocyte Growth Factor(HGF)seems to be the main mitogenic factor and its role has been established in liver regeneration[4].
The first case ofin vivohuman hepatic regeneration was described by Packet al[5]in 1962.Starting from animal models in the first half of the 20thcentury,it was recognized that liver regeneration could also be induced by portal vein ligation(PVL)[6].In 1986,the first cases of percutaneous transhepatic portal vein embolization(PVE)were performed before liver resection in the setting of hepatocellular carcinoma[7],and a few years later Makuuchiet al[8]reported the utility of PVE in promoting FLR hypertrophy prior to hepatic resection in patients with hilar cholangiocarcinoma.Since those initial reports,preoperative PVE has been established as the standard procedure for obtaining FRL hypertrophy,increasing the eligibility of patients for major hepatectomy as well as improving postoperative outcomes and safety.However,concerns regarding the insufficient increase of FLR and/or concomitant tumoral progression after PVE have led to the development of recent alternative techniques to push further the limits of liver surgery.
The aim of this article is to review the techniques available for preparing the liver for major hepatectomy,and to depict their advantages and limitations.
LIVER REGENERATION
The liver’s unique capacity for regeneration was first recorded in the legend of Prometheus in Greek mythology and it represents the basis of the treatment of many liver diseases.Regeneration of the liver is a pathophysiological process,embracing both hypertrophy(increase in cell size or protein content in the prereplicative phase)and hyperplasia(increase in cell numbers).Both events can take place independently[9].The mechanisms of liver regeneration have mainly been studied after extensive hepatectomy.The players of regeneration following the different techniques exposed in this article are thought to be similar to those after hepatectomy,but the precise mechanism remains unknown.Basically,the regeneration process is a cytokine- and growth-factor-mediated pathway.The main cytokine-mediated pathways include members of the innate immune system,tumor necrosis factor(TNF)α and interleukin(IL)-6,and growth-factor-mediated pathways are regulated by HGF and transforming growth factor(TGF)α[10].It is a multi-step process,starting from the “priming” of hepatocytes,the moment they acquire replicative capacity,followed by the proliferative step in which an adequate cell mass is attained,and a termination stage in which liver cell proliferation is ended once the necessary functional mass has been reached[11].Proliferation of hepatocytes advances from periportal to pericentral areas of the lobule,as a wave of mitoses[12].Proliferation of biliary epithelial cells occurs a little later than hepatocytes.The particularity of liver regeneration is that replacement of the lost hepatic mass is not mediated by selected stem cells proliferation but it entirely depends on mature adult hepatocytes and other hepatic cell types.Concerning the time interval,as far as we know,normal liver weight is reestablished within 8-15 d in humans[13].
POST-HEPATECTOMY LIVER FAILURE
Although morbidity and mortality after liver surgery have improved over the past 10 years,PHLF is still reported in up to 8%,ranging from 1.2% to 32%,and depends on the patient’s condition and functional reserve of the liver before resection[1].Different definitions of PHLF are available.In 2011,the International Study Group of Liver Surgery(ISGLS)defined PHLF as “a post-operatively acquired deterioration in the ability of the liver to maintain its synthetic,excretory,and detoxifying functions,which are characterized by an increased International Normalized Ratio(INR)and concomitant hyperbilirubinemia on or after postoperative day 5”[14].It is worth pointing out that severe PHLF is associated with a mortality rate of 54%.
A related syndrome that results in a transient but sometimes fatal form of liver failure has been described following liver transplantation(LT)but also after extensive liver resection.This is the so-called Small For Size Syndrome(SFSS).In 2005,Dahmet al[15]defined SFSS as a graft to recipient weight ratio < 0.8% alongside two of the following for three consecutive days;bilirubin > 100 mmol/L,INR > 2 and encephalopathy grade 3 or 4.In this definition,SFSS is a clinical syndrome characterized by post-operative liver dysfunction,prolonged cholestasis and coagulopathy,portal hypertension and ascites.It can lead to a higher rate of hemorrhage,sepsis and gastrointestinal bleeding[16].The key point of SFSS is the presence of portal hypertension and intra-hepatic portal congestion as the underlying cause of liver failure[17].
PREDICTION OF PHLF RISK
Despite improvements in surgical and postoperative management,parameters determining the degree of possible hepatectomy remain largely uncertain.Different patient related and surgical factors have to be considered to decrease PHLF incidence.Surgical factors include the extent of resection and volume of FLR,duration of intraoperative liver ischemia during portal pedicle clamping,duration of surgery and the need for blood transfusion.The risk of PHLF is highly influenced by the quality of underlying liver parenchyma.The type of underlying liver parenchyma is frequently assessed by preoperative liver biopsy,but noninvasive methods,such as liver stiffness,are now available.For example,liver stiffness measurement by transient elastography(Fibroscan)predicts persistent hepatic decompensation in patients undergoing resection for hepatocellular carcinoma[18].
It is generally thought that the minimal functional liver mass needed for adequate postoperative liver function is estimated to be 20%-25% in patients with normal liver parenchyma,whereas those with chemotherapy-induced liver injury require a FLR volume of approximately 30%,while those with cirrhosis at least a 40% minimal functional liver mass[19].Therefore,standardized FLR volume can be easily evaluated by a tridimensional computed tomography(CT)reconstruction method,as FLR/ estimated total liver volume[20].Estimated total liver volume is generally calculated using a formula based on body surface area[21].
In addition to volume,estimation of FLR function is an important factor.Typical biochemical parameters,such as liver function tests,albumin,and clotting factors must be evaluated.The old but effective Child-Turcotte-Pugh score,which was introduced in 1964,still represents a simple system for grading liver function[22].The model for end-stage liver disease score,which is mainly used in liver transplantation,can also predict the survival rate of cirrhotic patients to better select ideal candidates for surgery[23].A recent study also showed that mean serum level of hyaluronic acid can be a useful tool,especially when liver biopsy is not feasible[24].
Dynamic tests of liver function can also be used.The most well-known is indocyanine green(ICG)clearance.ICG is a water soluble,inert,fluorescent tricarbocyanine dye with protein binding close to 95%(mainly,alpha1- and betalipoproteins and albumin),a hepatic extraction rate above 70%,and is almost completely excreted in its unchanged form by the liver.ICG elimination can be expressed as ICG plasma disappearance rate(ICGPDR)or retention rate at 15 min(ICGR15),reflecting liver function.Use of the ICG test for patient selection has been shown to decrease postoperative mortality[25].
In recent years,there have been several attempts to assess hepatobiliary magnetic resonance imaging(MRI)as a tool to predict liver dysfunction.Since it was first described in 1991 by Weinmannet al[26],MRI has been showed to provide both global and segmental liver function information,and postoperative remnant liver function thanks to the measurement of liver signal intensity in the hepatobiliary phase.
Liver function evaluation by nuclear medicine techniques is also more and more used.Dynamic 99mTc-mebrofenin hepatobiliary scintigraphy has been used to provide quantitative information on total and regional liver function.The hepatic uptake of 99mTc-mebrofenin is similar to the uptake of organic anions such as bilirubin[27].This technique efficiently estimates the risk of postoperative liver failure especially in patients with uncertain quality of liver parenchyma[28].The 99m Tc-GSA is another recently proposed agent that is not affected by hyperbilirubinemia and can be used for liver function assessment in cholestatic patients[29].Finally,the LiMAx test allows real-timein vivodetermination of liver Cytochrome P450 1A2(CYP1A2)activity.The CYP1A2 is not influenced by cholestasis or drugs and is ubiquitous in liver parenchyma.Intravenous administration of 13C methacetin,a substance exclusively metabolized by CYP1A2,with continuous real-time breath analysis represents the basis of the LiMAx test[30].
PORTAL VEIN EMBOLIZATION
Since the first report in 1986,PVE has progressively become the gold standard for inducing liver hypertrophy with satisfying safety and efficacy[31].Initially described by laparotomy,the portal system access is now obtained by percutaneous puncture of the portal vein.According to the operator’s preference,an ipsilateral or contralateral approach can be chosen,in reference to the segment bearing the tumor.The ipsilateral approach has the main advantage of protecting the FLR from injury[2]whereas the contralateral approach facilitates embolization[32].Irrespective of the approach chosen,PVE is performed in a retrograde manner(Figure 1).Many embolic materials have been used for PVE without significant differences in terms of hypertrophy.Embolic materials include fibrin glue,N-butyl-2-cyanoacrylate and ethiodized oil,gelatin sponge and thrombin,coils,microparticles[e.g.,polyvinyl alcohol(PVA)particles or tris-acryl gelatin microspheres]and absolute alcohol[33].A nonabsorbable material is generally used.However,interesting results were reported with the use of an absorbable powder material(Gelfoam®powder,Pfizer,New York,USA)that lasts approximately 2 wk,leading to temporary PVE.In an animal model,this method showed efficient and stable liver regeneration[34].These results were confirmed in a limited preliminary series in clinical practice[35]and a prospective study is undergoing(EMBORES study,NCT02945059).One of the advantages of temporary PVE is that it can theoretically be repeated several times to boost more liver hypertrophy,as has been suggested in an animal model[36].
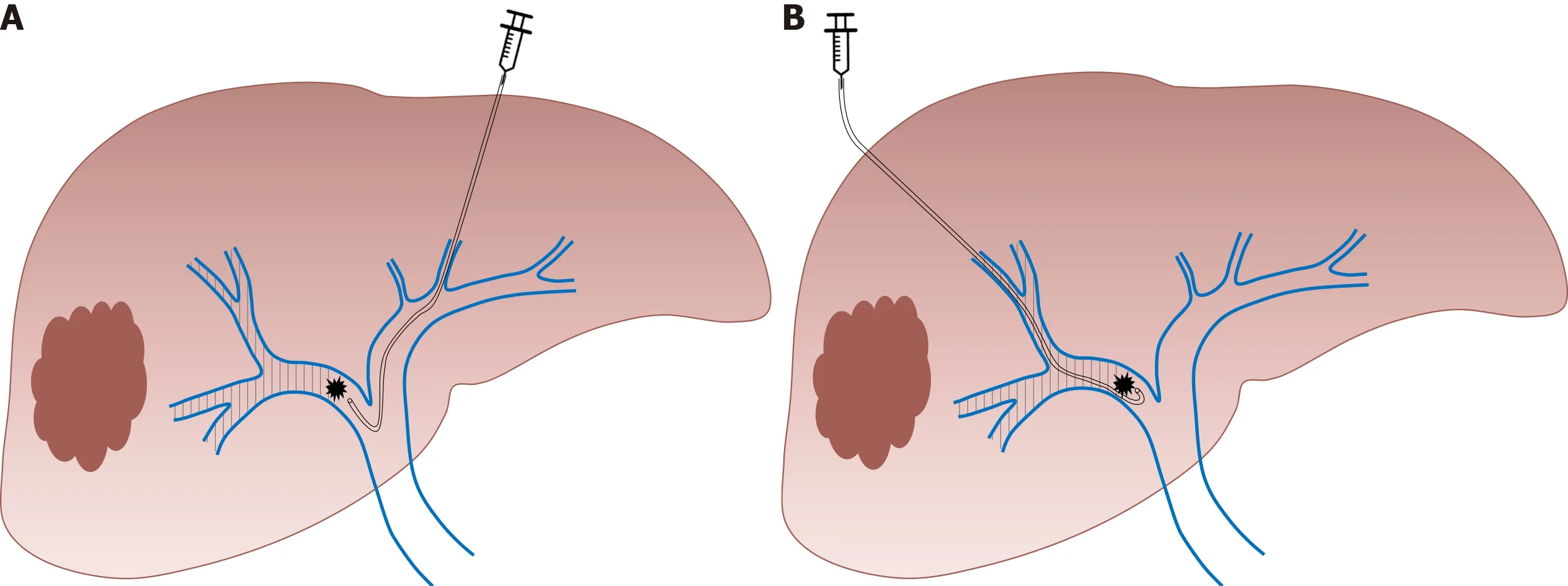
Figure 1 Right portal vein embolization using.A:Contralateral;B:Ipsilateral approach(authors’ own work).
PVE is successfully performed in more than 90% of cases[37].A computed tomography scan with volumetric evaluation is generally performed between 4 and 8 wk after embolization.PVE induces a FLR hypertrophy than can reach 40%[37],with a low 2% morbidity rate and no mortality in the vast majority of studies[37-39].PVE is considered an efficient method,allowing successful hepatectomy in more than 70% of cases[37,38,40].
Contraindications to PVE are extensive portal thrombus and important portal hypertension[41].Another potential limit of PVE is the risk of tumor growth during the 4 to 8 wk separating PVE and liver surgery.In addition,several authors have suggested that PVE itself could promote tumor growth within the embolized liver[42-45].Among others,these reasons have led to the development of alternative strategies.
PORTAL VEIN LIGATION(PVL)AND TWO-STAGE HEPATECTOMY
As it requires a surgical procedure with portal pedicles dissection,PVL is nowadays mainly indicated in the setting of two-stage hepatectomy(TSH)for the treatment of bilobar liver disease[46,47].In the TSH strategy,the first surgical step includes tumoral clearance of the FLR(usually by parenchymal spearing resections or locoregional treatment like radiofrequency ablation)and concomitant PVL that allows FLR growth.In the second step,after liver regeneration(approximately 4 to 8 wk later),major liver resection is performed(usually right or right extended hepatectomy)(Figure 2).Similarly,PVL can be performed for the management of patients presenting synchronous colorectal metastases or neuroendocrine tumors[47].The first surgical step associates colorectal resection with PVL,followed by major liver surgery in the second procedure.However,many centers have adopted PVE(performed by the percutaneous approach after FLR clearance or colorectal resection)for two-step procedures,avoiding portal pedicle dissection and facilitating the second procedure[48].
It was initially suggested that PVE resulted in superior FLR growth compared to PVL[49]as in theory PVE allows distal portal obstruction which decreases the possibility of intrahepatic collateral development.Several studies demonstrated that the results are globally similar[50,51].In fact,the debate concerning the efficiency of PVL compared to PVE is no longer relevant.PVL requires a surgical procedure and can appear as an alternative to PVE only when a two-step surgery is planned.In other cases,percutaneous PVE is clearly a simpler and better tolerated approach.
ASSOCIATING LIVER PARTITION AND PORTAL VEIN LIGATION FOR STAGED HEPATECTOMY
The aim of this alternative strategy,described by Schnizbaueret al[52]in 2012,is to induce rapid and massive liver hypertrophy,to allow liver surgery in a short period of time in patients with initially very limited FRL volume.The first step of the associating liver partition and portal vein ligation for staged hepatectomy(ALPPS)procedure consists of performing PVL and anin situsplitting of the liver parenchyma,leaving the hepatic artery,bile duct,and hepatic vein intact until the subsequent operation.This first surgical step can be associated with tumoral clearance of the FRL.During the second operation(that can be performed one to two weeks later)the remaining hepatic artery,bile duct,and hepatic vein are divided and the liver specimen is extracted(Figure 3).
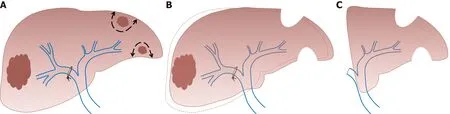
Figure 2 Two-stage hepatectomy procedure starts with tumoral clearance of the future liver remnant.A:Concomitant right portal vein ligation;B:Allowing left liver growth;C:Ends with right hepatectomy(authors’ own work).

Figure 3 Associating liver partition and portal vein ligation for staged hepatectomy procedure.A:Starts with in situsplitting of the liver parenchyma with concomitant right portal vein ligation;B:Ends with right hepatectomy(authors’ own work).
The first report demonstrated a morbidity rate of 44% and a mortality rate of 12%[52],and triggered an intense debate on the safety of this procedure,limiting its promotion worldwide.The morbi-mortality rate decreased with experience but remains high,with approximately 40% of major postoperative complications and 9% of mortality[53].Nevertheless,the ALPPS technique induces more than 65% of FLR growth in approximately 7 days[52-55]and the second procedure is feasible in more than 90% of cases[56].The main advantage of the ALPPS procedure is the rapid increase in FLR volume in a short interval and therefore a shorter interval between the two stages.Although the volumetric results of this technique are impressive,several authors suggested that FLR volume hypertrophy is not correlated to functional improvement[57,58]which could partly explain the high morbidity of the procedure.Besides,concerns have been raised by some authors regarding potentially poorer oncological results comparing to the classical TSH[59].The results of a meta-analysis comparing ALPPS to TSH showed that the extent of FLR increase was not different between the two groups[60].The time needed to reach final liver volume was shorter in ALPPS than in the TSH approach[60].In this meta-analysis,ALPPS was associated with a higher incidence of major and overall morbidity and mortality compared to TSH[60].However,in a recent randomized controlled trial,Hasselgrenet al[61]observed similar morbidity between ALPPS and classical TSH and an improved survival in the ALPPS group.
To decrease complication rate,a variety of technical modifications have been proposed such as partial-ALPPS,mini-ALPPS,tourniquet-ALPPS,hybrid-ALPPS,microwave ablation-assisted ALPPS and radiofrequency ablation-assisted ALPPS.Huanget al[62]suggested in a systematic review that a partial ALPPS technique in which only partial parenchymal sparing is performed during the first surgical step could achieve lower morbidity and mortality rates,reaching the same FLR hypertrophy rate as ALPPS in non-cirrhotic patients.
SEQUENTIAL TRANS-ARTERIAL EMBOLIZATION(TAE)AND PORTAL VEIN EMBOLIZATION
Although PVE remains the gold standard for FLR hypertrophy,two concerns persist with this approach:An insufficient contralateral hypertrophy,particularly in patients with underlying liver disease(steatosis,fibrosis or cirrhosis),and the eventuality of tumor progression while waiting for the non-embolized liver to hypertrophy.In particular,portal flow interruption may induce a compensatory increase in arterial blood flow of embolized segments and result in a paradoxical growth of tumors vascularized by arterial blood flow.In this context,it has been postulated that the addition of trans-arterial embolization(TAE)or trans-arterial chemoembolization(TACE)would produce more rapid and extensive FLR growth(by obtaining obliteration of intrahepatic arterioportal shunts)and may help to counteract the stimulating effect on tumor growth[63].Therefore,hepatocellular carcinomas,which are tumors particularly vascularized by arterial blood flow and develop generally in underlying pathological liver parenchyma,are the main target of this combined strategy[64].
During TAE,a catheter is directly insertedviaeither the common femoral or left radial artery and an intra-arterial injection of a combination of microspheres and PVA particles is performed in the arterial branches of the segments to be resected.During TACE,an intra-arterial injection of a cytotoxic drug is performed such as doxorubicin,epirubicin,idarubicin,mitomycin C,or cisplatin,that is emulsified in ethiodized oil(Lipiodol®Ultra-Fluid,Guerbet).This is followed by intra-arterial injection of an embolic agent,such as gelatin sponge,PVA particles,or microspheres[65](Figure 4).TACE can also be performed using recently developed drug-eluting beads(DEB)that allow the slow release of chemotherapeutic agents,and increase ischemia intensity and duration[65].
A sequential approach,with a time interval of a few days,is recommended to limit the risk of nontumoral liver ischemic necrosis[66]and TAE is mostly performed before PVE[66,67].Although the number of patients reported in studies that evaluated this approach is limited,observed FLR hypertrophy is generally superior to that observed after isolated PVE.For example,Yooet al[68]reported a statistically significant increase of 7.3% and 5.8% in FLR(over the total liver volume)for sequential TACE/PVE and isolated PVE,respectively.
An important elevation of transaminases is generally observed after this sequential approach without important clinical consequences.In the largest series reporting this approach,Penget al[64]reported 29 procedures without deaths and only one complication and 27 patients(93%)underwent subsequent hepatectomy.Posthepatectomy morbidity and mortality among these patients was 27.5% and 6.9%,respectively.
Theoretical contraindications of this method include extensive portal thrombus,important portal hypertension or previous biliary surgery(biliodigestive anastomosis)which exposes the patient to hepatic abscess formation after arterial embolization.
LIVER VENOUS DEPRIVATION
This technique consists of performing conventional PVE and ipsilateral hepatic vein obstruction(Figure 5).By associating hepatic vein embolization,the aim is to eliminate any residual portal vein flow and reduce hepatic artery inflow which can further encourage liver regeneration.Initially described as a sequential approach in which hepatic vein embolization is secondarily performed in case of insufficient FLR growth after PVE,it was demonstrated that both procedures(portal and hepatic vein embolization)can be performed simultaneously[69,70].This novel approach is particularly interesting as it allows important liver regeneration with good tolerance.Although no study comparing ALPPS to LVD is available,it has been suggested that LVD could overcome the limits of ALPPS,abolishing the necessity of two major surgical interventions in close sequence.
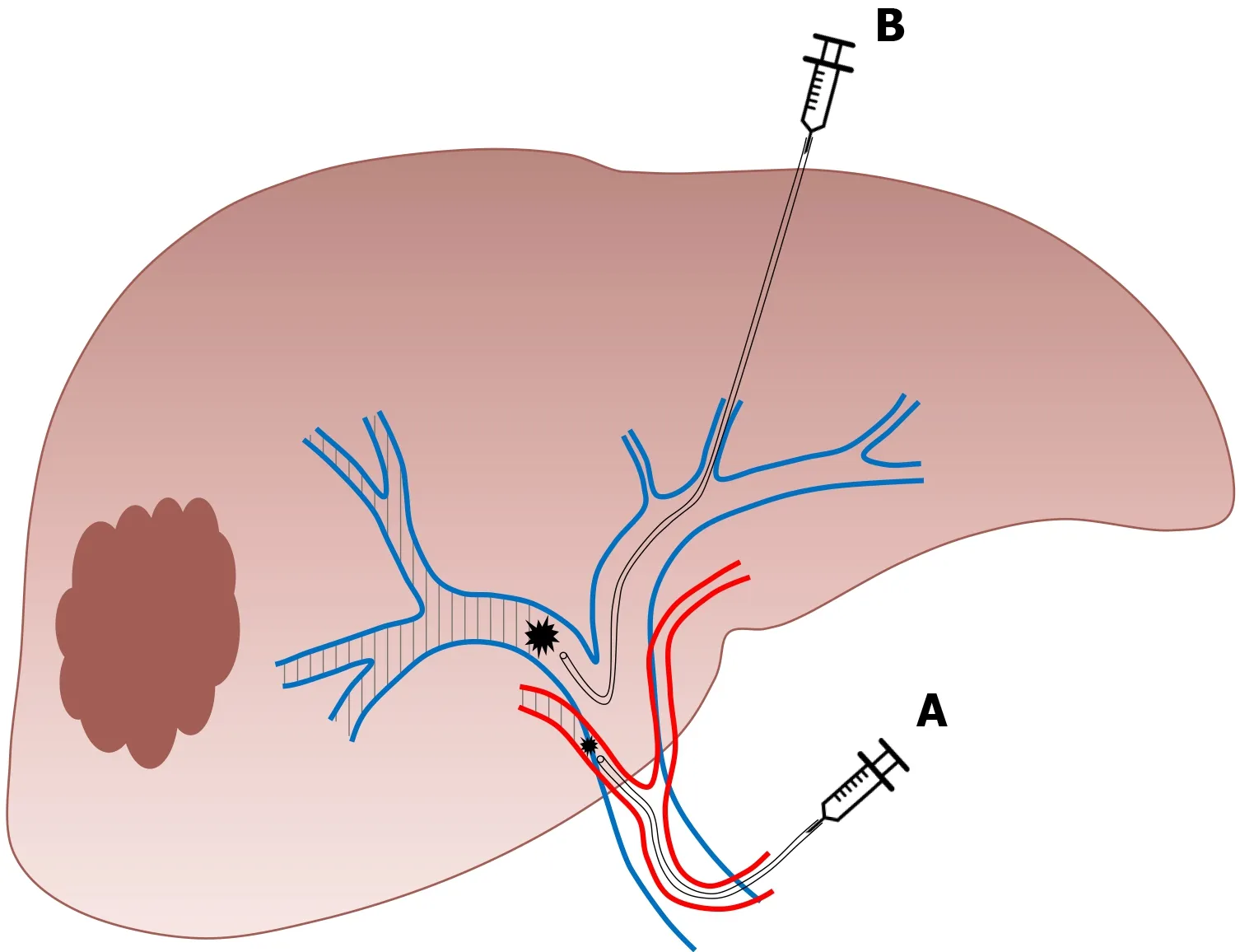
Figure 4 Sequential embolization.A:Trans-arterial embolization;B:Portal vein embolization of the right liver(authors’ own work).

Figure 5 Right liver venous derivation associates in a sequential or concomitant approach.A:Right portal vein embolization;B:Ipsilateral hepatic vein embolization(authors’ own work).
Firstly,PVE is performed as previously described.For hepatic vein embolization,a vascular plug is placed in the proximal part of the hepatic vein to avoid migration of embolization agent.The vein is then embolized with a mixture of ethiodized oil and N- butyl cyanoacrylate[71].The term “extended LVD” is used for concomitant embolization of the right and middle hepatic vein with the right portal branch[57].
The results of this approach on FLR increase are superior to those observed after isolated PVE.In a recent large comparative study,Laurentet al[71]observed a FLR volume increase of 28.9% after PVE compared to 61.2% after LVD(P< 0.0001).In this study,LVD allowed surgery in 86.4% of patients and no PHLF was reported.Kobayashiet al[72]observed similar results with a superior FLR hypertrophy after LVD compared to PVE(35%vs24%,P= 0.034).In addition,the tolerance of LVD seems to be similar to the tolerance of isolated PVE[71,72].
RADIATION LOBECTOMY
This recent approach is derived from trans-arterial radioembolization with yttrium-90[73].In radiation lobectomy(RL),radioembolization of both the tumor and the nontumoral liver parenchyma that will be secondarily resected is performed,which requires higher radiation doses[74,75].This technique allows concomitant tumoral control and FLR increase.One major advantage of this approach is that it could be carried out in patients with portal vein thrombosis[75].
The procedure is well-tolerated[74]with transient moderate adverse events.Results in terms of FLR volume growth are very similar to those observed after PVE.Voucheet al[74]reported 45% of FLR hypertrophy and observed a correlation between the presence of a portal vein thrombosis and FLR growth.However,series reporting major liver resection after RL are scarce[76,77].Andelet al[77]recently reported 10 major hepatectomies in patients that were initially treated with RL for insufficient functional FLR.The RL allowed a 41% increase in FLR volume with 84% of FLR function increase(evaluated on scintigraphy).All resections were performed without major intraoperative problems.Only one patient developed a serious complication not directly related to the liver surgery and other complications were mild.
CONCLUSION
Careful initial evaluation of FLR volume and function is crucial before planning major liver resection.When required,several approaches are now available to decrease the risk of PHLF(Table 1)and thus postoperative mortality.Although PVE remains the gold standard,recent techniques that are derived from PVE might play an increasingly important role in future years.

Table 1 Indication,advantages,and disadvantages of existing approaches to induce liver remnant hypertrophy before major liver resection
杂志排行
World Journal of Hepatology的其它文章
- Incidence of umbilical vein catheter-associated thrombosis of the portal system:A systematic review and meta-analysis
- Role of endoscopic ultrasound in the field of hepatology:Recent advances and future trends
- Porta-caval fibrous connections—the lesser-known structure of intrahepatic connective-tissue framework:A unified view of liver extracellular matrix
- Promising diagnostic biomarkers of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis:From clinical proteomics to microbiome
- Fatty acid metabolism and acyl-CoA synthetases in the liver-gut axis
- Liver involvement in inflammatory bowel disease:What should the clinician know?
